Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.102397
Revised: December 4, 2024
Accepted: February 6, 2025
Published online: April 24, 2025
Processing time: 161 Days and 3.2 Hours
Gastric cancer (GC) is one of the most common malignancies worldwide, and Helicobacter pylori (HP) infection is a well-established risk factor for its develo
To perform a meta-analysis to assess the relationship between HP and PD-L1 expression in patients with GC.
A systematic literature review was conducted using PubMed, Embase, Cochrane Library, and Web of Science databases. Observational studies that examined the association between HP infection and PD-L1 expression in patients with GC were included. Odds ratios and 95% confidence intervals were calculated to estimate the association. Heterogeneity was assessed using Cochrane’s Q test and I² statistic. A random-effects model was used due to significant heterogeneity across studies.
Fourteen studies involving a total of 3069 patients with GC were included. The pooled analysis showed a significant association between HP infection and increased PD-L1 expression in GC tissues (odd ratio = 1.69, 95% confidence interval: 1.24-2.29, P < 0.001, I2 = 59%). Sensitivity analyses confirmed the robustness of these findings. Subgroup analyses did not show significant variation based on geographic region, sample size, or method of PD-L1 assessment. Publication bias was minimal, as shown by funnel plots and Egger’s regression test.
HP infection is associated with increased PD-L1 expression in GC, suggesting that HP status may influence the response to programmed cell death protein 1/PD-L1 blockade therapy.
Core Tip: This meta-analysis investigated the relationship between Helicobacter pylori infection and programmed death-ligand 1 expression in gastric cancer (GC). Fourteen studies with 3069 GC patients were included. Results showed a significant association. Sensitivity and subgroup analyses confirmed the robustness. Publication bias was minimal. Helicobacter pylori infection may influence the response to programmed cell death protein 1/programmed death-ligand 1 blockade therapy in GC patients, but further research is needed to clarify the underlying mechanisms and clinical implications.
- Citation: Yang HC, Fu CF, Qiao LJ, Long GH, Yang LF, Yao B. Relationship between Helicobacter pylori infection and programmed death-ligand 1 in gastric cancer: A meta-analysis. World J Clin Oncol 2025; 16(4): 102397
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/102397.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.102397
Gastric cancer (GC) is among the top five most frequently occurring malignant tumors on a global scale and is the fourth major reason for deaths related to cancer. In 2022, there were approximately 960000 new cases of GC globally and about 650000 deaths[1-3]. Although surgery and postoperative adjuvant radiochemotherapy are considered the main treatment methods for patients with early-stage GC, many are diagnosed at an advanced stage of cancer, limiting the benefits of the aforementioned treatments and patient survival. The continued development of immune checkpoint blocking therapy, such as programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, has changed treatment approaches for GC, showing significant effects either as monotherapy or in combination with chemotherapy[4,5].
PD-L1 is a type I transmembrane protein that inhibits immune responses through its interaction with its receptor PD-1, which is expressed on activated T and B cells and other immune cells[6]. Therefore, PD-L1 upregulation in tumors allows them to evade immune surveillance by inhibiting immune cell activation. In contrast to traditional treatments that target cancer cells directly, PD-1/PD-L1 inhibitors reactivate the patient’s immune system for neoplasm treatment[7]. High PD-L1 expression is not only associated with reduced overall survival (OS) in GC but is also a strong predictive marker for the response of GC patients to immunotherapy[8,9]. In summary, it is important to test for PD-L1 in GC tissue for pro
Approximately 50% of the world’s population is infected with Helicobacter pylori (HP)[10], which is classified as a group 1 carcinogen by the World Health Organization[11], and over 95% of patients with GC have a history of HP infection[12]. Although most individuals infected with HP are asymptomatic, long-term HP infection can lead to chronic gastritis, producing reactive oxygen species that may cause DNA damage, thereby initiating a cancer cascade reaction[13]. Meta-analyses have shown that GC patients infected with HP may have a longer OS rate compared to uninfected patients[14,15]. In vitro and in vivo studies have demonstrated that HP infection may increase the expression of PD-L1 in gastric tissue[16,17], which indicates a potential correlation between HP infection and PD-L1 expression in GC. However, other studies have demonstrated that HP does not increase the risk of PD-L1 expression in GC tissue[18]. Therefore, we performed a systematic analysis to figure out whether HP infection is related to PD-L1 expression in GC patients. The findings from these studies may help us understand the potential interaction between HP infection and the efficacy of immunotherapy in patients with GC.
This study adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[19,20] and the Cochrane Handbook[21] for the conception, conduct, and reporting of the study. The study report conforms to the broad EQUATOR guidelines[22].
Electronic databases, including PubMed, Embase, Cochrane Library, and Web of Science, were searched from inception to June 30, 2024 using a combination of search terms related to the following: (1) “Helicobacter pylori” or “H. pylori” or “HP”; (2) “gastric” or “stomach” or “gastroesophageal junction”; (3) “cancer” or “tumor” or “carcinoma” or “neoplasm”; and (4) “PD-1” or “PD-L1” or “programmed death”. The search was restricted to human studies, and no language restrictions were applied. The reference lists of relevant original and review articles were also manually screened for potential relevant studies.
In accordance with the objectives of the meta-analysis, we established inclusion criteria and adopted the recommended PICOS criteria: P (Patients): Adult patients diagnosed with GC; I (Exposure): Patients infected with HP; C (Control): Patients not infected with HP. The method of verifying HP infection is consistent with the method used in the original study; O (Outcome): Comparing the number of patients with positive PD-L1 expression between HP-infected and uninfected patients. The method and criteria for defining tumor PD-L1 expression are consistent with those applied in the included studies; and S (Study design): Observational studies, such as cross-sectional studies, case-control studies, and cohort studies. Exclude review articles, preclinical studies, studies including other malignant tumor patients, studies not assessing HP infection, or studies not reporting the results of interest. Through these detailed inclusion and exclusion criteria, researchers can ensure that the studies included in the meta-analysis are consistent and comparable in design, execution, and results. This helps to improve the reliability and validity of the results of the meta-analysis and provides a solid evidence base for clinical decision-making and future research.
Literature search, data collection, and study quality assessment were independently conducted by two authors. In the case of disagreement, a third author was contacted for discussion and consensus. We collected the following data from each study that were included: Study information, patient demographics, cancer staging and treatment, HP infection diagnostic methods, and tumor PD-L1 expression. The quality of the studies was assessed using the Newcastle-Ottawa scale[23], which scores participant selection criteria, comparability between groups, and outcome validity. The scale scores range from 1 to 9 stars, with more stars indicating higher study quality.
The numbers of HP-positive and HP-negative patients with GC whose tumors expressed PD-L1 were extracted from each study. The association between HP infection and tumor PD-L1 expression was presented as odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Cochrane’s Q test and I2 statistic were used to assess heterogeneity among studies, with I2 > 50% reflecting significant heterogeneity[24]. A random-effects model was used to combine the results, taking into account the impact of heterogeneity[21]. Sensitivity analysis was performed by excluding one dataset at a time to assess the impact of individual studies on the meta-analysis results[25]. Publication bias was estimated visually by constructing a funnel plot and supplemented by Egger’s regression asymmetry test[26]. Data analysis was performed using R.
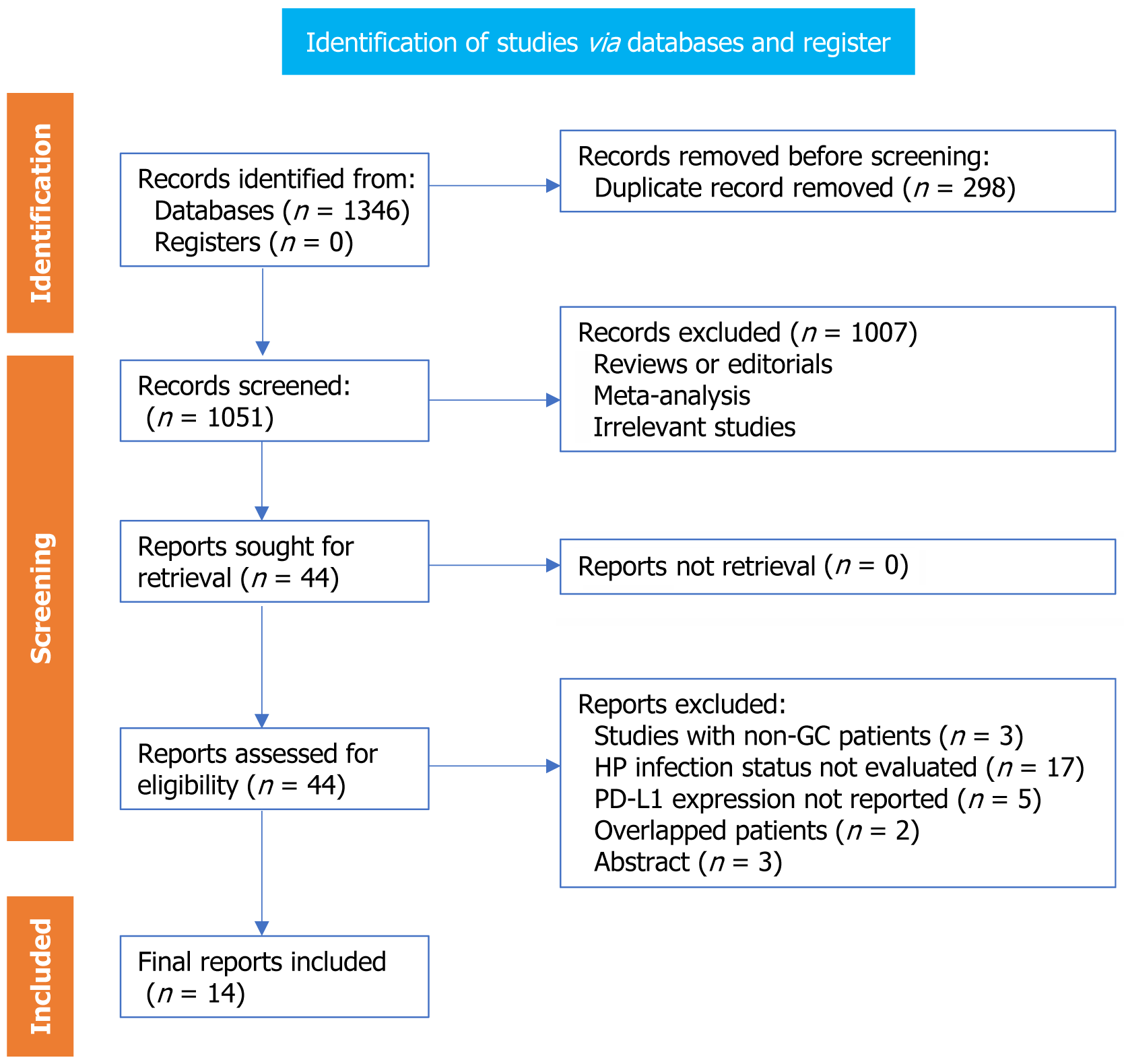
Figure 1 presents the flowchart of our process of searching for literature and conducting studies inclusion process. We obtained 1349 records through the database search and then eliminated 298 duplicate ones. After screening by title and abstract, we excluded an additional 1007 studies, mainly because they didn’t align with the objectives of the meta-analysis. Eventually, we carefully examined the full texts of the remaining 44 studies and removed 30 of them for the reasons detailed in Figure 1. Therefore, we included a total of 14 studies for meta-analysis. Therefore, we included a total of 14 studies for meta-analysis (Table 1).
| Ref. | Design | Country | Diagnosis | Sample size | Mean age (years) | Men (%) | Stage | HP evaluation | HP positive | PD-L1 evaluation and cutoffs | PD-L1 expression |
| Koizumi et al[27], 2022 | CS | Japan | Patients with GC after resection who underwent R0 gastrectomy | 491 | 69 | 67.0 | I-III | Immunohistochemistry | 175 (35.6) | IHC (≥ 10%) | 152 (30.9) |
| Shen et al[28], 2019 | CS | China | Patients with early GC | 54 | 65 | 46.3 | I | Pathological evaluation or histological immunostaining | 19 (35.2) | IHC (≥ 1%) | 24 (44.4) |
| Yoshida et al[29], 2022 | CS | Japan | Patients with GC or GEJC for total or partial | 106 | 68 | 67 | I-IV | Biopsy, serum test, or breath antibody test | 53 (50.0) | CPS (≥ 1) | 73 (68.9) |
| Böger et al[30], 2016 | CS | Germany | Patients with GC or GEJC for total or partial gastrectomy | 392 | 68 | 62.4 | I-IV | Histological PCR | 61 (15.9) | IHC (≥ 5%) | 93 (23.7) |
| Che et al[18], 2022 | CS | China | Patients with GC or GEJC | 25 | NA | 70.1 | III-IV | Histopathology, HpSA, or breath antibody test | 14 (56.0) | NA | 8 (32.0) |
| Magahis et al[31], 2023 | CS | United States | Patients with stage IV GC | 121 | 59 | 65.1 | IV | Histopathology, serum test, or breath antibody test | 25 (20.7) | CPS (≥ 1%) | 76 (62.8) |
| Wu et al[32], 2017 | CS | China | Patients with GC for total or partial gastrectomy | 102 | NA | 74.7 | I-IV | Serum test | 62 (60.8) | IHC (≥ 5%) | 62 (60.8) |
| Jia et al[17], 2024 | CS | China | Patients with GC | 562 | NA | 48.9 | I-IV | Breath antibody test | 290 (51.6) | CPS (≥ 1%) | 331 (58.9) |
| Liu et al[33], 2020 | CS | Korea | Patients with GC for gastrectomy | 127 | 64 | 66.0 | I-IV | Pathological evaluation or histological | 55 (43.3) | CPS (≥ 1%) | 74 (58.3) |
| Di Bartolomeo et al[34], 2015 | CS | Italy | Patients with GC after resection | 55 | 62 | 64 | II-III | Pathological evaluation or histological immunostaining | 23 (41.8) | IHC (≥ 5%) | 37 (67.3) |
| Kuo et al[35], 2017 | CS | Korea | Patients with GC after radical resection | 112 | NA | NA | I-IV | Histological PCR | 43 (38.4) | IHC (≥ 5%) | 35 (31.3) |
| Sughayer et al[36], 2020 | CS | Jordan | Patients with GC for total or partial gastrectomy | 92 | 63 | 61 | I-IV | Pathological evaluation or histological immunostaining | 10 (10.9) | CPS (≥ 1%) | 63 (68.5) |
| Tseng et al[37], 2020 | CS | China | Patients with GC after radical resection | 370 | NA | 81 | I-III | Histological PCR | 97 (26.2) | CPS (≥ 1%) | 78 (21.4) |
| Fang et al[38], 2020 | CS | China | Patients with GC after radical resection | 460 | NA | 71 | I-III | Histological PCR | 157 (34) | CPS (≥ 1%) | 140 (30.0) |
Overall, 14 studies included a total of 3069 patients with GC in this meta-analysis[17,18,27-38]. These studies were all cross-sectional studies, published between 2015 and 2023, conducted in China, South Korea, Japan, Jordan, the United States, Italy, and Germany. All studies included GC patients, most of whom underwent surgical resection. In eight studies[27,28,33-38], HP infection was detected by histological immunohistochemistry (IHC); in two studies[29,30], 13C breath tests, serological tests, and tissue IHC were used to detect HP infection; in two studies[18,31], 13C breath tests, fecal antigen detection methods, and tissue IHC were used to detect HP infection; in 1 study[32], serological tests were used to detect HP infection; and in 1 study[17], 13C breath tests were used to detect HP infection. The overall prevalence of HP infection was 34.7% (1066/3069). Among all included studies, 6 studies[27,28,30,32,34,35] assessed tumor PD-L1 expression by IHC, with one study defining PD-L1 positivity as ≥ 10% positive cells[27], and the other five studies defining PD-L1 positivity as ≥ 5% positive cells[28,30,32,34,35]. Seven studies assessed tumor PD-L1 expression by combined positive score (≥ 1%)[17,29,31,33,36-38], while one study did not define PD-L1 positivity[18]. Overall, out of all the patients with GC, 41.7% (1279 patients precisely) exhibited positive PD-L1 expression. The Newcastle-Ottawa scale scores of the included studies were consistently within the range of six to seven, which signified that the quality of these studies was at a medium or even better level (Table 2).
| Ref. | Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for age and sex | Control for other confounders | Exposure ascertainment | Same methods for events ascertainment | Non-response rates | Total |
| Koizumi et al[27], 2022 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Shen et al[28], 2019 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Yoshida et al[29], 2022 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 6 |
| Böger et al[30], 2016 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Che et al[18], 2022 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 6 |
| Magahis et al[31], 2023 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 6 |
| Wu et al[32], 2017 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 6 |
| Jia et al[17], 2024 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Liu et al[33], 2020 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 6 |
| Di Bartolomeo et al[34], 2015 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Kuo et al[35], 2017 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Sughayer et al[36], 2020 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Tseng et al[37], 2020 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Fang et al[38], 2020 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
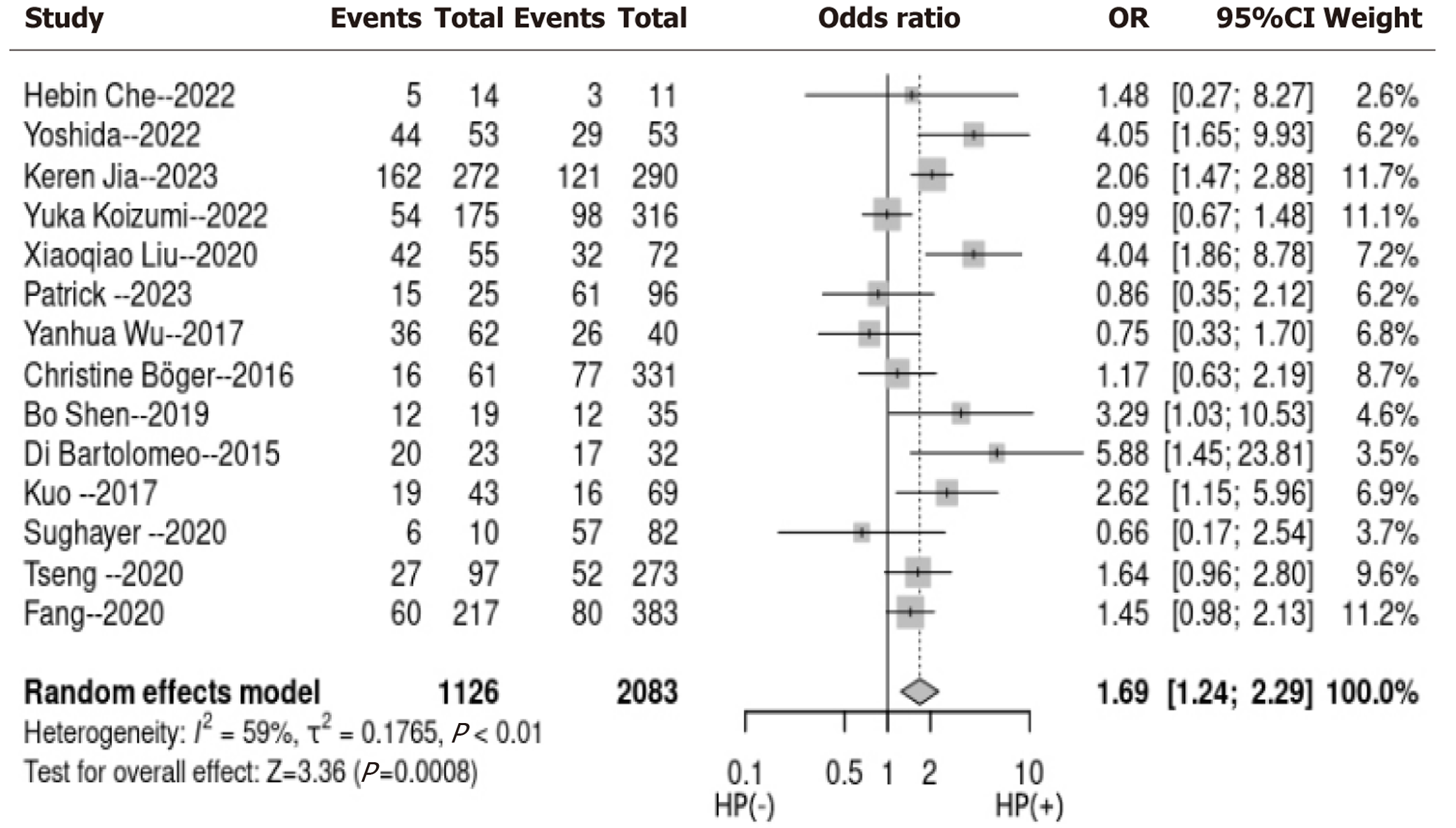
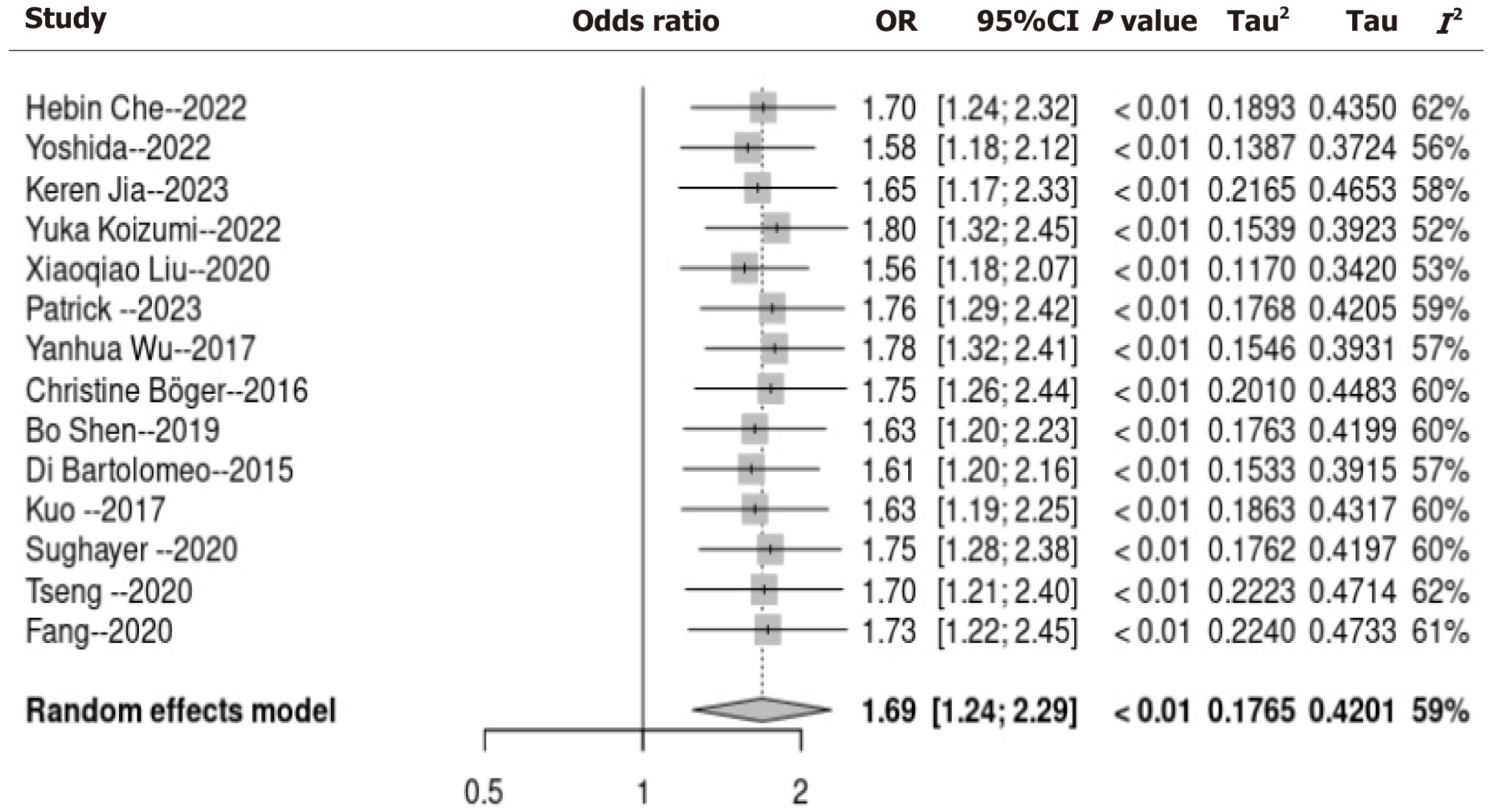
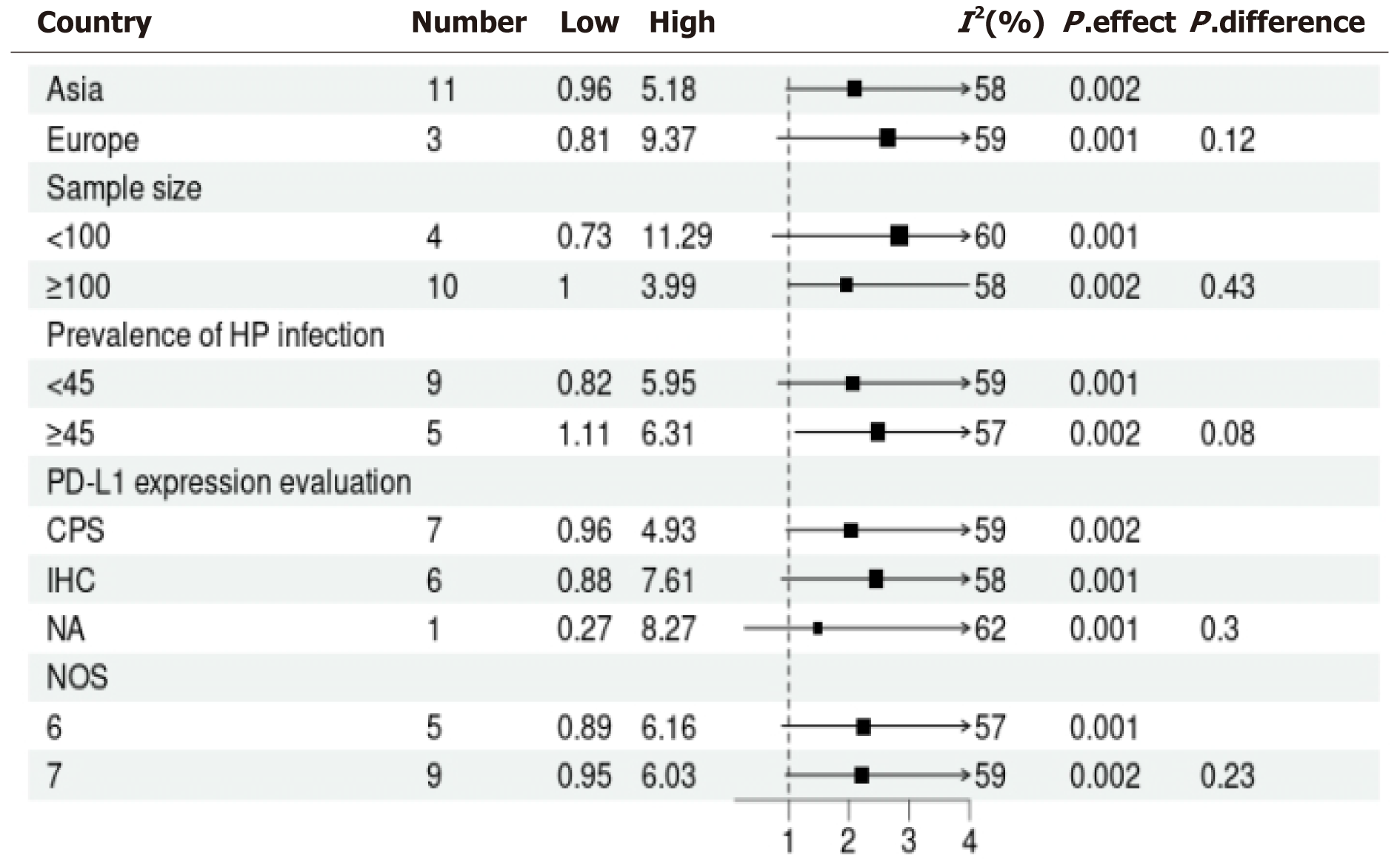
Utilizing a random-effects model to conduct a pooled analysis, it was revealed that HP infection exhibited a statistically significant correlation with tumor PD-L1 expression among patients with GC (OR = 1.69, 95%CI: 1.24-2.29, P < 0.001; I2 = 59%; Figure 2). For the sensitivity analysis, wherein each dataset was sequentially excluded, the outcomes remained consistent (OR = 1.24-2.19, P < 0.01; Figure 3). In the subgroup analysis, it was demonstrated that the relationship between HP infection and PD-L1 expression in GC was not substantially influenced by factors such as the country of origin, sample size, prevalence rate of HP infection, PD-L1 assessment methodology, or quality assessment score (all subgroup analyses, P > 0.05; Figure 4).
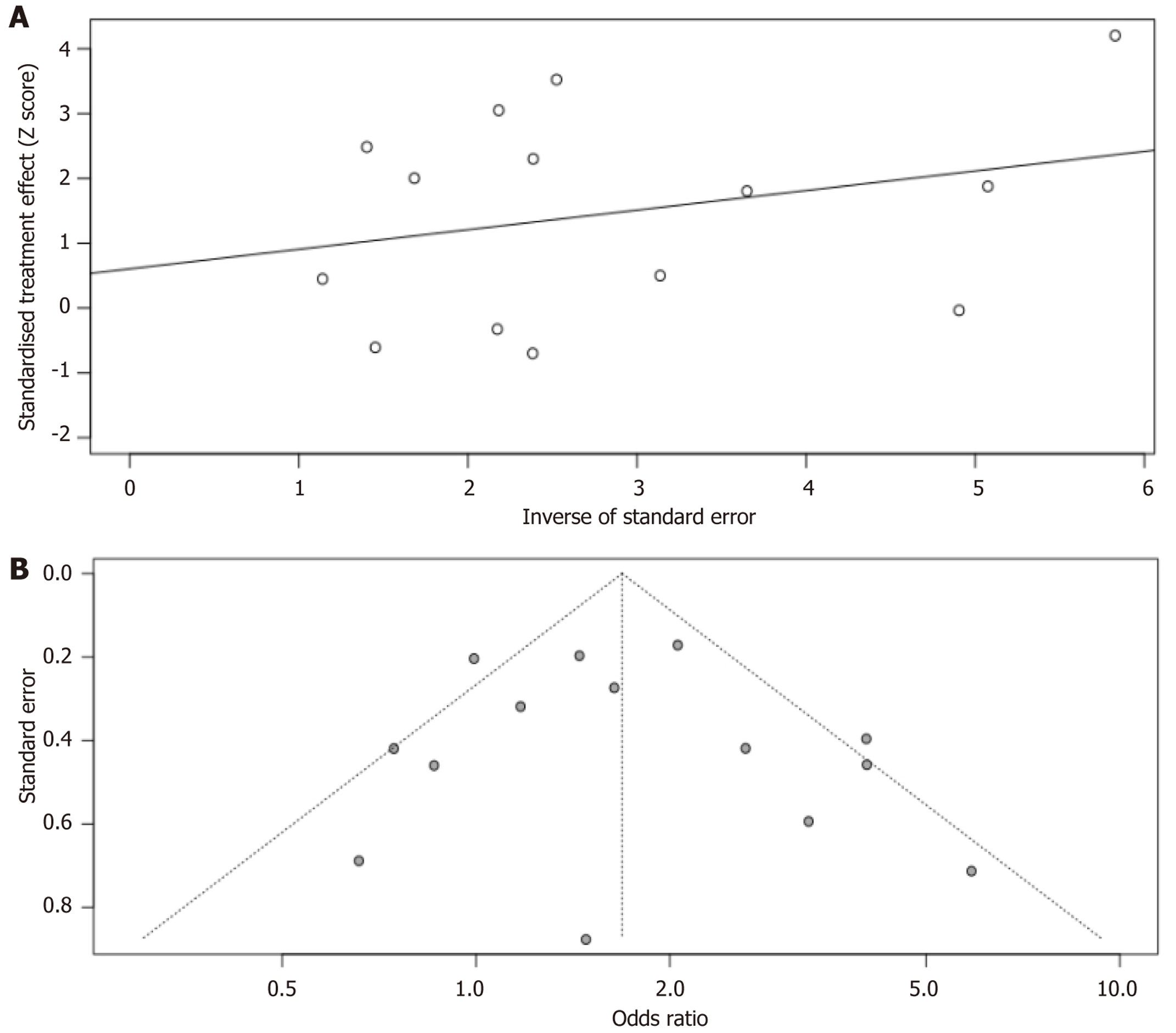
Figure 3 depicts the funnel plot for the meta-analysis of the link between HP infection and PD-L1 expression in GC patients’ neoplasms. Visual inspection reveals symmetry, and the Egger’s test shows a low probability of publication bias, bolstering the credibility of the analysis (Figure 5).
Our meta-analysis of 14 observational studies showed that HP infection in patients with GC is associated with PD-L1 tumor expression. The finding was consistently validated through sensitivity analysis where one dataset was excluded at a time, and through subgroup analysis based on a variety of study characteristics, namely the study country, sample size, HP infection rate, PD-L1 assessment methodology, and quality assessment score. Overall, the results of the meta-analysis show that HP infection is linked to the tumor expression of PD-L1 in GC. These findings are consistent with previous meta-analysis results[39]. Immunotherapy provides survival benefits for cancer patients, but not all patients benefit. PD-L1 expression is an important biological marker for testing whether patients will respond to cancer immunotherapy[40]. Therefore, it is of high importance to determine whether HP infection status affects the efficacy of PD-1/PD-L1 blockade therapy in GC patients. Previous reports have suggested that HP infection may upregulate PD-L1 expression in GC[16,17], but contrary findings have also been reported[18]. Therefore, it is particularly important to use meta-analysis methods to assess the relationship between HP infection and PD-L1 expression in patients with GC.
HP is the first pathogenic factor for GC[11], and treatment to eradicate HP can significantly reduce the incidence of GC by 43%[41]. Therefore, it is conceivable that HP eradication should extend the survival of GC patients, but there are conflicting reports. Kim et al[42] reported that HP eradication did not extend the survival of GC patients. In contrast, Zhao et al[43] reported that patients receiving anti-HP treatment had a significant advantage in OS and disease-free survival compared to patients not receiving HP treatment. After propensity score matching, the advantage in OS and disease-free survival still existed. There may be two reasons for the different conclusions[15,44-47]: The gene expression profile of tumor tissue is distinct in HP-negative GC cases vs HP-positive GC cases after eradication treatment, and the genes that show differential expression are involved in cancer-related signaling pathways, namely the extracellular signal-regulated kinase/mitogen-activated protein kinase and Wnt/β-catenin signaling pathways. These transcriptional changes could be due to epigenetic changes and chronic inflammation caused by HP infection. In addition, HP bacterial virulence factors, for example CagA and VacA, not only promote bacterial colonization in the gastric mucosa but also induce innate and adaptive immune responses, activate helper T cells 1, and are associated with good prognosis of GC. Taking the relationship between HP and enhanced PD-L1 in GC derived from the meta-analysis can be translated to clinical observations, namely, whether the status of HP infection in GC patients correlates to better responses to immunotherapy. Surprisingly, the opposite has been reported. Che et al[18] showed that OS and progression-free survival (PFS) of the HP-negative group are longer than those of the positive group [mOS 17.5 months vs 6.2 months, hazard ratio (HR) = 2.85, 95%CI: 1.74-1.78, P = 0.021; mPFS 8.4 months vs 2.7 months, HR = 3.11, 95%CI: 1.96-5.07, P = 0.008]. Multivariate analysis revealed that HP is an independent risk factor for PFS (HR= 1.90, 95%CI: 1.10-3.30; P = 0.022). Magahis et al[31] and other studies also found that the mPFS (P < 0.01) and OS (P = 0.02) of the HP-positive group were significantly shorter than those of the HP-negative group, and the OS of the positive group continued to be shorter after excluding patients receiving co-occurring chemotherapy (6.2 months vs 16.7 months). The same multivariate analysis confirmed that HP is an independent determinant of PFS (HR = 3.04, P < 0.01) and OS (HR = 2.24, P = 0.01). Similar findings have been reported for other tumors, such as lung cancer[48] and melanoma[49]. There is no exact mechanism to explain this phenomenon, though it may be related to the tumor microenvironment. In fact, in addition to HP being the most common bacterium in the stomach, there are other common microbial communities in the stomach, and the Proteobacteria phylum is the second most common bacterial community in HP-positive GC[50]. Increasing evidence continues to suggest that other microbial communities in the stomach also promote GC development and the abundance and diversity of the gastrointestinal microbiome affects the efficacy of immunotherapy[51,52]. HP alters the gastrointestinal microbiome, and the abundance of some bacteria returns to normal after eradication of HP[53]. In fact, some studies have shown that the abundance of the gastrointestinal microbiome in HP-positive GC and HP-negative GC cases is different[54]. Therefore, we speculate that the long-term colonization of HP infection causes changes in the abundance of the gastrointestinal microbiome, leading to a negative correlation between immune treatment effects. Of course, the specific mechanism is not yet clear and further research is needed.
This meta-analysis also has some limitations. First, the number of studies available was limited, and they each included small cohort sizes. The relationship between HP infection and tumor PD-L1 expression in patients with GC needs to be verified by large sample studies, and it is best to have prospective double-blind controlled studies to support the data in the future. Second, there was significant heterogeneity in the included studies because this study is based on the meta-analysis of single-variate analysis studies. Other characteristics of HP infection and GC PD-L1 expression relationships were not analyzed, such as HP detection method, HP-related virulence factors (CagA, VagA), GC tissue type, microsatellite instability, and GC tumor-node-metastasis staging, among others. Finally, the conclusions of this study should be interpreted with caution as observational results. HP is a well-established GC pathogenic factor, and the importance of its eradication to prevent GC has been recognized. Our study suggests that that HP increases PD-L1 expression in GC. Based on this result, whether patients with GC receiving immunotherapy should receive HP eradication treatment requires further verification.
In summary, the outcomes of the meta-analysis demonstrate that HP infection is related to tumor PD-L1 expression in GC patients. These results suggest that the status of HP infection may be beneficial in influencing the therapeutic efficacy of PD-1/PD-L1 blockade therapy in these patients, indicating that HP can be a potential indicator of GC immunotherapy prognosis. Nonetheless, the relationship between HP and immunotherapy requires further confirmation in the future.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11931] [Article Influence: 2982.8] [Reference Citation Analysis (4)] |
| 3. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8084] [Article Influence: 8084.0] [Reference Citation Analysis (2)] |
| 4. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1887] [Article Influence: 471.8] [Reference Citation Analysis (1)] |
| 5. | Rha SY, Wyrwicz LS, Weber PEY, Bai Y, Ryu MH, Lee J, Rivera F, Alves GV, Garrido M, Shiu KK, Fernández MG, Li J, Lowery M, Cil T, Cruz F, Qin S, Yin L, Bordia S, Bhagia P, Oh DY. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;34:319-320. [RCA] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 6. | Horita H, Law A, Hong S, Middleton K. Identifying Regulatory Posttranslational Modifications of PD-L1: A Focus on Monoubiquitinaton. Neoplasia. 2017;19:346-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 1345] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 8. | Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 9. | Xie T, Zhang Z, Zhang X, Qi C, Shen L, Peng Z. Appropriate PD-L1 Cutoff Value for Gastric Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:646355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 11. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobacter pylori. Lyon: International Agency for Research on Cancer, 1994. |
| 12. | Iizasa H, Kartika AV, Fekadu S, Okada S, Onomura D, Wadi AFAA, Khatun MM, Moe TM, Nishikawa J, Yoshiyama H. Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis. World J Gastroenterol. 2022;28:6249-6257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 13. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 14. | Fang WL, Huang KH, Chang SC, Lin CH, Chen MH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. Comparison of the Clinicopathological Characteristics and Genetic Alterations Between Patients with Gastric Cancer with or Without Helicobacter pylori Infection. Oncologist. 2019;24:e845-e853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Jia Z, Zheng M, Jiang J, Cao D, Wu Y, Zhang Y, Fu Y, Cao X. Positive H. pylori status predicts better prognosis of non-cardiac gastric cancer patients: results from cohort study and meta-analysis. BMC Cancer. 2022;22:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Koh V, Chakrabarti J, Torvund M, Steele N, Hawkins JA, Ito Y, Wang J, Helmrath MA, Merchant JL, Ahmed SA, Shabbir A, Yan So JB, Yong WP, Zavros Y. Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 2021;518:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 17. | Jia K, Chen Y, Xie Y, Wang X, Hu Y, Sun Y, Cao Y, Zhang L, Wang Y, Wang Z, Lu Z, Li J, Zhang X, Shen L. Helicobacter pylori and immunotherapy for gastrointestinal cancer. Innovation (Camb). 2024;5:100561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Che H, Xiong Q, Ma J, Chen S, Wu H, Xu H, Hou B. Association of Helicobacter pylori infection with survival outcomes in advanced gastric cancer patients treated with immune checkpoint inhibitors. BMC Cancer. 2022;22:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4658] [Article Influence: 1164.5] [Reference Citation Analysis (0)] |
| 20. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 21. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. [cited 10 October 2024]. Available from: https://training.cochrane.org/handbook. |
| 22. | Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 901] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 23. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 15 September 2024]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 24. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25779] [Article Influence: 1120.8] [Reference Citation Analysis (0)] |
| 25. | Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 846] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 26. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40493] [Article Influence: 1446.2] [Reference Citation Analysis (2)] |
| 27. | Koizumi Y, Ahmad S, Ikeda M, Yashima-Abo A, Espina G, Sugimoto R, Sugai T, Iwaya T, Tamura G, Koeda K, Liotta LA, Takahashi F, Nishizuka SS; Northern Japan Gastric Cancer Study Consortium. Helicobacter pylori Modulated Host Immunity in Gastric Cancer Patients With S-1 Adjuvant Chemotherapy. J Natl Cancer Inst. 2022;114:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Shen B, Qian A, Lao W, Li W, Chen X, Zhang B, Wang H, Yuan F, Sun Y. Relationship between Helicobacter pylori and expression of programmed death-1 and its ligand in gastric intraepithelial neoplasia and early-stage gastric cancer. Cancer Manag Res. 2019;11:3909-3919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Yoshida T, Ogura G, Tanabe M, Hayashi T, Ohbayashi C, Azuma M, Kunisaki C, Akazawa Y, Ozawa S, Matsumoto S, Suzuki T, Mitoro A, Fukunaga T, Shimizu A, Fujimoto G, Yao T. Clinicopathological features of PD-L1 protein expression, EBV positivity, and MSI status in patients with advanced gastric and esophagogastric junction adenocarcinoma in Japan. Cancer Biol Ther. 2022;23:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269-24283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 31. | Magahis PT, Maron SB, Cowzer D, King S, Schattner M, Janjigian Y, Faleck D, Laszkowska M. Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2023;11:e007699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8:64066-64082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Liu X, Choi MG, Kim K, Kim KM, Kim ST, Park SH, Cristescu R, Peter S, Lee J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract. 2020;216:152881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | Di Bartolomeo M, Pellegrinelli A, Iacovelli R, Pietrantonio F, Berenato R, Caporale M, Niger M, Barbera M, Labianca R, Martoni A, Rosati G, Nitti D, Boni C, Amadori D, Cantore M, de Braud F, Bajetta E. Association with programmed death ligand-1 (PDL-1) expression and Helicobacter Pylori infection in patients with non-diffuse type gastric carcinoma. Ann Oncol. 2015;26:vi93. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Kuo SH, Wu MS, Liou JM, Shun CT, Wei MF, Zeng YS, Hsu PN, Cheng AL. Helicobacter pylori CagA expression is closely associated with tumor PD-L1 expression and the better prognosis of gastric cancer patients. Ann Oncol. 2017;28:xi10. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Sughayer MA, Dabbagh TZ, Battah AH. PD-L1 Expression Is a Favorable Prognostic Marker in Gastric Carcinoma. Appl Immunohistochem Mol Morphol. 2020;28:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Tseng CH, Fang WL, Huang KH, Chen MH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. The clinicopathological characteristics and genetic alterations of mucinous carcinoma of the stomach. J Chin Med Assoc. 2020;83:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Fang WL, Chen MH, Huang KH, Lin CH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. The Clinicopathological Features and Genetic Alterations in Epstein-Barr Virus-Associated Gastric Cancer Patients after Curative Surgery. Cancers (Basel). 2020;12:1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Zhu Y, Zhu F, Ba H, Chen J, Bian X. Helicobacter pylori infection and PD-L1 expression in gastric cancer: A meta-analysis. Eur J Clin Invest. 2023;53:e13880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 40. | Walk EE, Yohe SL, Beckman A, Schade A, Zutter MM, Pfeifer J, Berry AB; College of American Pathologists Personalized Health Care Committee. The Cancer Immunotherapy Biomarker Testing Landscape. Arch Pathol Lab Med. 2020;144:706-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 674] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 42. | Kim YI, Cho SJ, Lee JY, Kim CG, Kook MC, Ryu KW, Kim YW, Choi IJ. Effect of Helicobacter pylori Eradication on Long-Term Survival after Distal Gastrectomy for Gastric Cancer. Cancer Res Treat. 2016;48:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Zhao Z, Zhang R, Chen G, Nie M, Zhang F, Chen X, Lin J, Chen Z, Lin F, Wei C, Zheng Z, Ruan S, Huang B, Chen Y, Nie R. Anti-Helicobacter pylori Treatment in Patients With Gastric Cancer After Radical Gastrectomy. JAMA Netw Open. 2024;7:e243812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Hu Y, He C, Liu JP, Li NS, Peng C, Yang-Ou YB, Yang XY, Lu NH, Zhu Y. Analysis of key genes and signaling pathways involved in Helicobacter pylori-associated gastric cancer based on The Cancer Genome Atlas database and RNA sequencing data. Helicobacter. 2018;23:e12530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Lian G, Wei C, Wang D, Cui M, Wang Z, Liu X, Li W, Wang L, Wang Q, Zhang DY, Suo J, Ye F. Protein profiling of Helicobacter pylori-associated gastric cancer. Am J Pathol. 2014;184:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (6)] |
| 47. | Jafarzadeh A, Larussa T, Nemati M, Jalapour S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microb Pathog. 2018;116:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, Richard C, Leblond MM, Messaoudene M, Machremi E, Limagne E, Ghiringhelli F, Routy B, Verdeil G, Velin D. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71:457-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 49. | Tonneau M, Nolin-Lapalme A, Kazandjian S, Auclin E, Panasci J, Benlaifaoui M, Ponce M, Al-Saleh A, Belkaid W, Naimi S, Mihalcioiu C, Watson I, Bouin M, Miller W, Hudson M, Wong MK, Pezo RC, Turcotte S, Bélanger K, Jamal R, Oster P, Velin D, Richard C, Messaoudene M, Elkrief A, Routy B. Helicobacter pylori serology is associated with worse overall survival in patients with melanoma treated with immune checkpoint inhibitors. Oncoimmunology. 2022;11:2096535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Yu G, Torres J, Hu N, Medrano-Guzman R, Herrera-Goepfert R, Humphrys MS, Wang L, Wang C, Ding T, Ravel J, Taylor PR, Abnet CC, Goldstein AM. Molecular Characterization of the Human Stomach Microbiota in Gastric Cancer Patients. Front Cell Infect Microbiol. 2017;7:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 51. | Wang M, Yang G, Tian Y, Zhang Q, Liu Z, Xin Y. The role of the gut microbiota in gastric cancer: the immunoregulation and immunotherapy. Front Immunol. 2023;14:1183331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 52. | Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. The Diversity of Gut Microbiome is Associated With Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J Thorac Oncol. 2019;14:1378-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 377] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 53. | Chen X, Wang N, Wang J, Liao B, Cheng L, Ren B. The interactions between oral-gut axis microbiota and Helicobacter pylori. Front Cell Infect Microbiol. 2022;12:914418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 54. | Kolahi Sadeghi L, Vahidian F, Eterafi M, Safarzadeh E. Gastrointestinal cancer resistance to treatment: the role of microbiota. Infect Agent Cancer. 2024;19:50. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |