Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.103564
Revised: December 24, 2024
Accepted: January 18, 2025
Published online: April 24, 2025
Processing time: 123 Days and 8.5 Hours
Pseudomyxoma peritonei (PMP) is a distinct form of peritoneal malignancy characterized by diffuse intra-abdominal gelatinous ascites, with an estimated incidence of 1-3 per 1000000. PMP is predominantly secondary to appendiceal mucinous neoplasms, with rarer origins including the ovaries, colon, and urachus. However, PMP originating from small intestine is extremely rare.
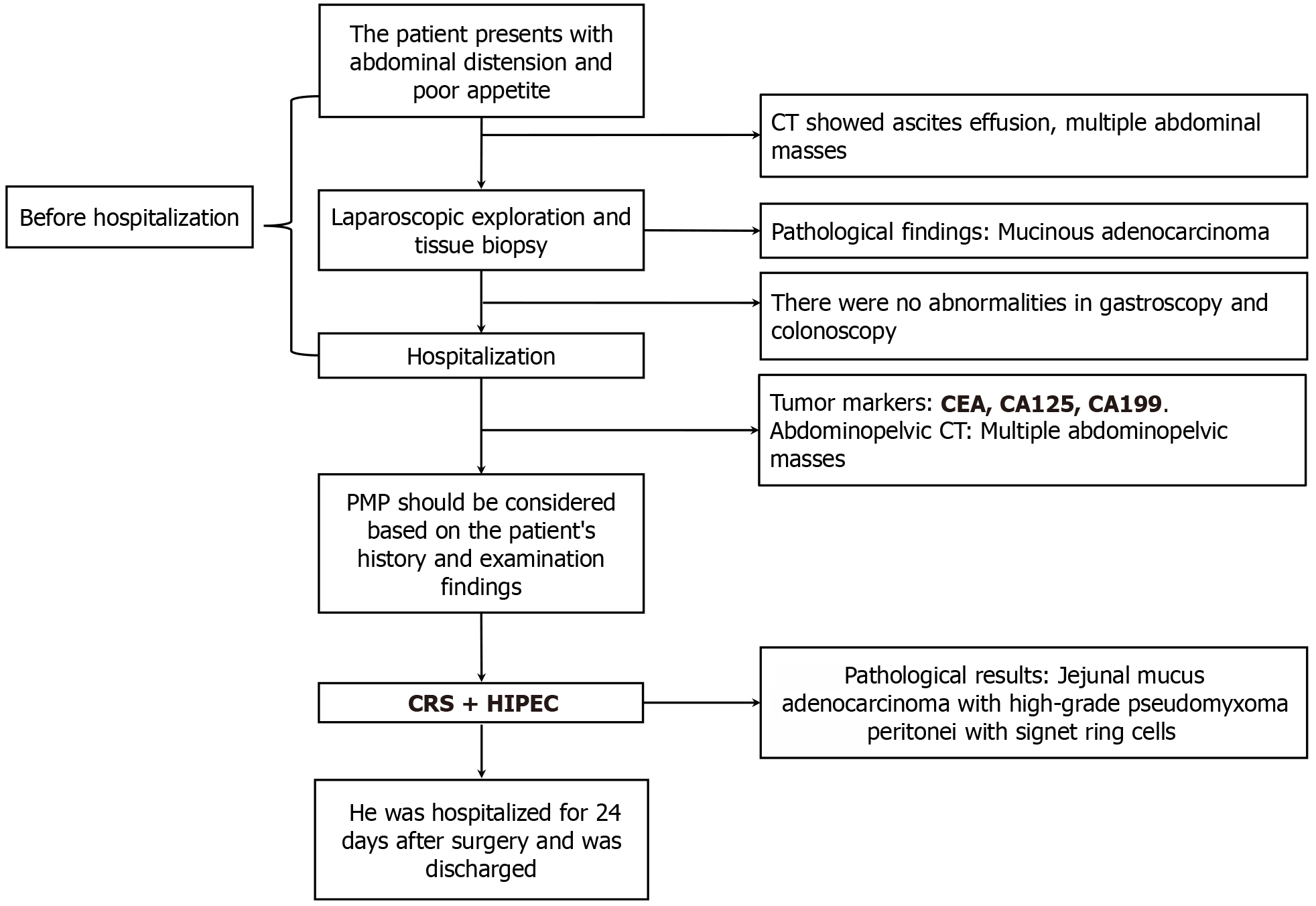
A 60-year-old male patient presented with anorexia and abdominal distension. Computed tomography revealed the presence of abdominopelvic effusions and multiple intra-abdominal space-occupying lesions. Ultrasound-guided aspiration indicated that the aspirated tissue was mucinous. Exploratory laparoscopy and tissue biopsy identified diffuse tumor nodules in peritoneum, omentum, pelvic region, intestinal walls, and mesentery. Histopathological analysis of the resected tumors confirmed the presence of mucinous adenocarcinoma, but the primary lesion was difficult to determine. The patient was referred to our center for further treatment and underwent cytoreductive surgery (CRS) combined with hyper
PMP originating from small intestine is an exceptionally rare entity that exhibits non-specific clinical features. The preferred treatment is CRS + HIPEC.
Core Tip: Pseudomyxoma peritonei (PMP) is a distinct type of peritoneal malignancy that predominantly originates from appendiceal mucinous neoplasms, and PMP originating from small intestine is extremely rare. Here we report a case of PMP originating from the jejunum in an attempt to enhance our understanding of this disease.
- Citation: Shi GJ, Wang C, Zhang P, Lu YY, Zhou HP, Ma RQ, An LB. Pseudomyxoma peritonei originating from small intestine: A case report and review of literature. World J Clin Oncol 2025; 16(4): 103564
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/103564.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.103564
Pseudomyxoma peritonei (PMP) is a distinct form of peritoneal malignancy characterized by diffuse intra-abdominal gelatinous ascites, with an estimated incidence of 1-3 per 1000000[1-3]. Unlike other tumor types, PMP usually stays confined to the abdominal cavity and seldom metastasizes to lymph nodes or distant organs. However, as a malignancy characterized by implantation metastasis, PMP can ultimately lead to severe and potentially fatal complications such as intestinal fistulas and obstructions if not adequately treated[4]. In 2016, the Peritoneal Surface Oncology Group International established a new PMP classification system, which subdivided PMP into four groups according to histopathological features: Acellular mucin, low-grade mucinous carcinoma peritonei, high-grade MCP (HGMCP), HGMCP with signet-ring cells[5]. Currently, the “gold standard” treatment for PMP is cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC). Patients who undergo successful CRS typically exhibit favorable survival outcomes[1,6].
PMP is predominantly secondary to appendiceal mucinous neoplasms, with rarer origins including the ovaries, colon, stomach, pancreas, gallbladder, urachus, and intestinal duplication[7-13]. Nevertheless, the small intestine-originated PMP is extremely rare. Here we report a case of PMP originating from the small intestine. The patient had a successful recovery after CRS + HIPEC.
A 60-year-old male patient presented with a one-month history of anorexia and abdominal distension.
The patient exhibited anorexia and abdominal distension, and computed tomography (CT) revealed abdominopelvic effusions and space-occupying lesions in the upper abdominal region. Ultrasound-guided aspiration indicated that the aspirated tissue was mucinous. Exploratory laparoscopy identified diffuse tumor nodules in the peritoneum, omentum, pelvic region, intestinal walls, and mesentery. The resected nodules were submitted for histopathological analysis, which confirmed the presence of mucinous adenocarcinoma, with the primary source remaining undetermined. The patient was admitted to our center for further treatment.
The patient‘s past medical history was unremarkable, without any significant underlying diseases.
The patient had no history of smoking or alcohol consumption, and there was no family history of malignancies or genetic disorders.
The abdomen was flat and soft, with no tenderness, rebound tenderness, or muscular tension. Firm masses with indistinct margins were palpable in the epigastric region. The masses were non-mobile and did not exhibit signs of shifting dullness. Bowel sounds were normal.
The results of serum tumor marker tests were as follows: Carcinoembryonic antigen (CEA): 4.38 ng/mL (normal range: 0-5 ng/mL); carbohydrate antigen (CA) 19-9: 1152.7 U/mL (normal range: 0-30 U/mL); and CA-125: 108.45 U/mL (normal range: 0-35 U/mL).
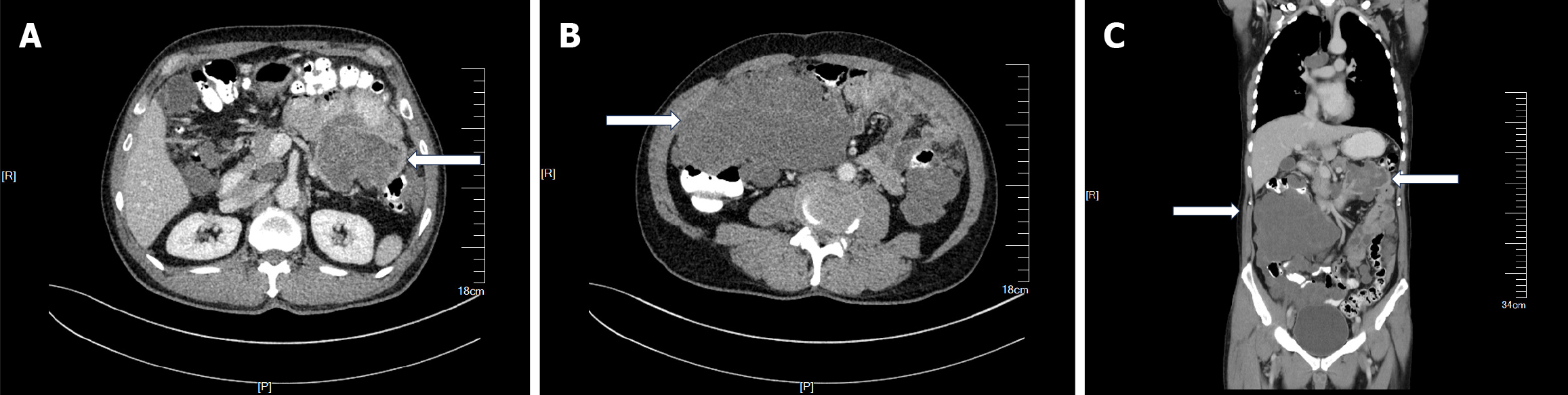
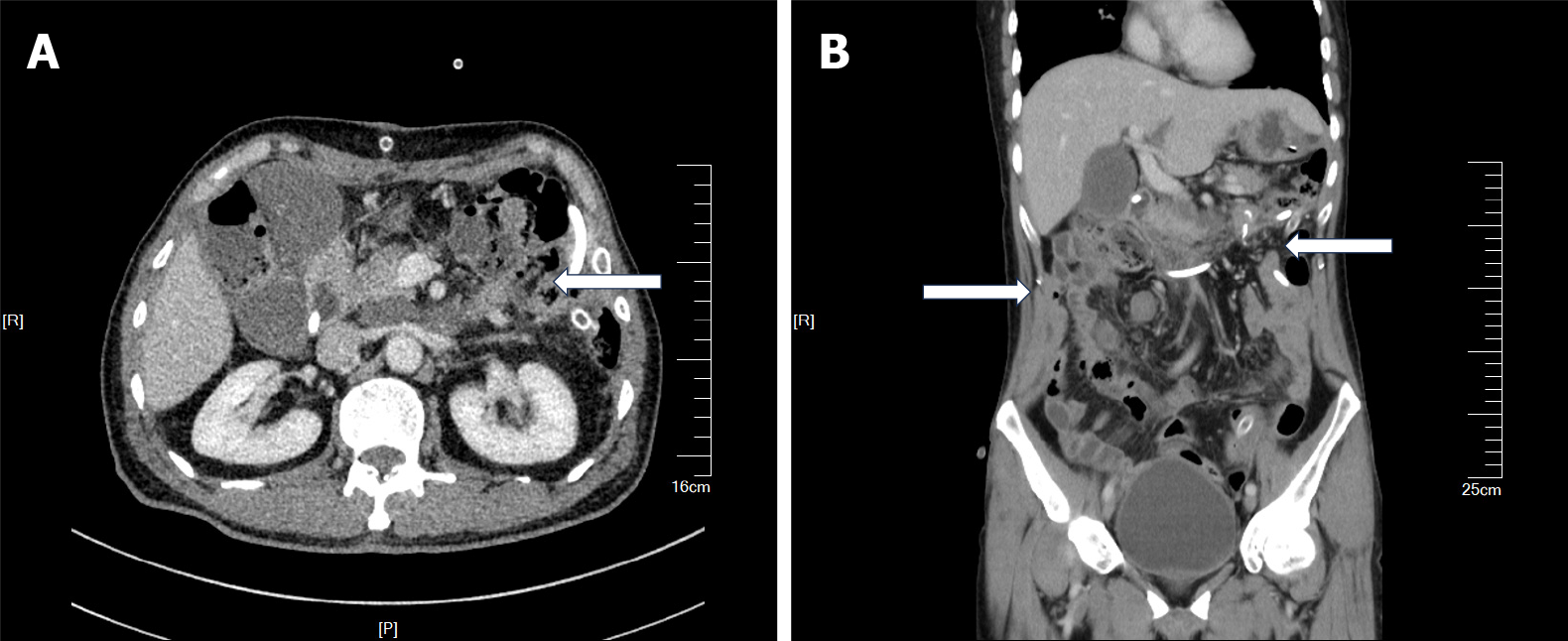
Contrast-enhanced CT identified multiple space-occupying mass-like lesions within the abdominal cavity. An irregularly shaped mass in the left upper quadrant was considered a jejunal tumor (Figure 1A). A large irregular mass in the right abdomen was considered a thickened omentum (Figure 1B). Specific location of the major tumor is shown in Figure 1C.
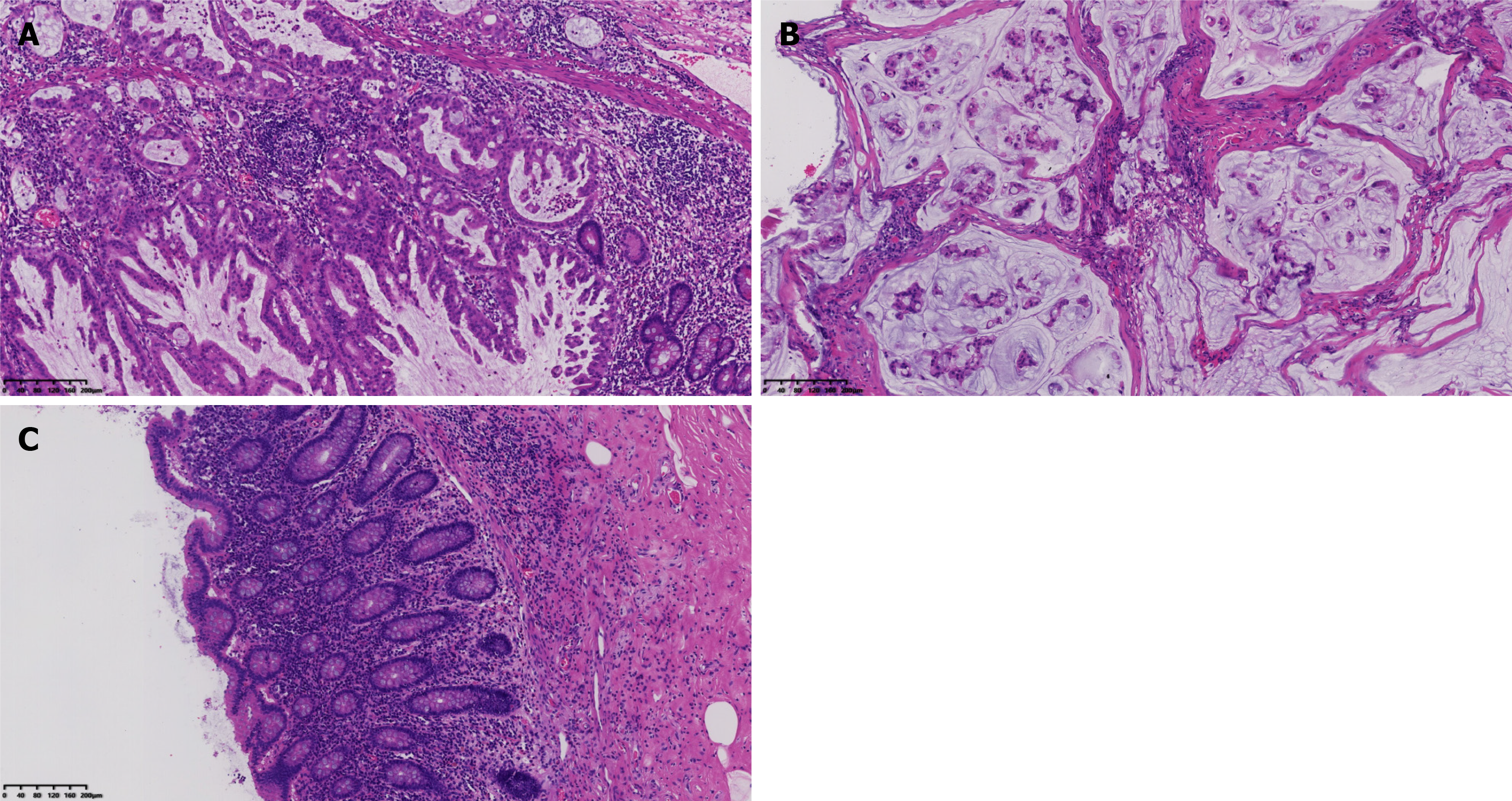
Serrated lesions, along with high-grade dysplasia, were seen within the jejunal mucosa (Figure 2A). Mucinous adenocarcinoma invasion and the formation of mucin lakes were present in the peritoneum (Figure 2B). No lesion was observed in the appendiceal mucosa (Figure 2C). The immunohistochemical analysis yielded the following results: CK7, +; CK20, +; MUC-2, +; and Ki-67 index, 40%, which suggested gastrointestinal (GI) origin, and combined with microscopic features, the diagnosis could be confirmed.
The final diagnoses included mucinous adenocarcinoma of the small intestine, originating from the proximal jejunum, complicated by high-grade PMP.
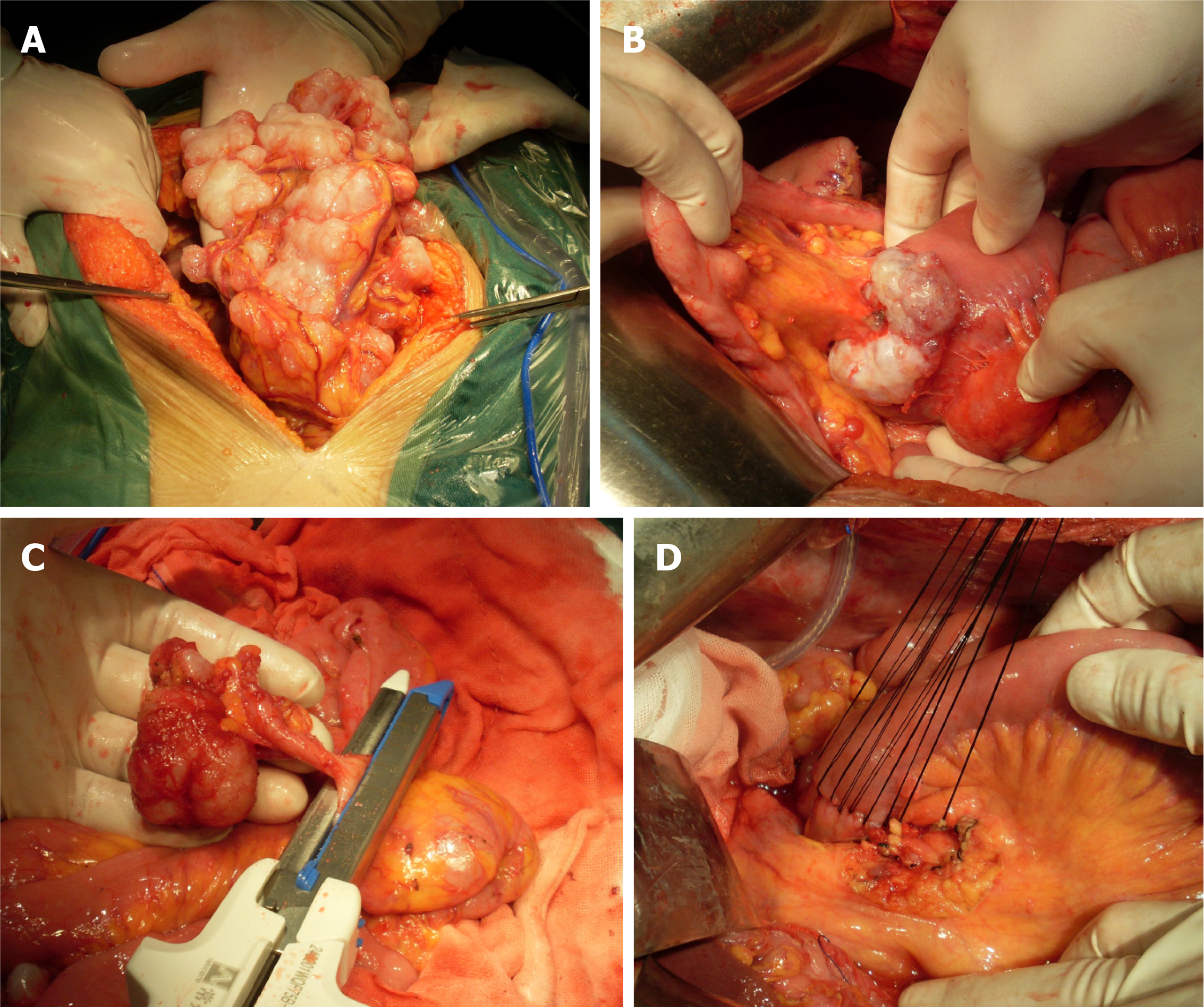
Following a comprehensive diagnostic workup, the patient underwent CRS + HIPEC under general anesthesia. Because the patient had previously undergone laparoscopic exploration, with a preliminary understanding of his abdominal condition, we chose open surgery directly. During the operation, a small amount of yellowish ascites about 500 mL in the abdominal cavity was shown. The surface of the omentum was covered with tumor nodules, which were clustered in the right upper abdomen (Figure 3A). A cauliflower-like tumor was found at the proximal jejunum, about 3cm away from the flexor ligament, with a size of about 5 × 5 cm, involving approximately two-thirds of the intestinal wall's diameter (Figure 3B). The appendix appeared macroscopically normal (Figure 3C).
Implantation of scattered tumor nodules was found on the surface of the mesentery, hepatic circular ligament, and bilateral diaphragmatic peritoneum. Dense tumor nodules were found in the ascending colon sulcus, descending colon sulcus, and bladder rectum peritoneal fold. The peritoneal cancer indexes (PCIs) in each region during surgery were 0:3; 1:3; 2:3; 3:3; 4:3; 5:3; 6:3; 7:3; 8:3; 9:3; 10:0; and 11:0; 12:0 respectively, with a total score of 30. During the surgical procedure, we adopted the extraperitoneal space approach at the incision site. The omentum, appendix, right colon, and the proximal jejunum were excised. The surgery lasted 8 h with a blood loss of 600 mL. Following the completion of CRS, a single session of closed HIPEC was performed intraoperatively. Two inflow and two outflow catheters were placed in the abdomen and connected to a hyperthermia chemotherapy perfusion machine (Jilin Minda Company, RHL - 2000B), and the treatment lasted 60 minutes. The circulation speed was controlled at 800-1000 mL/min, and the outlet tem
The surgical procedure was smooth, and complete cytoreduction (CCR0) was achieved. The patient experienced a favorable recovery postoperatively, without any significant surgical complications. The patient was hospitalized for 24 days after surgery. Postoperative abdominal CT showed disappearance of the initial jejunal tumor and omentum tumor (Figure 5). One week after discharge, the patient reported being in generally good condition during the telephone follow-up. We recommended that the patient should receive follow-up examinations every six months, including blood tumor markers and enhanced CT in the abdominal and pelvic cavity.
PMP has no distinct clinical manifestations in its early stages. As the disease progresses, patients may progressively exhibit atypical symptoms including abdominal distension and an increase in abdominal circumference. There are few specific diagnostic examinations or tests for PMP. Serum tumor markers are less sensitive for PMP diagnosis[14]. However, elevated postoperative tumor marker levels can be indicative of tumor recurrence[15]. An elevated CEA level often suggests a tumor originating from lower GI tract (including the appendix). In the present case, the preoperative serum CEA level was within the normal range, which, during preoperative deliberations, led to the consideration of an extra-appendiceal origin for the tumor. On the contrary, CA199 and CA125 levels were significantly elevated in this case, which may indicate a tumor originating from the patient's pancreatic and biliary system or a rare primary peritoneal tumor. Imaging studies are instrumental in the diagnosis of PMP. CT findings including scallopping sign around the liver and spleen, omental thickening, and diffuse hypodense masses within the abdominal and pelvic cavities are highly indicative of PMP[16,17]. Positron emission tomography/CT offers limited diagnostic utility for PMP; however, it can be effective in identifying extra-abdominal metastases[18]. While histopathology serves as a crucial foundation for confirming the diagnosis of PMP, exfoliative cytology of ascites is less helpful as tumor cells are often scarce in ascites. Nevertheless, gelatinous ascites aspirated via peritoneal puncture is highly valuable for PMP diagnosis.
PMP is predominantly secondary to appendiceal mucinous tumors, accounting for approximately 90%-95%[8,19,20], although other origins can also be observed in rarer cases. In general, the pathophysiological progression of PMP is fundamentally consistent. Firstly, the mucus produced by tumor cells accumulates progressively. Elevated pressure causes perforation or rupture, allowing the tumor cell-containing mucus to enter the abdominal cavity. These cells then disseminate extensively throughout the abdominal cavity via the circulation of peritoneal fluid, resulting in PMP. At this advanced stage, the opportunity for complete tumor debulking is often lost, which significantly elevates the rates of complications and mortality[5,21,22].
The small intestine constitutes approximately two-thirds of the total length of the digestive tract and plays a pivotal role in digestion and absorption. Interestingly, the incidence of small intestinal tumors is relatively low, comprising only 1% to 3% of all GI tumors[23]. Several mechanisms may explain this phenomenon. First, the rapid peristalsis of the small intestine facilitates the swift transit of intestinal contents, resulting in shorter exposure of the small intestinal mucosa to potential carcinogens. Second, the rich lymphoid tissues and high levels of immunoglobulins in the small intestine contribute to its robust immune defense mechanisms. Third, the weakly alkaline environment of the small intestine is unfavorable for the formation of nitrosamines, an important group of carcinogens[24]. Due to the unique anatomical location and large mobility of the small intestine, coupled with the absence of distinct clinical symptoms, the early detection of small bowel tumors remains challenging. With advancements in CT and capsule endoscopy, the detection rate for small bowel tumors has dramatically increased[25-27]. The most prevalent pathological types of small bowel tumors are adenocarcinoma and carcinoid tumors, which constitute approximately 80% of all small bowel tumors, with stromal tumors and lymphangiomas ranking as subsequent common types[28,29].
Despite the varied origins of PMPs, their biological behaviors are fundamentally similar, characterized by diffuse intra-abdominal implantation and local invasion. In addition to the primary lesions, all the metastatic tumors and the involved organs should also be resected during the treatment. The prognosis of PMP is closely associated with the extent of CRS, pathological type, and PCI score. A PCI score of > 19, palliative debulking surgery, high-grade mucinous adenocarcinoma, and mucinous adenocarcinoma with signet ring cells have been identified as independent risk factors for a poor prognosis[6,30,31]. Currently, it is generally accepted that the optimal treatment for PMP is CRS combined with HIPEC[1,6]. The five-year and ten-year survival rates for patients with appendiceal PMP treated with CRS + HIPEC could reach 87.4% and 70.3%, respectively[32]. In addition, the combination of CRS and HIPEC has shown great potential in the treatment of PMP from colorectal and ovarian sources[33,34]. Based on the biological characteristics of PMP and the superiority of CRS+HIPEC, we adopted this treatment mode in our case.
PMP originating from the small intestine is exceptionally rare, and there is a scarcity of large-sample studies. It has been proposed that CRS + HIPEC offers a novel therapeutic approach for peritoneal metastasis from small intestinal tumors, with satisfactory debulking potentially leading to improved survival outcomes[35-37]. However, the three-year survival rate following treatment for patients with peritoneal metastasis from small intestinal tumors was approximately 50%, which was notably lower than that of appendix-derived PMP[37]. Therefore, small intestine-derived PMP may have a poorer prognosis, which may be related to its biological characteristics. However, this finding requires further validation by large-scale multicenter and basic studies.
Notably, for patients with confirmed PMP, careful exploration and exclusion of the origin of the appendix should be conducted. In the present case, the appendix appeared normal during the surgical procedure, and the histopathological examination confirmed the absence of tumor in the appendiceal mucosa. The serrated lesions and high-grade dysplasia were found in the mucosa of the jejunum, along with mucin lakes and severely dysmorphic mucus glands in the peritoneal fibrous tissue. All point to the jejunum as the origin of the primary lesion, leading to rupture of the lumen and formation of PMP.
PMP originating from the small intestine is exceptionally rare, and its clinical manifestations are generally indistinguishable from those originating from the appendix. CRS combined with HIPEC represents the preferred treatment approach, with the goal of achieving optimal tumor debulking. However, the long-term survival outcomes or alternative therapeutic approaches warrant further research.
| 1. | Ye S, Zheng S. Comprehensive Understanding and Evolutional Therapeutic Schemes for Pseudomyxoma Peritonei: A Literature Review. Am J Clin Oncol. 2022;45:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 2. | Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 366] [Article Influence: 20.3] [Reference Citation Analysis (4)] |
| 3. | Patrick-Brown TDJH, Carr NJ, Swanson DM, Larsen S, Mohamed F, Flatmark K. Estimating the Prevalence of Pseudomyxoma Peritonei in Europe Using a Novel Statistical Method. Ann Surg Oncol. 2021;28:252-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12:291-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J, Pai RK, Rodriguez-Justo M, Sobin LH, van Velthuysen MF, Yantiss RK. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. 2017;71:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 6. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 780] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 7. | Guo AT, Song X, Wei LX, Zhao P. Histological origin of pseudomyxoma peritonei in Chinese women: clinicopathology and immunohistochemistry. World J Gastroenterol. 2011;17:3531-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Han XD, Zhou N, Lu YY, Xu HB, Guo J, Liang L. Pseudomyxoma peritonei originating from intestinal duplication: A case report and review of the literature. World J Clin Cases. 2021;9:7459-7467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Shi GJ, Xia A, Ma RQ, Wang B, Zhai XC, An LB, Xu HB. Diagnosis and treatment of pseudomyxoma peritonei of extra-appendiceal origin: analysis of 34 cases. Zhongguo Zhongliu Linchuang. 2019;46:897-902. [DOI] [Full Text] |
| 10. | Allievi N, Samuel VM, Carr N, Shah N, Di Fabio F, Dayal S, Tzivanakis A, Cecil T, Moran B, Mohamed F. Pseudomyxoma Peritonei Arising from Urachal Mucinous Neoplasms: a Case Series and Updated Literature Review. Indian J Surg Oncol. 2023;14:144-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Kataoka A, Ito K, Takemura N, Inagaki F, Mihara F, Gohda Y, Kiyomatsu T, Yamada K, Kojima N, Igari T, Yamakawa M, Yano H, Kokudo N. Immunohistochemical staining as supportive diagnostic tool for pseudomyxoma peritonei arising from intraductal papillary mucinous neoplasm: A report of two cases and literature review. Pancreatology. 2020;20:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wang W, Meng L, Crespo E, Adams J, Manoucheri M. Gelatinous Abdomen: A Rare Case of Pseudomyxoma Peritonei Arising from Metastatic Gastric Adenocarcinoma. Cureus. 2019;11:e4666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Giang TH, Ngoc TT, Hassell LA. Carcinoma involving the gallbladder: a retrospective review of 23 cases - pitfalls in diagnosis of gallbladder carcinoma. Diagn Pathol. 2012;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Wagner PL, Austin F, Sathaiah M, Magge D, Maduekwe U, Ramalingam L, Jones HL, Holtzman MP, Ahrendt SA, Zureikat AH, Pingpank JF, Zeh HJ 3rd, Bartlett DL, Choudry HA. Significance of serum tumor marker levels in peritoneal carcinomatosis of appendiceal origin. Ann Surg Oncol. 2013;20:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Menassel B, Duclos A, Passot G, Dohan A, Payet C, Isaac S, Valette PJ, Glehen O, Rousset P. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur J Surg Oncol. 2016;42:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Fonseca EKUN, Martins AN, Tridente CF, Yamauchi FI, Baroni RH. Liver scalloping in pseudomyxoma peritonei. Abdom Radiol (NY). 2017;42:2003-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Passot G, Glehen O, Pellet O, Isaac S, Tychyj C, Mohamed F, Giammarile F, Gilly FN, Cotte E. Pseudomyxoma peritonei: role of 18F-FDG PET in preoperative evaluation of pathological grade and potential for complete cytoreduction. Eur J Surg Oncol. 2010;36:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Spyropoulos C, Rentis A, Alexaki E, Triantafillidis JK, Vagianos C. Appendiceal mucocele and pseudomyxoma peritonei; the clinical boundaries of a subtle disease. Am J Case Rep. 2014;15:355-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (3)] |
| 20. | Darr U, Renno A, Alkully T, Khan Z, Tiwari A, Zeb W, Purdy J, Nawras A. Diagnosis of Pseudomyxoma peritonei via endoscopic ultrasound guided fine needle aspiration: a case report and review of literature. Scand J Gastroenterol. 2017;52:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Li Y, Xu HB, Peng Z, Cui SZ, Wu W, Liang H, Zhu ZG, Lu WQ, Li ZY, Xu HM, Liu Q, Zhao YB, Liang B, Ding KF, Wu YM, Zhang ZY, Yang GL, Yang XJ, Wei SZ. Expert consensus on cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy in the treatment of pseudomyxoma peritonei. Zhonghua Yixue Zazhi. 2019;99:1527-1535. [DOI] [Full Text] |
| 22. | Evans T, Aziz O, Chakrabarty B, Wilson MS, Malcomson L, Lavelle C, O'Dwyer ST. Long-term outcomes for patients with peritoneal acellular mucinosis secondary to low grade appendiceal mucinous neoplasms. Eur J Surg Oncol. 2021;47:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cheung DY, Choi MG. Current advance in small bowel tumors. Clin Endosc. 2011;44:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Cao GX, Tian XF, Jiang XH, Wang D. Clinical analysis of 52 cases of primary intestinal tumours. Jiaotong Yixue. 2022;36:416-417. [DOI] [Full Text] |
| 25. | Schmidt S, Felley C, Meuwly JY, Schnyder P, Denys A. CT enteroclysis: technique and clinical applications. Eur Radiol. 2006;16:648-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Cheon JH, Kim YS, Lee IS, Chang DK, Ryu JK, Lee KJ, Moon JS, Park CH, Kim JO, Shim KN, Choi CH, Cheung DY, Jang BI, Seo GS, Chun HJ, Choi MG; Korean Gut Image Study Group. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy. 2007;39:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 28. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 29. | Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol. 2011;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 30. | Ma R, Lu D, Wang B, Zhai X, Xia A, An L, Shi G, Cai Y, Lu Y, Pang S, Chen F, Xu H. Complete Cytoreductive Surgery vs. Debulking Surgery for pseudomyxoma peritonei of appendiceal origin: A propensity score-matched study based on a single-center experience. Eur J Surg Oncol. 2021;47:2369-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Shamavonian R, Lansom JD, Karpes JB, Alzahrani NA, Morris DL. Impact of signet ring cells on overall survival in peritoneal disseminated appendix cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2021;47:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. 2017;33:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 33. | Drittone D, Schipilliti FM, Arrivi G, Mazzuca F. Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy applications in upper and lower gastrointestinal cancer, a review. Oncol Rev. 2024;18:1496141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Trecourt A, Bakrin N, Glehen O, Gertych W, Villeneuve L, Isaac S, Benzerdjeb N, Fontaine J, Genestie C, Dartigues P, Leroux A, Quenet F, Marchal F, Odin C, Khellaf L, Svrcek M, Thierry S, Augros M, Omar A, Devouassoux-Shisheboran M, Kepenekian V; RENAPE Group. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy to Treat Pseudomyxoma Peritonei of Ovarian Origin: A Retrospective French RENAPE Group Study. Ann Surg Oncol. 2024;31:3325-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Saxena A, Valle SJ, Liauw W, Morris DL. Recurrence and Survival Outcomes After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Small Bowel Adenocarcinoma. Anticancer Res. 2017;37:5737-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | van Oudheusden TR, Lemmens VE, Braam HJ, van Ramshorst B, Meijerink J, te Velde EA, Mehta AM, Verwaal VJ, de Hingh IH. Peritoneal metastases from small bowel cancer: Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery. 2015;157:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Yonemura Y, Levine EA, Glehen O, Goere D, Elias D, Morris DL, Sugarbaker PH, Tuech JJ, Cashin P, Spiliotis JD, de Hingh I, Ceelen W, Baumgartner JM, Piso P, Katayama K, Deraco M, Kusamura S, Pocard M, Quenet F, Fushita S; BIG-RENAPE Group. Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastases From a Small Bowel Adenocarcinoma: Multi-Institutional Experience. Ann Surg Oncol. 2018;25:1184-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |