Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.107987
Revised: April 29, 2025
Accepted: June 18, 2025
Published online: August 24, 2025
Processing time: 140 Days and 15.7 Hours
Gastric signet ring cell carcinoma (SRCC) is a rare, aggressive subtype of gastric cancer characterized by poor prognosis and distinctive biological behavior. Despite advances in gastric cancer treatment, SRCC remains difficult to diagnose early and manage effectively due to its infiltrative pattern and molecular varia
To investigate the prognostic factors, including human epidermal growth factor receptor 2 (HER2) expression, associated with survival outcomes in patients with gastric SRCC.
A retrospective analysis of 100 cases diagnosed between 2015 and 2019 was conducted, assessing demographic, clinical, and pathological data. HER2 expre
With a median follow-up of 43 months, the median patient age was 50 years, and males exhibited a higher mortality rate (P = 0.0107). Elevated serum carbohydrate antigen 19-9 and carcinoembryonic antigen levels were significantly associated with increased mortality (P = 0.00149 and P = 0.00163, respectively). Advanced tumor-node-metastasis stage and lymphovascular invasion were strong predictors of poor outcomes (P < 0.001 and P = 0.019). HER2 positivity correlated with higher mortality (P = 0.00882) but was not significantly linked to recurrence (P = 0.53). Surgical treatment significantly improved survival compared with non-surgical approaches (P = 0.0226).
These findings highlight the aggressive nature of SRCC with advanced disease stage, elevated tumor markers, and lymphovascular invasion contributing to poor outcomes. HER2 expression, though infrequent, may indicate worse prognosis, reinforcing the role of surgical intervention in survival improvement.
Core Tip: This study provided a comprehensive analysis of gastric signet ring cell carcinoma, emphasizing its unique histopathological features, clinical implications, and prognostic significance. By examining key tumor characteristics, including infiltration patterns and molecular alterations, the research highlighted the challenges in early diagnosis and treatment. The findings contributed to a deeper understanding of the aggressive nature of signet ring cell carcinoma and its impact on patient outcomes, underscoring the need for improved diagnostic strategies and targeted therapeutic approaches.
- Citation: Ebrahim NAA, Othman MO, Tahoun NS, Salama RA, Arafat A, Amin NH. Deciphering prognostic markers in gastric signet ring cell carcinoma: Human epidermal growth factor receptor 2 and other key factors. World J Clin Oncol 2025; 16(8): 107987
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/107987.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.107987
Gastric signet ring cell carcinoma (SRCC) is a rare but aggressive subtype of gastric cancer and is characterized by unique histopathological features and challenging clinical behavior. The hallmark of SRCC is its histological appearance in which tumor cells have mucin-filled cytoplasm displacing the nucleus to the periphery, forming a “signet ring” structure. This subtype is predominantly associated with diffuse gastric cancer and is known for its aggressive biological behavior, including a propensity for early dissemination and peritoneal metastasis. These traits distinguish SRCC from other gastric cancer subtypes and contribute to its poor prognosis despite advancements in gastric cancer treatment[1].
One of the critical challenges in managing SRCC lies in its dismal prognosis, which remains inferior to that of other histological subtypes. SRCC often presents at an advanced stage due to vague symptoms and its diffuse growth pattern, which eludes early detection. Even with advancements in diagnostic imaging and endoscopy, SRCC is frequently diagnosed at later stages when treatment options are limited. Studies revealed that SRCC demonstrated higher recurrence rates and mortality compared with other forms of gastric adenocarcinoma, underscoring the need for innovative therapeutic strategies tailored to its unique biology[2]. Current research highlights significant heterogeneity in the clinical behavior of SRCC, ranging from relatively better outcomes in early-stage disease to poor survival rates in advanced stages.
Unlike intestinal-type gastric cancer, SRCC exhibits a distinct pattern of metastasis, favoring peritoneal dissemination over hematogenous spread. This unique metastatic behavior necessitates a nuanced approach to staging and treatment. Additionally, SRCC is often resistant to conventional chemotherapy and radiotherapy, further complicating its mana
A growing area of focus in SRCC research is the exploration of molecular pathways and potential therapeutic targets. Emerging research into molecular markers and genomic profiling identified actionable targets, providing hope for more personalized treatment approaches[4]. Large-scale cohort studies, such as those analyzing data from international cancer registries, play a crucial role in advancing the understanding of SRCC. These studies not only provide insights into the clinical and molecular features of SRCC but also pave the way for the development of robust prognostic models. Such findings are critical in guiding clinical decision-making and tailoring treatment protocols to individual patient needs[1,2].
In summary, gastric SRCC remains a formidable challenge in oncology, demanding a multidisciplinary approach that integrates advanced diagnostic techniques, novel therapeutic strategies, and ongoing research into its unique molecular characteristics. By leveraging data from cohort studies and exploring innovative treatment avenues, the field moves closer to improving the prognosis and quality of life for patients affected by this aggressive malignancy.
The present study aimed to investigate key prognostic factors influencing survival outcomes in patients with gastric SRCC with particular emphasis on human epidermal growth factor receptor 2 (HER2) expression, serum tumor markers, and pathological features such as tumor-node-metastasis (TNM) stage, and lymphovascular invasion. A secondary objective was to evaluate the impact of different therapeutic approaches, particularly surgical intervention, on overall and disease-free survival. By clearly defining these objectives, the study contributed to a deeper understanding of SRCC behavior and to inform more effective, individualized management strategies for affected patients.
This retrospective study was conducted to explore prognostic factors in patients diagnosed with gastric SRCC. The study included patients treated and followed up over 5 years (from 2015 to 2019). Patients with a histopathologically confirmed diagnosis of gastric SRCC were included in the study. Eligibility criteria required that patients had undergone surgical or oncological treatment at the National Cancer Institute, Cairo University and had complete clinical, pathological, and follow-up data for a minimum of 12 months or until death. Patients were excluded if they had incomplete data, a dia
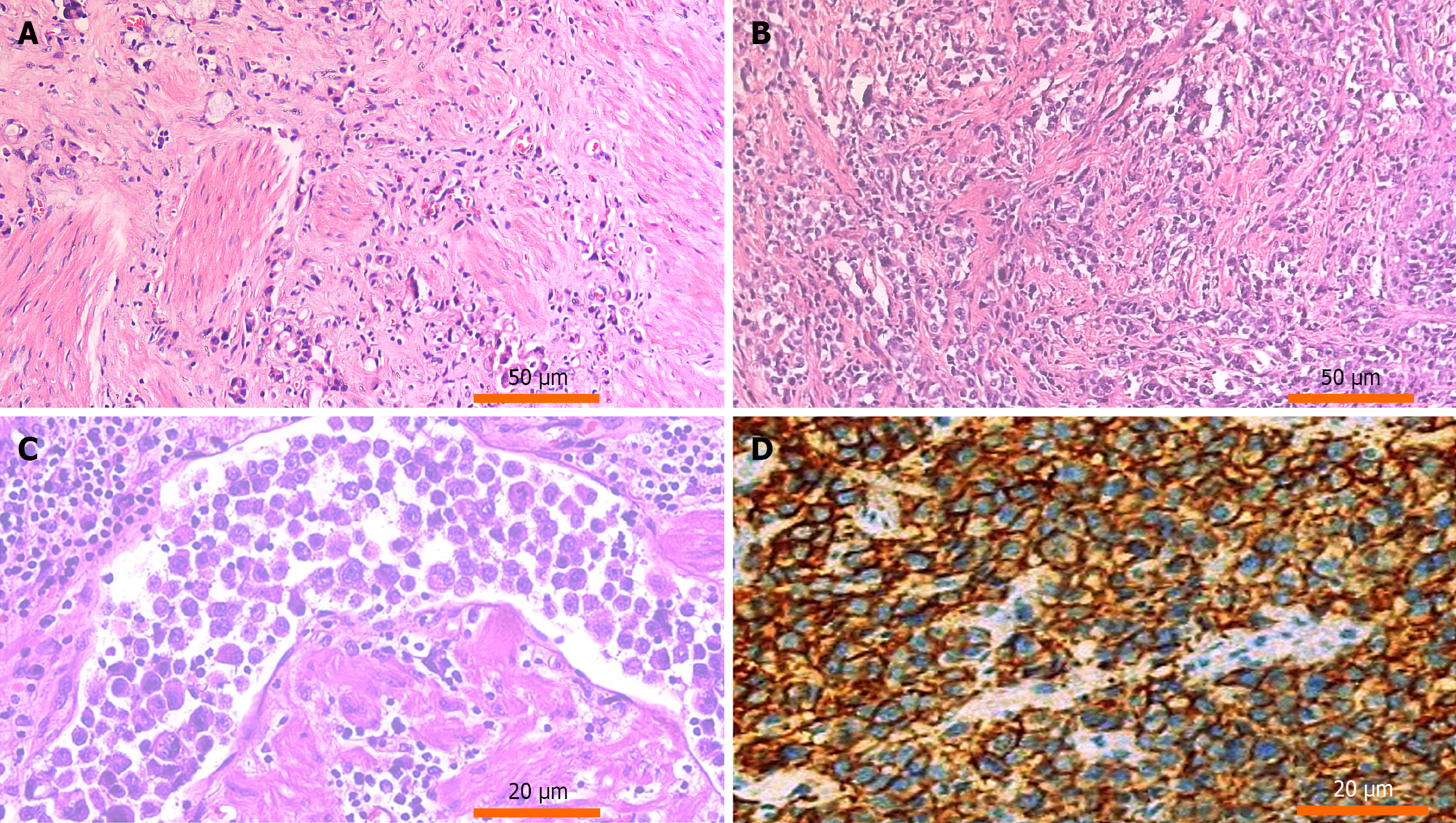
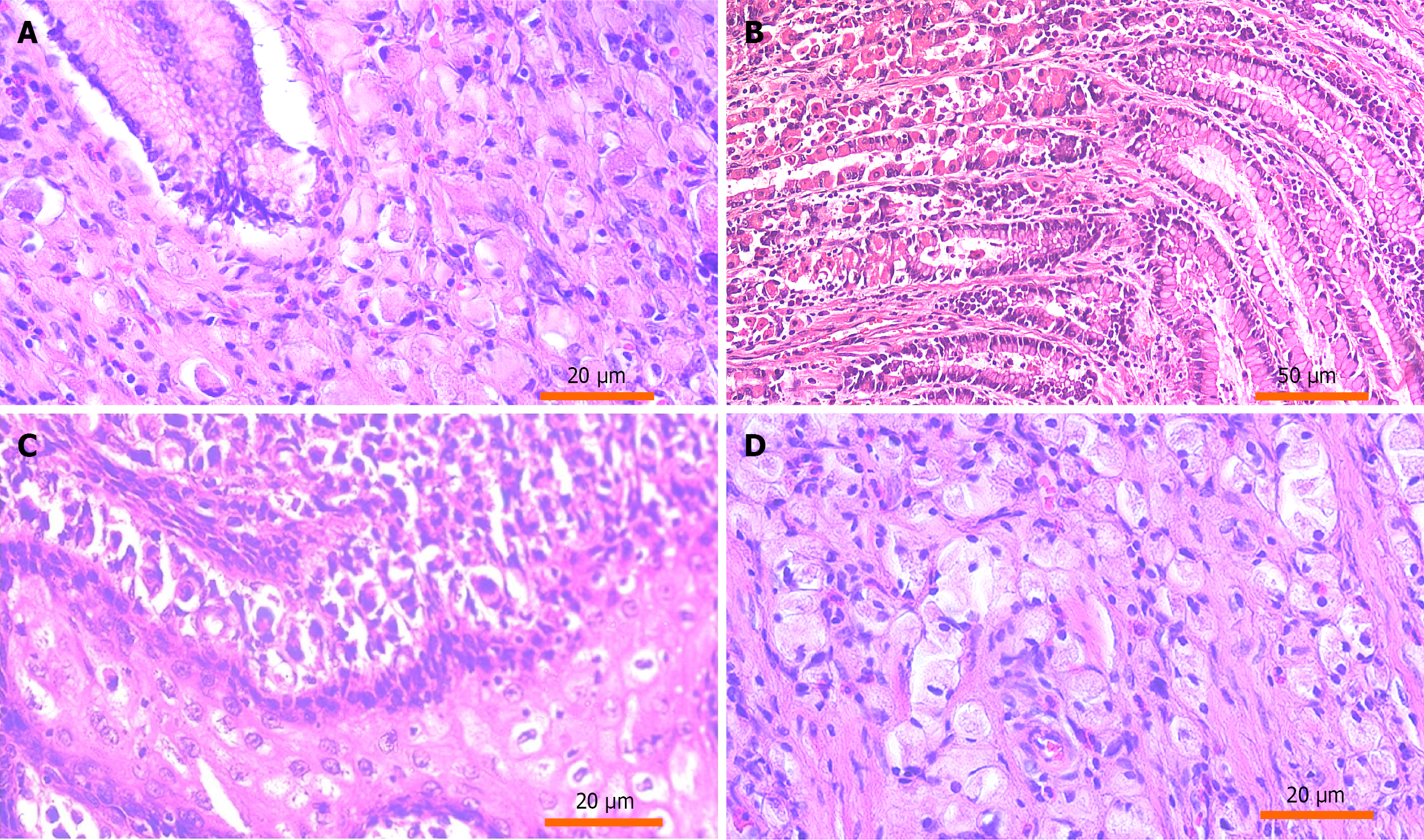
Hematoxylin and eosin-stained slides of tissue samples were retrieved. Histopathological evaluation confirmed the diagnosis and classification of gastric SRCC according to the World Health Organization classification of tumors of the digestive system.
HER2 expression in gastric SRCC was assessed using immunohistochemistry with rabbit anti-HER2 oncoprotein antibody (DAKO, Fujifilm, Japan) at a 1:200 dilution. Control slides from HER2-overexpressing breast carcinoma were used for comparison with positivity defined by lateral or basolateral membranous staining in ≥ 10% of tumor cells, following the American Society of Clinical Oncology/College of American Pathologists guidelines. The preparation involved tissue collection, formalin fixation, paraffin embedding, and sectioning into 4-5 μm slices. After deparaffinization the slides underwent antigen retrieval using heat-induced epitope retrieval. This process involves heating the tissue sections in an antigen retrieval solution to unmask potential antigenic sites that may have been masked during formalin fixation, improving antibody binding. The slides were then incubated with the 4B5 monoclonal antibody for HER2 detection. Positive staining was visualized using diaminobenzidine, and scoring was based on intensity. For equivocal results (score 2+), silver in situ hybridization was performed to confirm HER2 gene amplification.
Demographic, clinical, and pathological data were collected including: Age;sex; tumor characteristics (size, location, stage, and lymph node involvement); and treatment modalities (surgical, non-surgical, or combined therapy). Overall survival was calculated as the duration of time from diagnosis or treatment to death from any cause, while disease-free survival was calculated as the length of time after treatment during which a patient remained free from any signs or symptoms of the disease.
Data analysis was performed using SPSS (version 29) and R programming (version 4.3.2) to identify significant prognostic factors. Categorical variables were summarized as frequencies/percentage, while continuous variables were presented as medians. Kaplan-Meier survival curves were constructed, and the log-rank test was used to compare survival distributions. The association between HER2 expression and survival outcomes was evaluated.
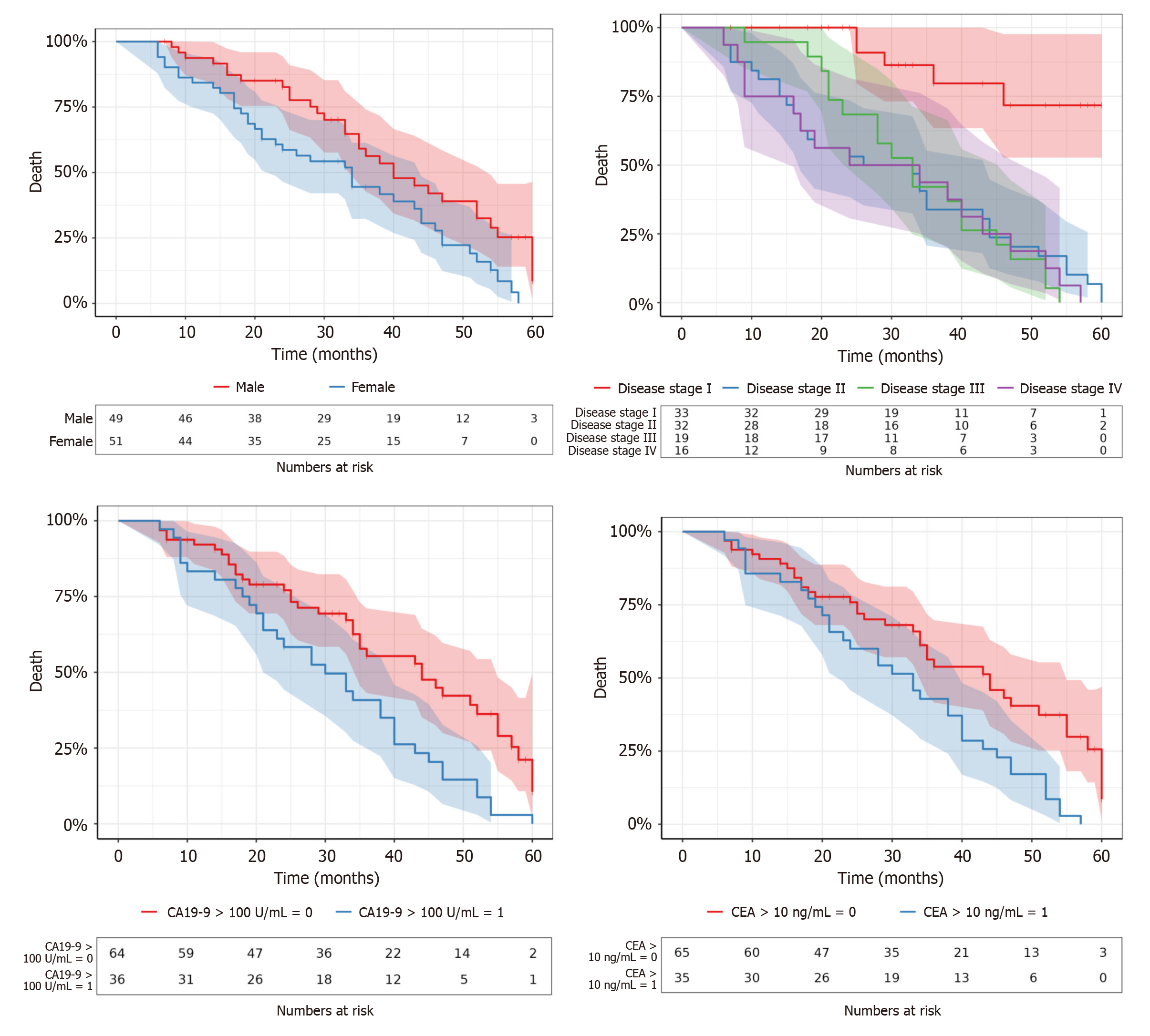
The analysis of clinicopathological factors in gastric SRCC (Table 1, Figures 1, 2, 3, and 4) revealed several key associations with recurrence and mortality, emphasizing the multifaceted nature of this aggressive disease. The follow-up period ranged from 6-60 months with a median of 43 months. The age of patients ranged from 35-65 years with a median of 50 years. Although male patients had a higher incidence of mortality (P = 0.0107), no significant difference was observed for recurrence between genders (P = 0.17). This highlighted the gender disparity in survival with males potentially having poorer outcomes.
| Clinicopathological variable | Number | Recurrence | P value | Death | P value | |
| Age | ≤ 50 | 51 | 27 | 0.62000 | 41 | 0.11000 |
| > 50 | 49 | 25 | 30 | |||
| Sex | Male | 49 | 24 | 0.17000 | 30 | 0.01070a |
| Female | 51 | 28 | 41 | |||
| CA 19-9 serum level | > 100 ng/mL | 36 | 16 | 0.79000 | 35 | 0.00149a |
| ≤ 100 ng/mL | 64 | 36 | 36 | |||
| CEA serum level | > 10 U/mL | 35 | 15 | 0.50000 | 35 | 0.00163a |
| ≤ 10 U/mL | 65 | 37 | 36 | |||
| TNM stage | I | 33 | 21 | 0.77000 | 5 | < 0.00100a |
| II | 32 | 16 | 31 | |||
| III | 19 | 10 | 19 | |||
| IV | 16 | 5 | 16 | |||
| Stage | Advanced | 35 | 15 | 0.50000 | 35 | 0.00169a |
| Localized | 65 | 37 | 36 | |||
| T stage | Mucosa | 22 | 11 | 0.50000 | 15 | 0.23000 |
| Submucosa | 19 | 8 | 13 | |||
| Muscularis propria | 31 | 15 | 24 | |||
| Serosa | 28 | 18 | 19 | |||
| Tumor size | < 5.75 cm | 49 | 23 | 0.89000 | 37 | 0.24000 |
| ≥ 5.75 cm | 51 | 29 | 34 | |||
| Lymph node status | Positive | 71 | 34 | 0.80000 | ||
| Negative | 29 | 18 | ||||
| Tumor location | Diffuse | 46 | 24 | 0.62000 | 33 | 0.52000 |
| Antrum | 35 | 16 | 27 | |||
| Body | 12 | 7 | 6 | |||
| Fundus | 3 | 2 | 2 | |||
| Cardia | 4 | 3 | 3 | |||
| Tumor morphology | Infiltrative | 52 | 26 | 0.94000 | 44 | 0.06800 |
| Ulcerative | 41 | 23 | 24 | |||
| Polypoid | 7 | 3 | 3 | |||
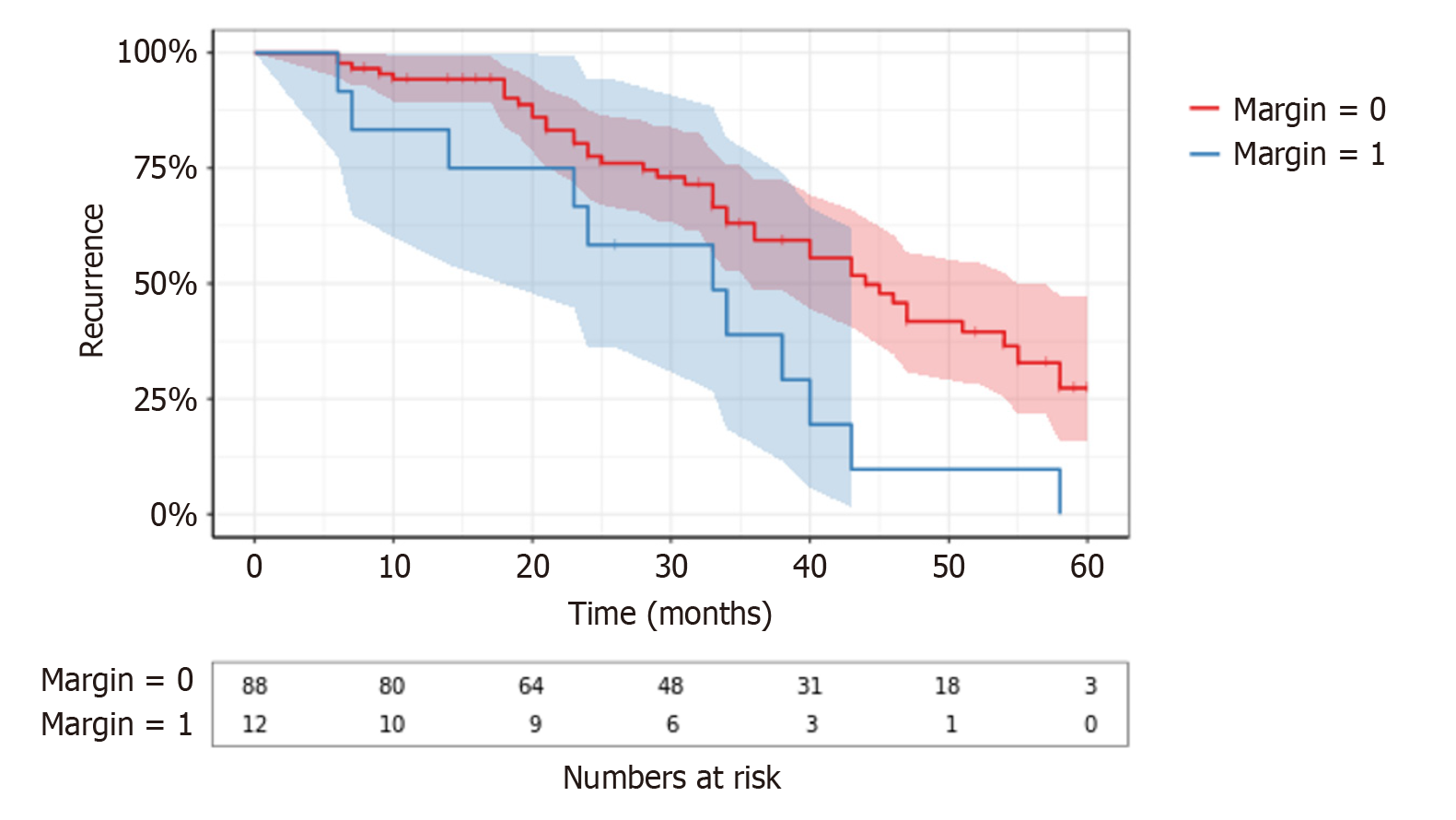
| Surgical margin | Positive | 12 | 41 | 0.00956a | 8 | 0.64000 |
| Negative | 88 | 11 | 63 | |||
| Lymphovascular invasion | Present | 80 | 41 | 0.51000 | 61 | 0.01900a |
| Absent | 20 | 11 | 10 | |||
| Perineural invasion | Present | 59 | 33 | 0.62000 | 41 | 0.68000 |
| Absent | 41 | 19 | 30 | |||
| HER2 | Positive | 15 | 7 | 0.53000 | 15 | 0.00882a |
| Negative | 85 | 45 | 56 | |||
| Treatment | Non-surgical | 38 | 19 | 0.62000 | 31 | 0.02260a |
| Surgical | 27 | 14 | 16 | |||
| Combined | 35 | 19 | 24 | |||
| Distant metastases | Present | 58 | 28 | 0.22000 | 41 | 0.46000 |
| Absent | 42 | 24 | 30 | |||
Elevated serum carbohydrate antigen 19-9 (CA 19-9) levels (> 100 ng/mL) showed no significant association with recurrence (P = 0.79). However, higher levels of CA 19-9 were strongly correlated with increased mortality (P = 0.00149), indicating its potential as a prognostic marker for SRCC. The carcinoembryonic antigen (CEA) serum level did not significantly correlate with recurrence (P = 0.50), but elevated levels (> 10 U/mL) were significantly associated with increased mortality (P = 0.00163), underscoring its role as a prognostic biomarker in SRCC.
The TNM stage showed no significant association with recurrence (P = 0.77), but a significant relationship was found with mortality (P < 0.001). This suggests that advanced TNM stage is a critical determinant of survival in SRCC. The stage of the disease, whether localized or advanced, did not significantly correlate with recurrence (P = 0.50). However, advanced stage was significantly linked to increased mortality (P = 0.00169), highlighting the poor prognosis of advanced SRCC. The T stage of the tumor (mucosa, submucosa, muscularis propria, and serosa) did not show a significant relationship with recurrence (P = 0.50). However, advanced T stages, especially tumors in the serosa, were associated with higher mortality although not reaching statistical significance (P = 0.23). Tumor size ranged from 2.10 cm to 9.90 cm with a median of 5.75 cm. It did not significantly affect recurrence (P = 0.89), but larger tumors (≥ 5.75 cm) were associated with increased mortality (P = 0.24), suggesting a trend towards worse outcomes in patients with larger tumors.
Positive lymph node status did not significantly correlate with recurrence (P = 0.80) but was associated with an increased risk of mortality. This supports the importance of lymph node involvement as a prognostic factor in SRCC. Tumor morphology (infiltrative, ulcerative, or polypoid) did not show a significant correlation with recurrence (P = 0.94). However, infiltrative tumors exhibited a higher mortality rate (P = 0.068), pointing to the potential aggressiveness of infiltrative SRCC.
Positive surgical margins were significantly associated with recurrence (P = 0.00956), indicating the importance of achieving negative margins in SRCC surgery. However, no significant correlation was found with mortality (P = 0.64). Lymphovascularinvasion,was not significantly associated with recurrence (P = 0.51), but it was significantly correlated with increased mortality (P = 0.019), emphasizing its role in poor survival outcomes in SRCC.
HER2-targeted therapies were not administered in this study. HER2-positive tumors showed worse survival outcomes. HER2 positivity did not correlate significantly with recurrence (P = 0.53) but was significantly associated with reduced mortality (P = 0.00882), indicating that HER2 may serve as an unfavorable prognostic marker in SRCC. Surgical treatment, whether alone or combined with other modalities, was significantly associated with lower mortality (P = 0.0226). Moreover, Pearson’s χ2 test showed a significant association between CA19-9 Levels greater than 100 U/mL and tumor stage (early vs late) (P < 0.001) as well as HER2 status (P < 0.001).
Multivariate analysis identified the following factors as significant predictors of poor prognosis: Advanced tumor stage (stage IV) was associated with a 70% increased risk of death within 5 years (P < 0.001); Elevated CEA levels > 10 IU/mL were linked to a 50% increased risk of death within 5 years (P < 0.001); and lymphovascular invasion was associated with a reduction in 5-year survival (P = 0.019). Significant predictors of HER2 status were late-stage tumors (P < 0.001), positive lymph node status (P = 0.007), increased depth of tumor invasion (P = 0.026), and elevated serum biomarkers [CEA > 10 ng/mL (P < 0.001) and CA 19-9 > 100 U/mL] (P < 0.001).
Gastric SRCC is recognized as a particularly aggressive subtype of gastric cancer and is marked by high recurrence rates, poor prognosis, and significant therapeutic challenges. The prognosis, recurrence risk, and overall mortality in SRCC are influenced by a complex array of clinicopathological factors. Among these gender-related differences in survival have been consistently reported with male patients exhibiting significantly higher mortality rates than females. This disparity may reflect variations in immune system function, hormonal influences, and lifestyle-associated comorbidities that differentially affect tumor progression. In contrast females often experience better outcomes, particularly in early disease stages, possibly owing to more robust immune responses, favorable environmental exposures, and earlier initiation of treatment interventions[5-7].
Biomarkers such as CA19-9 and CEA have been extensively studied in the context of SRCC prognostication. Elevated levels of these markers are strongly associated with increased mortality, suggesting their potential utility in identifying patients who are highrisk. However, neither CA19-9 nor CEA has demonstrated reliable predictive value for recurrence, limiting their effectiveness in anticipating disease relapse[8,9]. This observation underscores the need to identify additional molecular or histopathological markers that could better predict recurrence risk.
Tumor staging, particularly according to the TNM classification, remains one of the most powerful prognostic indicators in SRCC. Advanced stages (III and IV) correlate with substantially worse outcomes, largely due to factors such as larger tumor size, deeper invasion (especially T4 tumors), and the presence of distant metastases[5,6,10,11]. These features highlight the critical importance of early detection strategies and the necessity of timely, aggressive treatment to improve survival outcomes. Unfortunately, the often subtle and nonspecific symptoms associated with SRCC contribute to diagnostic delays, exacerbating its aggressive clinical course.
Management strategies for SRCC continue to evolve. Surgical resection with negative margins remains the cornerstone of curative-intent therapy and is associated with significantly improved survival rates. Nonetheless, given the high likelihood of systemic dissemination, adjuvant therapies are crucial. Multimodal treatment approaches combining surgery with chemotherapy and/or radiotherapy have demonstrated superior outcomes compared with monotherapies, particularly in advanced-stage disease[6,12-14]. Tailoring these therapies to the individual patient’s disease characteristics and overall condition is essential for optimizing survival.
The role of HER2 in SRCC has garnered increasing attention despite its relatively low expression rate compared with other gastric cancer subtypes. When it is present, HER2 overexpression can meaningfully influence prognosis and therapeutic decision-making. Targeted treatments, such as trastuzumab, have shown promise in improving outcomes for patients with HER2-positive SRCC[8,12]. These findings highlight the necessity of routine HER2 testing in the mana
Emerging research has shifted focus toward comprehensive molecular profiling as a tool for enhancing personalized therapy in SRCC. The incorporation of molecular data into clinical practice is poised to refine risk stratification, guide targeted therapy choices, and ultimately improve survival outcomes for patients with SRCC. As our understanding of SRCC biology deepens, the development and implementation of precision medicine strategies will be pivotal in addressing the formidable challenges posed by this aggressive malignancy.
Gastric SRCC remains a highly aggressive and clinically challenging malignancy with poor survival outcomes. This study highlighted the significant prognostic factors influencing SRCC progression, recurrence, and mortality, emphasizing the impact of tumor stage, lymphovascular invasion, and serum biomarkers such as CA19-9 and CEA on patient survival. Advanced tumor stage, elevated serum biomarkers, and lymphovascular invasion were strongly associated with increased mortality, reinforcing their roles as critical prognostic indicators.
| 1. | Graziosi L, Marino E, Natalizi N, Donini A. Prognostic Survival Significance of Signet Ring Cell (SRC) Gastric Cancer: Retrospective Analysis from a Single Western Center. J Pers Med. 2023;13:1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (3)] |
| 3. | Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2851] [Article Influence: 570.2] [Reference Citation Analysis (5)] |
| 5. | Yu C, Yang J, Li H, Wang J, Jin K, Li Y, Zhang Z, Zhou J, Tang Y. Prognostic prediction and treatment options for gastric signet ring cell carcinoma: a SEER database analysis. Front Oncol. 2024;14:1473798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Zaafouri H, Jouini R, Khedhiri N, Khanchel F, Cherif M, Mesbahi M, Daghmouri A, Mahmoudi W, Akremi S, Sabbah M, Benzarti Y, Hadded D, Gargouri D, Bader MB, Maamer AB. Comparison between signet-ring cell carcinoma and non-signet-ring cell carcinoma of the stomach: clinicopathological parameters, epidemiological data, outcome, and prognosis-a cohort study of 123 patients from a non-endemic country. World J Surg Oncol. 2022;20:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Li Y, Zhong YX, Xu Q, Tian YT. Protective effects of female reproductive factors on gastric signet-ring cell carcinoma. World J Clin Cases. 2022;10:5217-5229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Wang F, Ba Y. Treatment strategies for patients with HER2-positive gastric cancer. Cancer Biol Med. 2024;20:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Zhao S, Lv L, Zheng K, Tian Y, Zheng JC, Jiang CG. Prognosis and Biological Behavior of Gastric Signet-Ring Cell Carcinoma Better or Worse: A Meta-Analysis. Front Oncol. 2021;11:603070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Wang YF, Xu SY, Wang Y, Che GW, Ma HT. Clinical significance of signet ring cells in surgical esophageal and esophagogastric junction adenocarcinoma: A systematic review and meta-analysis. World J Clin Cases. 2021;9:10969-10978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Tian HK, Zhang Z, Ning ZK, Liu J, Liu ZT, Huang HY, Zong Z, Li H. Clinicopathological characteristics and prognosis of gastric signet ring cell carcinoma. World J Clin Cases. 2022;10:10451-10466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Gokon Y, Nakashima Y, Ohki Y, Ogino T, Hatoyama K, Shimizu K, Kashiwadate T, Katsura K, Abe T, Sato K. Prognostic significance of low HER2 expression in gastric cancer: a retrospective, single-center analysis. BMC Cancer. 2024;24:1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Tang YH, Ren LL, Mao T. Update on diagnosis and treatment of early signet-ring cell gastric carcinoma: A literature review. World J GastrointestEndosc. 2023;15:240-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Weng CY, Sun SP, Cai C, Xu JL, Lv B. Endoscopic submucosal dissection for early signet ring cell gastric cancer: A systematic review and meta-analysis. World J Clin Cases. 2022;10:6915-6926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |