Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.105077
Revised: February 4, 2025
Accepted: February 27, 2025
Published online: April 24, 2025
Processing time: 75 Days and 1.1 Hours
Drug-induced lung injury is a common adverse effect of chemotherapeutic agents. Diffuse alveolar hemorrhage (DAH) is a fatal complication associated with drug-induced lung injury. Early diagnosis and treatment of DAH is critical, as delayed management can lead to respiratory failure and poor outcomes. However, the diagnosis of DAH is difficult because of the nonspecific clinical manifestations; as such, bronchoscopy is necessary to establish a diagnosis.
The patient presented with fever and dry cough. He had been receiving fluo
Prompt DAH diagnosis and bronchoscopy in patients receiving oxaliplatin-containing chemotherapy presenting with acute respiratory failure are critical for improving outcomes.
Core Tip: This report describes a case of a serious complication of oxaliplatin-induced diffuse alveolar hemorrhage. The diagnosis of diffuse alveolar hemorrhage (DAH) is challenging because the clinical manifestations and radiographic findings of DAH, such as diffuse bilateral opacities, are non-specific, necessitating bronchoscopy with bronchoalveolar lavage fluid analysis to establish the diagnosis of DAH. As early diagnosis by bronchoscopy and prompt initiation of corticosteroid treatment were essential for saving the patient, clinicians should be aware that diffuse alveolar hemorrhage is a potential cause of respiratory failure in patients receiving oxaliplatin.
- Citation: Tsurui T, Mura E, Horiike A, Tsunoda T. Oxaliplatin-induced diffuse alveolar hemorrhage: A case report. World J Clin Oncol 2025; 16(4): 105077
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/105077.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.105077
Drug-induced lung injury is commonly associated with chemotherapeutic agents. Diffuse alveolar hemorrhage (DAH) is one of the most severe complications of drug-induced lung injury, with a mortality rate reaching over 50%[1]. Oxaliplatin is a platinum-derived agent that is widely applied to treat gastrointestinal cancers. Oxaliplatin-induced pulmonary toxicity is relatively uncommon but can be life-threatening. Early recognition of oxaliplatin-induced pulmonary toxicity is critical, as prompt intervention can improve survival and prevent respiratory failure[2]. In addition, older patients may be at an increased risk of infection due to age-related changes in immune function. This vulnerability may be of particular concern during the treatment of DAH using corticosteroids and other immunosuppressive agents. Herein, we present a case of oxaliplatin-induced DAH, which rapidly deteriorated necessitating the use of mechanical respiratory support. Early diagnosis with bronchoscopy and prompt initiation of high-dose corticosteroids improved the symptoms and saved the patient.
A 72-year-old man presented with fever and dry cough.
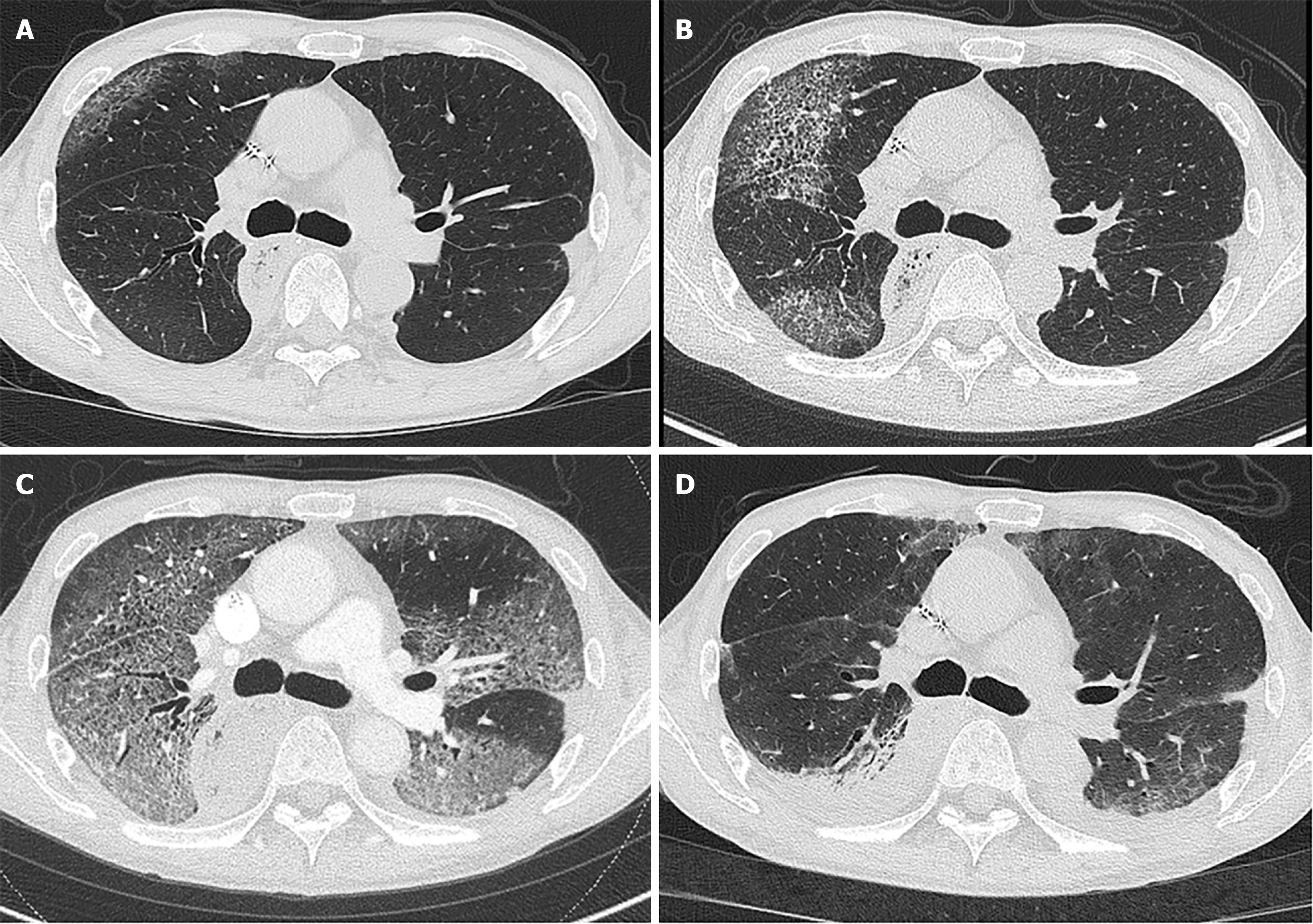
The patient completed eight cycles of treatment with folinic acid, fluorouracil, and oxaliplatin (modified FOLFOX-6 therapy) 1 month before presentation and then started oral fluoropyrimidine monotherapy for recurrent esophageal squamous cell carcinoma. Plain chest radiography showed opacification in the right lower lung, and computed tomography demonstrated bilateral ground-glass opacities (GGOs) (Figure 1A). Treatment with oral amoxicillin-clavulanate was empirically started. After 3 days, the patient developed a persistent fever. His dry cough progressively worsened, and he experienced moderate respiratory distress on exertion.
The patient had a history of advanced atrioventricular block and hypertrophic cardiomyopathy.
The patient had quit smoking, but had a smoking history of two packs a day lasting 25 years.
His oxygen saturation was 97% at rest, but decreased to < 93% with mild exertion. Fine crackles were heard in both the lower lung fields.
Leukocytosis (11900/μL) and elevated C-reactive protein (8.9 mg/dL) was noted. Arterial blood gas analysis showed mild hypoxemia (partial pressure of oxygen was 49 mmHg on room air).
Computed tomography revealed rapid worsening of diffuse bilateral GGOs involving approximately 20% of the total lung volume (Figure 1B).
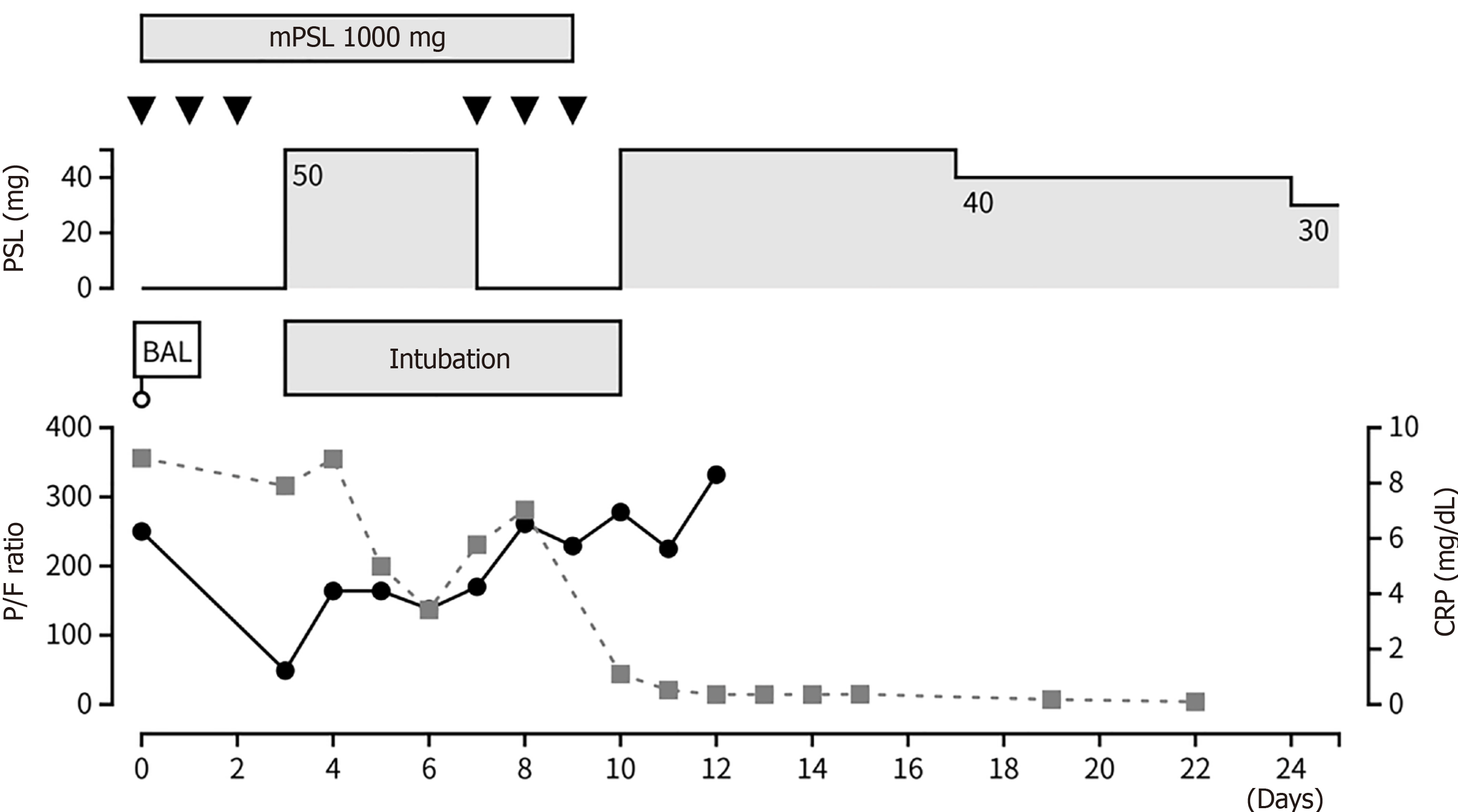
We consulted a pulmonologist and bronchoscopy was performed on the day of admission. The bronchoalveolar lavage fluid became progressively more hemorrhagic. An extensive workup did not reveal any infectious etiology, autoimmune disease, heart failure or coagulopathy to cause DAH.
A diagnosis of DAH was made, with oxaliplatin suspected as the causative agent.
High-dose methylprednisolone (1000 mg/day) was administered immediately after diagnosis. However, his respiratory status suddenly deteriorated on day 3 of the hospitalization. Computed tomography revealed marked enlargement of the GGO area without pulmonary embolism (Figure 1C). The patient was transferred to the intensive care unit and intubated. After the administration of the second dose of high-dose methylprednisolone, his respiratory status gradually improved, and he was eventually successfully extubated.
The patient was discharged home with full activities of daily living after the corticosteroids were tapered to 30 mg. The pulmonary opacities were markedly improved (Figure 1D). The clinical course during hospitalization was summarized in Figure 2. Corticosteroids were tapered to 5 mg over 6 months without any relapse; the patient was alive at the time of writing.
Herein, we present a case of oxaliplatin-induced DAH. This case highlights the importance of an early diagnosis using bronchoscopy and corticosteroid therapy. Oxaliplatin is an antineoplastic platinum derivative and widely used in gastrointestinal cancers including esophageal, gastric, pancreatic, and colorectal cancers. Interstitial lung disease (ILD) is a relatively uncommon, but serious adverse event of oxaliplatin. Although the incidence of oxaliplatin-induced ILD has been reported to be < 1%, the overall mortality rate is reportedly 30%[2,3]. Moreover, a review of previous literature showed that the mortality rate can reach up to 44.4% in patients requiring corticosteroids and 76.9% in patients requiring invasive respiratory support, respectively[4]. DAH is the most severe consequence of drug-induced lung injury. To date, several cases of oxaliplatin-induced DAH have been reported (Table 1)[2,5,6]. FOLFOX regimen was administered in two cases and oxaliplatin monotherapy in one case. In all of the three cases, invasive mechanical ventilation was applied, and corticosteroids were administered. Corticosteroids are the gold standard for treating DAH and they suppress inflammatory and immune responses, reducing lung damage[7,8]. Although information regarding the timing of corticosteroid therapy initiation was missing in two patients, corticosteroid treatment was delayed for 3 days after the diagnosis of DAH in one patient, who died without improvement. Several literature reviews on oxaliplatin-induced ILD suggested that steroid therapy should be immediately initiated because the time from symptom onset to steroid initiation has been correlated with treatment success[2,9]. However, diagnosing DAH is particularly challenging. Hemoptysis is a hallmark sign of DAH but may be absent in one-third of patients[10]. In addition, radiographic findings of DAH such as diffuse bilateral opacities are nonspecific[11]. Bronchoscopy with bronchoalveolar lavage fluid analysis is the gold standard for diagnosing DAH. As DAH can rapidly deteriorate within a few days, prompt bronchoscopy should be considered in patients at risk of DAH, such as those receiving potentially pulmotoxic chemotherapy including oxaliplatin. In this case, bronchoscopy could be performed on the day of admission, which confirmed the diagnosis of DAH soon after symptom onset. The early initiation of high-dose corticosteroid therapy may have improved the symptoms. However, older patients receiving corticosteroid therapy are at an increased risk of infections due to immunosuppression[12-14]. Given the vulnerability of this population, careful decision-making and close monitoring are essential for management of DAH.
| Characteristics | Kumaran et al[6] | Lou et al[5] | Watkins et al[2] | Present case |
| Types of cancer | Unknown primary | Esophageal | Colorectal | Esophageal |
| Regimen | Oxaliplatin | FOLFOX | FOLFOX | FOLFOX and S-1 |
| Chemotherapy before onset | 1 cycle | 1 cycle | 11 cycles | 8 cycles |
| Mechanical ventilation | Yes | Yes | Yes | Yes |
| Treatment | Corticosteroids | mPSL 1 mg/kg | mPSL 125 mg q6h | mPSL 1000 mg |
| Timing of corticosteroids | Not addressed | Not addressed | After 3 days of hospitalization | On the day of hospitalization |
| Outcome | Extubation | Extubation | Death | Extubation |
Oxaliplatin can cause DAH, a serious pulmonary adverse effect. Oncologists should suspect the possibility of DAH and perform bronchoscopy immediately when patients receiving chemotherapy containing oxaliplatin present with acute respiratory failure.
We thank the patient and the patient’s family for granting permission to share the patient’s clinical information.
| 1. | Schwarz MI, Fontenot AP. Drug-induced diffuse alveolar hemorrhage syndromes and vasculitis. Clin Chest Med. 2004;25:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Watkins J, Slade JH, Phan A, Eng C, Weissferdt A, Overman MJ. Fatal diffuse alveolar damage associated with oxaliplatin administration. Clin Colorectal Cancer. 2011;10:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Suthar KH, Al Mutar S, Venkatesan R. Oxaliplatin-induced Pulmonary Toxicity: A Rare but Serious Complication. Cureus. 2020;12:e7483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | De Weerdt A, Dendooven A, Snoeckx A, Pen J, Lammens M, Jorens PG. Prognosis and treatment of FOLFOX therapy related interstitial pneumonia: a plea for multimodal immune modulating therapy in the respiratory insufficient patient. BMC Cancer. 2017;17:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Lou B, Korotun M, Oks M. Diffuse Alveolar Hemorrhage and Folfox: An Unexpected Pair. Chest. 2018;154:451A. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Kumaran D, Rupa D, Haresh K, Gupta S, Sharma D, Rath G. Oxaliplatin induced pulmonary toxicity—a rare phenomenon. Cancer Treatment Communications. 2015;4:203-205. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Park MS. Diffuse alveolar hemorrhage. Tuberc Respir Dis (Seoul). 2013;74:151-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Park JA. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int J Mol Sci. 2021;22:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Shimura T, Fuse N, Yoshino T, Minashi K, Tahara M, Doi T, Joh T, Ohtsu A. Clinical features of interstitial lung disease induced by standard chemotherapy (FOLFOX or FOLFIRI) for colorectal cancer. Ann Oncol. 2010;21:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 11. | Reisman S, Chung M, Bernheim A. A Review of Clinical and Imaging Features of Diffuse Pulmonary Hemorrhage. AJR Am J Roentgenol. 2021;216:1500-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Waki D, Nishimura K, Tokumasu H, Kadoba K, Mukoyama H, Saito R, Murabe H, Yokota T. Initial high-dose corticosteroids and renal impairment are risk factors for early severe infections in elderly patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: A retrospective observational study. Medicine (Baltimore). 2020;99:e19173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Bartsch R, Aletaha D, Fuereder T, Aapro M, Jornayvaz FR, Lang PO, Migliorini D, Csajka C, Aretin MB, Dougoud-Chauvin V. Corticosteroid therapy in older adults with cancer: Expert recommendations from a task force of the International Society of Geriatric Oncology. J Geriatr Oncol. 2024;102077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 14. | Jung C, Wernly B, Fjølner J, Bruno RR, Dudzinski D, Artigas A, Bollen Pinto B, Schefold JC, Wolff G, Kelm M, Beil M, Sigal S, van Heerden PV, Szczeklik W, Czuczwar M, Elhadi M, Joannidis M, Oeyen S, Zafeiridis T, Marsh B, Andersen FH, Moreno R, Cecconi M, Leaver S, Boumendil A, De Lange DW, Guidet B, Flaatten H; and the COVIP study group. Steroid use in elderly critically ill COVID-19 patients. Eur Respir J. 2021;58:2100979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |