Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.101548
Revised: January 12, 2025
Accepted: February 21, 2025
Published online: April 24, 2025
Processing time: 188 Days and 19.8 Hours
The cytochrome P450 3A (CYP3A) gene family’s role in early progression of gastric cancer was comprehensively investigated. Its potential as a therapeutic target was evaluated. Upon literature review, aberrant expression of the CYP3A gene family has a strong correlation with gastric cancer onset, although the precise underlying mechanisms remain unclear. To assess its potential as a bio
Core Tip: Although the role of cytochrome P450 3A (CYP3A) enzymes in gastric cancer remains underexplored, this review synthesizes existing evidence to reveal their involvement in tumor growth, migration, and resistance to chemotherapy. By integrating insights into CYP3A polymorphisms and their influence on drug metabolism, the study provides a potential framework for developing personalized treatment strategies aimed at improving patient outcomes.
- Citation: Zhu JK, Wang J. Cytochrome P450 3A gene family in gastric cancer: Unveiling diagnostic biomarkers and therapeutic targets for personalized treatment. World J Clin Oncol 2025; 16(4): 101548
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/101548.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.101548
Gastric cancer remains one of the most common malignancies globally, with consistently high incidence and mortality rates. According to the World Health Organization, approximately one million people worldwide are diagnosed with gastric cancer annually, with about 70% of these patients losing the opportunity for curative surgery due to late-stage diagnosis. The early symptoms of gastric cancer are always nonspecific and subtle, typically presenting as indigestion or epigastric discomfort, leading to delayed diagnosis and missed treatment windows. Therefore, identifying effective biomarkers and therapeutic targets is crucial for early diagnosis and therapy of gastric cancer.
As a crucial member of the cytochrome P450 (CYP) superfamily, the CYP3A gene family has recently garnered significant attention in cancer research[1]. As early as 1995, McKinnon et al[2], in their study, used in situ hybridization to analyze formalin-fixed, routinely processed biopsy samples, establishing the distribution of CYP3A mRNA in human tissues, with high expression observed in the small intestine or liver. Additionally, in the study published by Klyushova et al[3], it was noted that the CYP3A family is involved in the metabolism of various endogenous and exogenous com
While many studies have preliminarily explored CYP3A gene family’s role in gastric cancer, there are still many gaps in the current research. First, most studies focus on the correlation between CYP3A enzyme expression levels and gastric cancer, as illustrated in the study published by Murray et al[6]. However, the specific mechanisms by which CYP3A propels gastric cancer progression, such as its regulation of signaling pathways, effects on the cell cycle, and involvement in apoptosis, have not been studied. Furthermore, we fail to understand the differential expression of the CYP3A gene family in various gastric cancer subtypes and its clinical significance; thus, its application as a biomarker in clinical settings is still limited. In addition, further investigation and validation regarding therapeutic strategies targeting the CYP3A gene family, such as the development of specific inhibitors[7] and the potential of gene therapy are required.
In light of this, we aim to systematically summarize and analyze the role of the CYP3A gene family in the early progression of gastric cancer and its therapeutic potential. We examine the biological functions of the CYP3A family, explore its regulatory mechanisms in gastric cancer, and analyze its relationship with early tumor progression. Additionally, we will evaluate the feasibility of CYP3A as a therapeutic target, along with the potential application of the CYP3A family in personalized treatment for gastric cancer. We seek to offer novel strategies for the early diagnosis and therapy of gastric cancer.
From a research perspective, we hope to shed light on processes underlying gastric cancer progression and provide potential biomarkers for early diagnosis via a deeper investigation into the mechanisms by which the CYP3A gene family influences gastric cancer. The CYP3A family serves as a key enzyme in drug metabolism; therefore, its role in gastric cancer therapy is of significant clinical value. In terms of research significance, the findings of this review will offer theoretical support along with practical guidance for the early diagnosis and therapy of gastric cancer, improve patient outcomes and quality of life, and offer considerable scientific and societal value. In conclusion, we will conduct an in-depth analysis of the role of the CYP3A gene family in the early progression of gastric cancer and its therapeutic potential. We aim to offer new strategies and directions for the early diagnosis and treatment of gastric cancer.
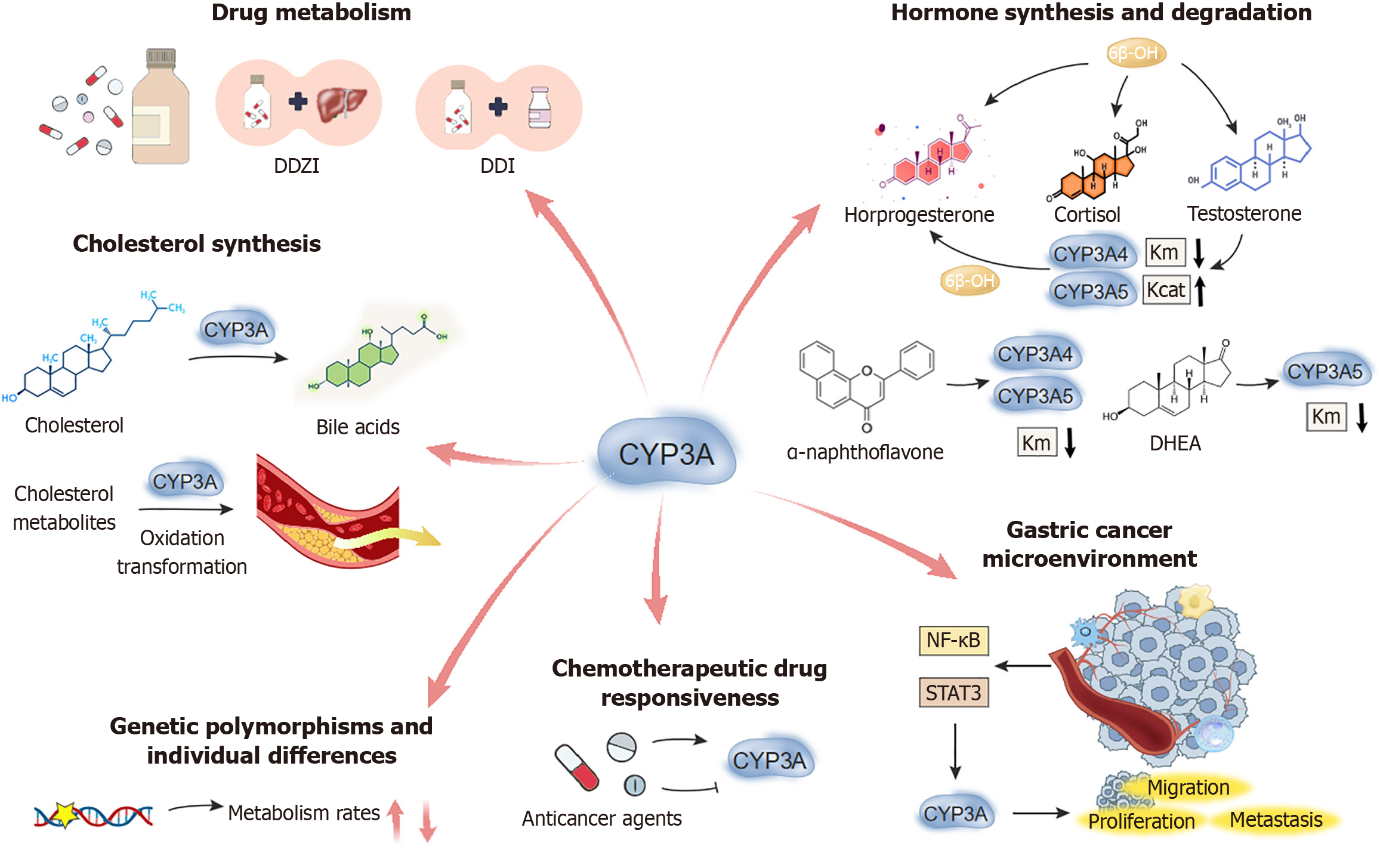
The CYP3A gene family is a key subfamily within the CYP enzyme system, and is widely distributed across various human tissues, such as the liver, kidney and small intestine. CYP enzymes were first mentioned by O. Hayaishi in 1958, and since then, numerous scholars have performed extensive research which has greatly expanded our understanding of the functions and mechanisms of this enzyme system[8-11]. These enzymes play a pivotal role in critical biochemical processes such as drug metabolism[12], hormone synthesis and degradation[13], and cholesterol synthesis (Figure 1). Aberrations in CYP3A enzyme activity have been strongly linked to the onset and progression of various illnesses, particularly in the tumor biology field. The CYP3A gene family is crucial in the initiation and progression of gastric cancer, and significantly influences metastasis and patient response to therapeutic agents. Figure 1 shows the role of CYP3A4/5 in drug metabolism, hormone synthesis/degradation, and cholesterol synthesis, with implications for gastric cancer and therapeutic response.
CYP3A enzymes are the predominant drug-metabolizing enzymes in the liver, which metabolize over 50% of the drugs (including many chemotherapeutic agents) used in clinical practice. For example, apatinib is an oral small-molecule tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor. It has gained approval from China for treating advanced gastric cancer or gastroesophageal junction adenocarcinoma. The metabolism of apatinib primarily occurs through CYP3A4/5 and CYP2D6 enzymes, and CYP3A4/5 plays a dominant role in the process[14]. Researchers evaluated the pharmacokinetic behavior of apatinib under various conditions, including drug-drug interactions related to CYP3A4/5 and CYP2D6, as well as drug-disease interactions in patients with liver or kidney impairment via a physiologically based pharmacokinetic model. Their results indicated that moderate CYP3A4 inhibitors and inducers could change 2-4-fold during apatinib exposure, whereas strong CYP2D6 inhibitors moderately increased apatinib exposure (1.25-2 times). These findings indicate that apatinib plasma exposure may be influenced by modulators of CYP3A4/5 and CYP2D6 activity.
CYP3A enzymes are critical for the synthesis and degradation of various hormones. The study by Niwa et al[15] elucidated the effects of different members of the CYP3A subfamily on sex hormones, including progesterone, cortisol, and testosterone. CYP3A5, CYP3A4, and CYP3A7 and other members of the CYP3A subfamily, are key players in the metabolism of various endogenous steroid hormones, particularly in the 6β-hydroxylation (6β-OH) reactions of testosterone, progesterone, and cortisol. Certain endogenous steroid hormones and many other compounds affect the metabolic activity of these CYP3A family members. The study found that testosterone, dehydroepiandrosterone, and α-naphthoflavone could activate the 6β-OH of cortisol and progesterone. However, this activation effect varied among different CYP3A subfamily members. For instance, testosterone was found to be the least easily activated among the three steroid hormones, which implied that testosterone 6β-OH could serve as a useful in vitro marker reaction in studies of CYP3A-related drug interactions.
Testosterone enhances the metabolic activity of CYP3A4, CYP3A5, and CYP3A7 in the 6β-OH of progesterone, as indicated by reduced substrate affinity (Km) values (increased substrate affinity) and/or increased catalytic efficiency values (maximum reaction rates). Dehydroepiandrosterone lowered the Km value in CYP3A5-mediated reactions, whereas α-naphthoflavone reduced the Km values in both CYP3A4 and CYP3A5-mediated reactions. These findings suggest that different CYP3A subfamily members exhibit varying sensitivities in steroid hormone metabolism, and their metabolic activity can be activated or inhibited by specific endogenous hormones and exogenous compounds. This finding is significant for understanding drug-drug interactions and inter-individual differences in drug responses.
Moreover, we also observed that certain compounds, such as azole antifungal agents like voriconazole and ke
Cholesterol is a vital component of cell membranes. It serves as a precursor for various bioactive substances, such as vitamin D, steroid hormones and bile acids. CYP3A enzymes are crucial to the cholesterol biosynthesis pathway[17], and take part in multiple steps, including the synthesis and conversion of cholesterol precursors. Cholesterol biosynthesis begins with acetyl-coenzyme A in the liver, proceeds through a series of enzymatic reactions, and ultimately yields cholesterol. In this process, CYP3A enzymes are particularly involved in the later stages of cholesterol synthesis, and regulate modification and transformation of cholesterol side chains. These reactions are essential for controlling the rate of cholesterol synthesis and for maintaining the balance of cholesterol levels in the body.
In addition to their role in cholesterol synthesis, CYP3A enzymes are critical in the generation of cholesterol metabolites. For instance, CYP3A enzymes are involved in the conversion of cholesterol into bile acids, the primary metabolites of cholesterol in the liver. Bile acids are essential for digesting and absorbing fats. By catalyzing the oxidation of cholesterol, CYP3A enzymes promote bile acid synthesis, and thereby influence cholesterol excretion and homeostasis. Moreover, CYP3A enzymes participate in the inactivation and clearance of cholesterol metabolites, and prevent their excessive accumulation in the body. The excessive buildup of cholesterol and its metabolites can lead to atherosclerosis, cardiovascular disorders and other diseases. CYP3A enzymes facilitate their elimination from the body by catalyzing the further oxidation and transformation of cholesterol metabolites, and prevent potential harm caused by cholesterol accumulation. Abnormal cholesterol metabolism can lead to changes in cell membrane function, which, in turn, may affect cell signaling and cell cycle regulation, and closely link cholesterol metabolism to tumorigenesis. Research has indicated a strong association between cholesterol metabolism levels and pancreatic cancer and other cancers[18,19]. Moreover, Wnt-dependent cancers may find new therapeutic avenues from cholesterol-targeted treatments[20].
Aberrant expression of CYP3A enzymes may enhance tumor cell proliferation, invasion and migration. Relevant mechanisms have been explored in one study which mainly investigated how hypoxic conditions influence chemoresistance and inhibit cell proliferation in human medulloblastoma DAOY cells[21]. The research noted that hypoxia is a common feature in many solid tumors and regulates various biological processes associated with tumor progression through the activation of hypoxia-inducible factor 1-alpha (HIF-1α) activation. In medulloblastoma, hypoxic conditions are strongly linked to the activation of HIF-1α, a process which might regulate the activity and expression of CYP3A enzymes, and thereby affect tumor cell responsiveness to chemotherapy drugs.
In tumor cells, CYP3A enzymes may promote cell proliferation by metabolizing substances, which stimulate cell growth or by regulating the expression of proteins involved in the cell cycle. Additionally, CYP3A enzymes may enhance tumor cell invasion and migration by influencing degradation and remodeling of the extracellular matrix. Expression of CYP3A enzymes in DAOY cells is reduced under hypoxic conditions. It correlates with increased resistance to che
Moreover, hypoxic conditions may also inhibit cell proliferation and arrest the cell cycle at the G1 phase via the HIF-1α pathway. Studies have shown that chemically inhibiting HIF-1α transcriptional activity can enhance the cytotoxicity of cyclophosphamide and ifosfamide, and promote apoptosis. This suggests that inhibiting HIF-1α could restore CYP3A enzyme activity and expression, and thereby increase drug metabolism efficiency and cytotoxicity, offering new therapeutic strategies for medulloblastoma. To sum up, aberrant expression of CYP3A enzymes is closely related to increased tumor cell proliferation, migration, and invasion, particularly under hypoxic conditions. Modulating CYP3A enzyme activity may provide new avenues for the chemotherapy of medulloblastoma.
Numerous studies have emphasized the significance of CYP3A enzymes in the tumor microenvironment[22-25]. The activity and expression of CYP3A enzymes in the tumor microenvironment could be affected by the tumor, immune system, vascular cells and other factors. For instance, inflammatory cytokines and growth factors in the tumor microenvironment may activate specific signaling pathways, including nuclear factor κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3), and accordingly regulate CYP3A gene expression. Moreover, CYP3A enzymes may regulate oxidative stress responses within the tumor microenvironment, and accordingly further modulate the biological behavior of tumor cells.
Aberrant expression of CYP3A enzymes is closely associated with tumor aggressiveness and poor prognosis in certain types of tumors. For example, the expression of CYP3A enzymes is directly linked to chemoresistance and the inhibition of cell proliferation in medulloblastoma. In hepatocellular carcinoma, CYP3A enzyme expression is tightly connected to inflammatory responses and immune cell function within the tumor microenvironment, and potentially promotes tumor development and metastasis as it can modulate inflammatory mediators and angiogenic factors. CYP3A enzymes may promote tumor growth and dissemination as it can regulate inflammatory responses, angiogenesis[26], and immune cell function in the tumor microenvironment. This mechanism provides new understanding on the role of CYP3A enzymes in different tumors, and offers potential targets for optimizing cancer therapeutic strategies.
The activity of CYP3A enzymes may influence the responsiveness of gastric cancer patients to chemotherapeutic drugs. Many anticancer agents, such as sorafenib and sunitinib, are substrates of CYP3A enzymes[27]. Sorafenib and sunitinib, both multi-targeted small molecule tyrosine kinase inhibitors developed in recent years, have demonstrated significant survival benefits in treating gastrointestinal stromal tumors, hepatocellular carcinoma, and advanced renal cell carcinoma. These drugs exert their antitumor effects by inhibiting key signaling pathways involved in tumor angio
Recent studies have explored the effects of sorafenib and sunitinib on the activity of the primary drug-metabolizing enzymes in the liver, CYP3A4 and CYP3A5, particularly their regulation of midazolam 1’-hydroxylation[27]. The findings revealed that both drugs significantly inhibited midazolam hydroxylation, which is mediated by recombinant CYP3A4. Similarly, the hydroxylation of midazolam in liver microsomes from individuals with the CYP3A53/3 genotype (characterized by low CYP3A5 expression) was also inhibited. However, the midazolam 1’-hydroxylation reaction catalyzed by recombinant CYP3A5 exhibited concentration-dependent enhancement in the presence of sunitinib and sorafenib, reaching saturation at approximately 10 μmol/L. Researchers found similar enhancement in liver microsomes from individuals with high CYP3A5 expression (CYP3A51/1 genotype).
Further metabolic studies showed that the primary metabolic pathways of sorafenib and sunitinib - sorafenib N-oxidation and sunitinib N-deethylation - are predominantly catalyzed by CYP3A4, not CYP3A5[28]. Pre-incubation of sorafenib and sunitinib prior to adding midazolam did not influence enhancement of midazolam hydroxylation catalyzed by CYP3A5, implying that this enhancement is directly induced by the parent drugs. Molecular docking studies, based on one homology model of CYP3A5 constructed from the structure of CYP3A4, revealed that midazolam binds more tightly to the heme group in CYP3A5, suggesting that sunitinib and sorafenib might act as activators rather than substrates of CYP3A5[27]. These results suggest that while sunitinib and sorafenib may enhance midazolam 1’-hydroxylation by activating CYP3A5, they are simultaneously inhibited by CYP3A4. This indicates the possibility of unexpected drug interactions due to variable activation of CYP3A5 by sunitinib and sorafenib, which warrants further investigation in future drug interaction studies. Thus, CYP3A enzyme activity could serve as an important biomarker for predicting the efficacy of chemotherapy in gastric cancer patients. For instance[29], CYP3A5 gene polymorphisms may lead to huge disparities in drug metabolism rates and responsiveness among individuals. This is particularly critical for personalized treatment in gastric cancer patients.
Polymorphisms in the CYP3A gene family[30,31] have been proved to result in variations in drug metabolism rates and responsiveness among individuals. To detect potential genetic variations, researchers used denaturing gradient gel electrophoresis and conducted a comparative analysis of CYP3A4 cDNA in the colorectal adenocarcinoma cell line HT-29 and its oxaliplatin-resistant derivative HT-29-OxR. The analysis revealed polymorphisms in key members of the CYP3A family, especially in CYP3A4 and CYP3A5 genes, within these cancer cell lines. Further sequencing confirmed that there was genetic diversity in the CYP3A4 gene in such cell lines.
Researchers have recorded differences in gene expression, protein expression levels, and CYP3A4 enzyme activity in studies involving 12 different cancer cell lines[30]. Notably, huge disparities in drug responsiveness were observed in the original HT-29 cell line and the oxaliplatin-resistant HT-29-OxR cell line. These findings suggest that, variations in the CYP3A genes may have a substantial impact on the efficacy of anticancer drugs, and may alter the drug metabolism process. In view of the existence of such polymorphisms in the CYP3A gene family, it is important to take genetic va
Regulation of CYP3A gene family expression in gastric cancer is a complicated biological process which involves various factors and mechanisms. These can include genetic predispositions, environmental influences, lifestyle choices, the tumor microenvironment, inflammatory responses, oxidative stress, and epigenetic modifications. A detailed description of these regulatory mechanisms is shown below.
Genetic factors significantly affect expression of the CYP3A gene family. Members of the CYP3A family, such as CYP3A4 and CYP3A5, exhibit various genetic polymorphisms, which may result in notable differences in enzyme activity and expression levels, and consequently affect drug metabolism rates and plasma drug concentrations. A study on the pharmacokinetics of phenytoin and clinical outcomes in populations from the Middle East and North Africa highlighted the impact of CYP3A polymorphisms on drug metabolism[32]. For example, certain genetic variants of CYP3A5, such as “CYP3A53”, may lead to a complete lack of expression in some individuals. This polymorphism is the primary genetic determinant of CYP3A5 expression. Although CYP3A5 expression in the liver and intestines is generally lower than CYP3A4, under specific circumstances - such as when CYP3A4 activity is limited or when a drug is predominantly metabolized by CYP3A5 - the genetic variations in CYP3A5 can have a critical impact on drug metabolism.
In oncology, in view of their roles in drug metabolism and hormone regulation, genetic polymorphisms of CYP3A enzymes, particularly CYP3A4 and CYP3A5, are strongly linked to cancer risk and treatment responses. CYP3A41B polymorphism (rs2740574 A > G) significantly increases prostate cancer risk, especially among African populations, and G allele and GG genotype are associated with higher susceptibility[33]. Similarly, the CYP3A53 polymorphism (rs776746 A > G), which reduces CYP3A5 activity, is linked to colorectal cancer, chronic leukemia, and acute leukemia, particularly in Caucasian or Asian populations. However, it shows no clear association with prostate or liver cancer[34]. These polymorphisms also affect chemotherapy responses, and CYP3A53 carriers have reduced drug clearance and higher toxicity risks for agents such as paclitaxel; thus, it is essential to adjust the genetic-based dose[35].
According to studies, the CYP3A gene is detected in approximately 28% of gastric cancer patients[6]. Changes in gene copy number are viewed as major mechanisms of regulating oncogene and tumor suppressor gene expression. However, such changes do not always exhibit a linear correlation with gene expression levels. Increased CYP3A4 gene copy number in gastric cancer patients is significantly associated with elevated expression levels[36]. Moreover, the prevalence of the CYP3A41A + CYP3A53 haplotype is significantly higher in patients with intestinal-type gastric cancer in comparison with healthy controls (95.3% vs 82.8%)[37].
Additionally, CYP3A4 expression was found to be minimal in healthy gastric tissues but progressively increases in Helicobacter pylori-associated chronic gastritis, chronic atrophic gastritis (CAG), and gastric cancer tissues, and positive expression rates were 10%, 55%, and 77.3%, respectively[38]. Further Kaplan-Meier analyses have demonstrated that CYP3A4 overexpression is significantly associated with poorer overall survival and advanced illness progression in patients suffering from gastric cancer. Thus, we hypothesized that CYP3A4 may facilitate the transition from CAG to gastric cancer by regulating chemical carcinogenesis. CYP3A4 might serve as a molecular biomarker for forecasting CAG progression and unfavorable prognosis of gastric cancer.
CYP3A4 is the most abundant phase I metabolic enzyme in the intestines and liver, and metabolizes approximately 50% of drugs on the market. The active site of CYP3A4 is relatively small but also has a peripheral binding site, allowing it to interact with a broad range of drug substrates. Recent studies have revealed that CYP3A4 undergoes significant conformational changes when bound to inhibitors like ketoconazole or erythromycin, with the active site volume increasing by over 80%, providing structural insights into its wide substrate specificity[39].
The genetic polymorphisms, environmental factors, and transcriptional regulation of CYP3A4 play key roles in drug metabolism. Genetic variations can influence CYP3A4 activity and expression, and accordingly affect an individual’s ability to metabolize drugs. Additionally, CYP3A4 expression can be regulated by environmental factors, such as drugs, food, chemicals, and toxins. For example, rifampicin acts as an inducer, which can increase CYP3A4 expression by activating pregnane X receptor (PXR) and other transcription factors, and accelerate drug metabolism. Conversely, ketoconazole serves as an inhibitor, and reduces CYP3A4 activity and slows drug metabolism.
Transcription factors, such as PXR and constitutive androstane receptor (CAR), are crucial to CYP3A4 regulation. They can be activated by drugs and subsequently bind to the promoter region of CYP3A4 gene to regulate related transcription. PXR activation induces CYP3A4 expression, whereas CAR activation inhibits its expression. This precise regulation provides adaptability and flexibility in drug metabolism. It is necessary to study the interactions between new com
Recently, many studies have implied that, the gene expression profiles of cellular systems and their responses to external stimuli heavily depend upon the interaction between genomic higher-order chromatin structure and gene products. Researchers have explored the role of such factors in gene expression changes mediated by foreign substances in primary and transformed human liver cells, such as the HuH7 cell line. They found that cultured liver cells like HuH7 often exhibit gene expression profiles different to primary hepatocytes. When exposed to CYP3A transcription activators such as rifampicin, dexamethasone, pregnane-16α-carbonitrile, and phenobarbital, primary hepatocytes showed a dose-dependent increase in CYP3A transcription. However, similar changes were not observed in HuH7 cells. By altering the expression levels of PXR and upstream promoter transcription factor I in HuH7 cells, or by disrupting their higher-order chromatin structure, CYP3A expression and/or activation became similar to primary hepatocytes. Such results offer proof of the potential role of these mechanisms in the in vivo regulation of CYP3A expression[41].
The expression of CYP3A genes is greatly affected by various lifestyle factors, such as smoking, alcohol consumption, and dietary habits. Vences-Mejía et al[42] carried out a randomized controlled trial to explore the influence of high-sodium chloride diets on the activity and expression of major CYP subfamilies in normal gastric mucosa and changed gastric mucosa in rats. Their findings indicated that compared with controls, rats fed a high-concentration sodium chloride diet dramatically increased CYP3A expression in the gastric mucosa. High-salt diets enhanced CYP3A expression and the activity of related enzymes (such as erythromycin-N-demethylase, associated with CYP3A), which may promote the occurrence and progression of gastric cancer. Similarly, a high-fat diet can also affect CYP3A expression[43]. Bile acids and cholesterol derivatives in the diet activate PXR. It is crucial to regulate CYP3A gene expression. Once activated, PXR forms a heterodimer with retinoid X receptor binding to the promoter region of the CYP3A gene, and promotes gene transcription and increases CYP3A enzyme mRNA and protein expression levels. It also enhances CYP3A enzyme activity, may significantly affect drug metabolism, and possibly alter drug efficacy and toxicity due to species differences.
Furthermore, certain foods can also affect the activity and expression of CYP3A enzymes. Researchers have shown that grapefruit juice dramatically increases the bioavailability of oral felodipine[44]. This is attributed to the selective reduction of CYP3A4 expression in the intestinal wall by grapefruit juice, and thereby decreases the metabolic conversion of felodipine’s precursor. Grapefruit juice may affect CYP3A4 activity for up to 24 hours. Further research assessed the inhibitory effects of flavonoids, furanocoumarins, and involved compounds in grapefruit juice on CYP3A4 activity in human liver microsomes. It was found that bergapten (5-methoxypsoralen) had the lowest half-maximal inhibitory concentration value and served as the most potent CYP3A4 inhibitor. This may explain the primary mechanism of gra
Chronic alcohol consumption and smoking may induce CYP3A enzyme expression. Studies have shown that chronic alcohol drinkers exhibit a significant increase in CYP3A4 microsomal expression in the prefrontal cortex, whereas the expression of CYP3A5 remains unchanged[45]. Therefore, alcohol intake may selectively induce CYP3A4 expression, thus alter drug metabolism. Research by Liu et al[14] revealed that the mechanism of alcohol affecting CYP3A activity is quite complicated. Alcohol may activate receptors, such as PXR and CAR, which are crucial to regulating drug-metabolizing enzymes, thereby increase CYP3A expression. Alcohol also influences CYP3A transcription and expression via cyclic adenosine monophosphate and other intracellular signaling pathways. Additionally, oxidative stress during alcohol metabolism may affect CYP3A activity and expression. Alcohol consumption may alter gut microbiota, and influence CYP3A expression in the intestines. Moreover, alcohol and its metabolites may directly interact with CYP3A enzymes, and affect their activity. Nicotine also affects the CYP3A enzyme system dramatically. Studies have shown that nicotine binds to intracellular nicotinic acetylcholine receptors, activating downstream signaling pathways, such as the phosphoinositide 3-kinase/protein kinase B pathway and cyclic adenosine monophosphate response element-binding protein, thereby enhances CYP3A gene transcription[46]. Furthermore, CYP3A expression may be increased by activating nuclear receptors such as PXR and CAR with nicotine.
With regard to gastric cancer, many cytokines and growth factors within the tumor microenvironment are crucial to CYP3A gene expression regulation, such as tumor necrosis factor-α (TNF-α)[47] and interleukin-6 (IL-6)[48], These factors modulate the expression of CYP3A genes through the activation of specific signaling pathways, like NF-κB and STAT3. Multifunctional cytokine TNF-α is core to immune regulation and to inflammation promotion. In gastric cancer, elevated TNF-α levels may enhance CYP3A gene expression via activation of the NF-κB signaling pathway, thereby affect drug metabolism. Key transcription factor NF-κB translocates to the nucleus responding to TNF-α and other inflammatory signals, and initiates the transcription of various genes related to inflammation and cell survival.
IL-6, another cytokine closely associated with tumor progression and immune regulation, activates the STAT3 signaling pathway to affect cell behavior within the tumor microenvironment. STAT3, a transcription factor, promotes tumor cell proliferation, survival, and angiogenesis and inhibits apoptosis once activated. In gastric cancer, IL-6 may regulate CYP3A gene expression through STAT3 activation. It is intricately linked to tumor cell sensitivity and resistance to therapeutics. Additionally, cell-cell interactions within the tumor microenvironment also affect CYP3A gene expre
Research indicates that inflammation is an essential factor in the initiation and development of gastric cancer[49]. Within the tumor microenvironment of gastric cancer, macrophages and T cells and other inflammatory cells secrete a variety of inflammatory mediators, such as prostaglandin E2 and IL-1β. These mediators regulate the expression of CYP3A genes by activating inflammation-related signaling pathways, like NF-κB, and mitogen-activated protein kinase. The NF-κB pathway plays a critical role in gastric carcinogenesis, and can influence processes such as cell differentiation, proliferation, apoptosis, and immune responses. When inflammatory mediators, like TNF-α and IL-1β, activate the NF-κB pathway, the NF-κB transcription factor is triggered and translocates to the nucleus, and promotes the transcription of various genes, including those of the CYP3A family. Moreover, the mitogen-activated protein kinase signaling pathway is also involved in regulation of inflammatory responses and interacts with the NF-κB pathway, and collectively influences biological behavior of gastric cancer cells.
Elevated levels of inflammatory factors like TNF-α and IL-1β in the tumor microenvironment of gastric cancer may induce the expression of CYP3A enzymes, thereby affecting drug metabolism. For example, CYP3A4, a member of the CYP3A family, is crucial to the first-pass metabolism and bioavailability of drugs in the liver and intestines. In gastric cancer patients, activation of inflammatory factors may upregulate CYP3A4 expression, alter the metabolic rate of chemotherapeutic agents and thus affect their efficacy and toxicity. Additionally, inflammatory cytokines may modulate other cells within the tumor microenvironment, such as cancer-related fibroblasts and endothelial cells, and accordingly indirectly regulate CYP3A gene expression. These cell interactions form a complex network, which collectively affects tumor growth, invasion, and response to therapy.
Oxidative stress, characterized by increased levels of reactive oxygen species (ROS) within cells, is crucial in the initiation and progression of gastric cancer[50]. According to a study which investigated the hepatotoxic effects of atorvastatin in diabetic rats induced by a high-fat diet and streptozotocin, ROS can regulate CYP3A gene expression by modulating the activity of oxidatively modified transcription factors and signaling molecules, like P53 and nuclear factor erythroid 2-related factor 2 (Nrf2), and thereby influencing the transcriptional activity of CYP3A genes[51]. P53 is a tumor suppressor protein. It is central to the cellular stress response, such as in case of DNA damage, oxidative stress, and nutrient deprivation. Under oxidative stress conditions, P53 can be activated by ROS, and lead to the regulation of downstream gene expression. Nrf2 is a major antioxidant transcription factor. It promotes the expression of antioxidant genes by binding to antioxidant response elements. Activating Nrf2 may help mitigate oxidative stress-induced liver damage under conditions of diabetes and high-fat diets.
In the study, atorvastatin, a statin drug, was adopted to evaluate its influences on CYP3A gene expression in diabetic rats’ livers. Statins are primarily used to lower cholesterol levels. They may also impact the activity of drug-metabolizing enzymes. Such findings imply that atorvastatin may reduce ROS production or directly interact with transcription factors, thereby protecting the liver from oxidative stress-induced damage and maintaining normal CYP3A gene expression.
Epigenetic modifications, such as DNA methylation and histone modifications, are crucial in CYP3A gene expression regulation. In general, DNA methylation suppresses gene expression, whereas histone acetylation facilitates gene expression. In gastric cancer, abnormal DNA methylation in the promoter region of CYP3A genes may result in gene silencing. Studies have identified significant hypermethylation in the CLEM4 region within the CpG sites of the upstream regulatory region of the CYP3A4 gene[5]. Specific regions near the core promoter are highly associated with the incidence of gastric cancer. This means that, methylation modifications of CYP3A4 may suppress its expression in normal gastric tissues. However, during the progression of gastric cancer, alterations in methylation status may relieve this suppression, thereby leading to a significant upregulation of CYP3A4 expression. Additionally, increased activity of histone deace
The CYP3A gene family is crucial in gastric cancer, and thus might be a promising therapeutic target. We may influence gastric cancer cell proliferation, invasiveness, and responsiveness to chemotherapy by modulating CYP3A enzyme activity. Therefore, we could provide novel strategies for gastric cancer treatment by developing inhibitors or activators targeting CYP3A enzymes.
Inhibitors of CYP3A enzymes can reduce enzyme activity, and thereby decrease the metabolism of certain drugs in gastric cancer cells. This leads to higher drug concentrations in tumor tissues, and can enhance the efficacy of chemotherapy. For instance, ketoconazole, a known CYP3A inhibitor, significantly suppresses CYP3A-mediated drug metabolism, and potentially improves the therapeutic outcomes of certain chemotherapeutic agents[5].
Conversely, CYP3A enzyme activators can increase enzyme activity, accelerate drug metabolism and reduce side effects, but potentially exert toxic effects on tumor cells. Rifampin, a CYP3A inducer, activates the enzyme and may be applied in the treatment of gastric cancer to enhance drug clearance and minimize adverse reactions[52-54].
Gene therapy is an emerging therapeutic approach, which directly intervenes in gene expression to treat diseases. Targeted gene therapy for the CYP3A gene, such as utilizing small interfering RNA or CRISPR/CRISPR-associated protein 9 technology, could selectively silence or edit the CYP3A gene, and inhibit the growth and invasiveness of gastric cancer cells[1,55,56].
CYP3A enzymes are primarily known for their role in drug metabolism, but they may also greatly affect the development and metastasis of gastric cancer by modulating inflammatory responses and immune cell functions within the tumor microenvironment. Research suggests that CYP3A enzymes are involved in the metabolism of prostaglandins and thromboxanes, key eicosanoids derived from arachidonic acid. These are critical in inflammation and tumor progression. For instance, we have proved that prostaglandin E2, a product of arachidonic acid metabolism, can promote tumor cell proliferation, invasion and migration in various cancers[57]. Thus, CYP3A enzymes may result in tumor growth and immune modulation in gastric cancer by influencing the levels of these bioactive molecules. More research is required to elucidate the mechanisms through which CYP3A enzymes interact with the gastric cancer microenvironment and their potential as therapeutic targets.
CYP3A enzymes may regulate the proliferation and invasiveness of gastric cancer cells by modulating expression of cell cycle proteins along with apoptosis-related proteins. For instance, CYP3A enzymes may metabolize substances that promote cell cycle progression, and thereby drive the proliferation of gastric cancer cells[5,38].
The metabolism of various chemotherapeutic drugs is closely linked to CYP3A enzyme activity. Modulating CYP3A enzyme activity could affect drug efficacy and toxicity. We can optimize the dosage and treatment regimen of che
Polymorphisms in the CYP3A gene may change individual responses to CYP3A inhibitors or activators. Understanding such polymorphisms can help predict patient responses to specific treatments, and enable personalized therapy[58-61]. In conclusion, the CYP3A gene family in gastric cancer treatment may be applied in the development of inhibitors and activators, gene therapy strategies, and future research into CYP3A enzyme activity and gene polymorphisms. These strategies could provide new therapeutic avenues for gastric cancer, and ultimately improve patient outcomes.
We evaluated the crucial role of the CYP3A gene family in gastric cancer and further explored its possible use as a biomarker for early diagnosis and as a therapeutic target. We explored the clinical significance of this research in the following aspects:
Potential for early diagnosis: Non-invasive liquid biopsy technologies could detect abnormalities in CYP3A gene expression levels and potentially be developed in the future to enable early screening for gastric cancer[62].
Guidance for personalized chemotherapy: Research has proved that CYP3A gene polymorphisms significantly affect the metabolic rate and efficacy of chemotherapeutic drugs. Hence, genetic testing could be used to predict patients’ sen
Core value in precision medicine: CYP3A is a key gene family involved in drug metabolism; therefore, research into this gene family will form the foundation of precision medicine. This will aid in optimizing the dosage and combination of chemotherapeutic drugs, and help to minimize drug-related toxicities[52-54]. Consequently, this research bridges the gap between pharmacogenomics and clinical application, and further advances the principles of precision medicine. In conclusion, this study effectively integrates fundamental research with clinical needs, and offers theoretical support and practical guidance for precision therapy and diagnosis of gastric cancer as well as for future drug development.
The relationship between the CYP3A gene family and gastric cancer, particularly its role in drug metabolism, has become a focal point of current research. However, several limitations and points of contention remain, which warrant further exploration. First, there are huge disparities in experimental design and data selection across studies, especially concerning the analysis of samples from different ethnicities and individual variations. These differences may limit the reproducibility and applicability of research findings. For example, some studies focus on specific populations or single gastric cancer subtypes, which restricts the generalizability of their results. Gastric cancer has a higher incidence in regions such as Eastern Europe, South America, South Asia, Central Asia, and East Asia. According to age-standardized data, East Asia has the highest incidence rates, followed by Eastern Europe, South America, and Western Asia. Mongolia has the highest gastric cancer incidence globally, followed by Japan and South Korea. In fact, over half of the global gastric cancer cases occur in East Asia[61]. Much of the research on the CYP3A gene family is based on samples from these populations. However, the distribution of CYP3A gene polymorphisms varies significantly across different ethnicities, which may affect drug metabolism efficiency and therapeutic responses. Future studies should include more diverse ethnic and geographic samples to assess the global applicability of CYP3A gene family findings.
Second, current literature has not adequately explored the influence of genetic polymorphisms on personalized treatment in the context of the CYP3A gene family and drug metabolism. While some studies suggest that CYP3A5 polymorphisms may affect patients’ responses to chemotherapy, conclusions remain inconsistent across studies. For instance, some studies indicate that high expression of CYP3A5 is related to enhanced drug metabolism, whereas other studies show the opposite/no important relation. In a study on gene polymorphisms and drug metabolism in he
Additionally, CYP3A gene family interactions with other tumor-related genes have yet to be fully explored. Most existing literature focuses on the singular role of CYP3A in drug metabolism, overlooking its interaction with other signaling pathways. For instance, the CYP3A gene family may influence gastric cancer progression through its regulation of oxidative stress and inflammatory responses in the tumor microenvironment. These complex molecular mechanisms remain insufficiently elucidated. Future research should integrate multi-omics data to explore the synergistic effects between the CYP3A gene family and other key genes, providing a more comprehensive understanding of its role in gastric cancer.
To further elucidate the critical role of the CYP3A gene family in gastric cancer, we should prioritize the integration of global cancer genomics data, particularly through multi-omics methods (such as proteomics, transcriptomics, and genomics) in future research. Multi-omics analysis provides a broader perspective for researchers, and propels the discovery of complex interactions between the CYP3A gene family and other genes or signaling pathways. This comprehensive understanding will deepen insights into the functions of the CYP3A gene in tumor progression and drug me
Moreover, advancements in technologies of the CYP3A gene family, such as single-cell sequencing and liquid biopsy, increase its potential applications in early cancer diagnosis. Single-cell sequencing can reveal the specific expression patterns of the CYP3A gene in different cell populations, and help researchers better understand tumor cell heterogeneity and its relationship with drug responses. Liquid biopsy offers a non-invasive method to monitor CYP3A gene expression and mutations by detecting circulating tumor DNA and exosomes in the bloodstream. This is particularly valuable for early cancer diagnosis and monitoring therapeutic responses.
Future research should also explore how these advanced technologies can be combined with gene editing tools (e.g., CRISPR/CRISPR-associated protein 9) to optimize functional studies of the CYP3A gene and drug development. Gene editing technology allows precise regulation of CYP3A gene expression, facilitating the validation of its specific roles in tumors and providing a solid theoretical foundation for new targeted drug development. As big data and cutting-edge technologies continue to merge, research on the CYP3A gene will play an increasingly significant part in early cancer diagnosis, personalized treatment, and long-term prognosis improvement.
This study thoroughly explores the critical role of the CYP3A gene family in early progression of gastric cancer and evaluates its potential as a therapeutic target. The central functions of CYP3A enzymes in drug metabolism, hormone regulation, and cholesterol synthesis, as well as their regulatory mechanisms within the tumor microenvironment, provide novel molecular markers and therapeutic strategies for the early diagnosis and treatment of gastric cancer.
With regard to drug metabolism, variations in CYP3A enzyme activity directly affect chemotherapeutic drug responsiveness in patients suffering from gastric cancer. This is a finding of considerable significance for personalized medicine. We observed a close association between CYP3A enzyme activity and the proliferation and invasiveness of gastric cancer cells, and have successfully provided new ideas. Regulatory mechanisms for governing CYP3A enzyme expression are also examined. The influence of genetic polymorphisms, environmental factors, and lifestyle on their expression is also discussed. These factors may collectively create a favorable microenvironment for gastric cancer progression. Additionally, our research identifies the significant roles of inflammatory responses and oxidative stress in regulating CYP3A gene expression, and offers new avenues for gastric cancer prevention and treatment.
We summed the multifaceted roles of the CYP3A gene family in gastric cancer, and analyzed its potential as a therapeutic target. We elucidated the relationship between CYP3A enzyme activity and gastric cancer progression, and provided theoretical support for the development of innovative therapeutic strategies targeting CYP3A enzymes. Furthermore, we underscored the importance of CYP3A gene polymorphisms in predicting treatment responses in gastric cancer patients, and suggested its potential as a novel molecular marker for personalized medicine.
Regarding therapeutic strategies, we advocated the development of CYP3A enzyme inhibitors and activators, as well as the potential application of gene therapy technologies. These strategies may be beneficial in the treatment of gastric cancer. It may be possible to optimize the efficacy of chemotherapeutic agents and reduce adverse reactions by mo
Overall, we conducted a comprehensive analysis of the role of the CYP3A gene family in gastric cancer, and provided new molecular markers and therapeutic pathways for its early diagnosis and treatment. Such findings enrich knowledge on the mechanisms underlying gastric cancer progression, but also provide a solid scientific basis for the advancement of future research and clinical practice in gastric cancer treatment.
| 1. | Jia Q, Ding Q, Shao K, Dang J, Zhang F. Research progress regarding CYP3A gene family in gastric cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023;48:1874-1881. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | McKinnon RA, Burgess WM, Hall PM, Roberts-Thomson SJ, Gonzalez FJ, McManus ME. Characterisation of CYP3A gene subfamily expression in human gastrointestinal tissues. Gut. 1995;36:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Klyushova LS, Perepechaeva ML, Grishanova AY. The Role of CYP3A in Health and Disease. Biomedicines. 2022;10:2686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Kruijtzer CM, Boot H, Beijnen JH, Lochs HL, Parnis FX, Planting AS, Pelgrims JM, Williams R, Mathôt RA, Rosing H, Schot ME, Van Tinteren H, Schellens JH. Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann Oncol. 2003;14:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Golestanian R, Barzegar A, Mianji GR, Ebrahimzadeh MA, Fatemi B. Evaluation of Alterations in DNA Methylation of CYP3A4 Gene Upstream Regulatory Elements in Gastric Cancer and in Response to Diazinon Treatment. Curr Drug Metab. 2022;23:242-250. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Murray GI, Taylor MC, Burke MD, Melvin WT. Enhanced expression of cytochrome P450 in stomach cancer. Br J Cancer. 1998;77:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Liu X, Zhang Y, Chen Q, Zhan Y, Wang Q, Hu C, Yu C, Guo Z, Chen X, Zhong D. Pharmacokinetic Drug Interactions of Apatinib With Rifampin and Itraconazole. J Clin Pharmacol. 2018;58:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8851] [Cited by in RCA: 8249] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 9. | Gonzalez FJ, Nebert DW. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990;6:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 309] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Conney AH. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967;19:317-366. [PubMed] |
| 11. | Omura T. Recollection of the early years of the research on cytochrome P450. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:617-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2251] [Cited by in RCA: 2759] [Article Influence: 229.9] [Reference Citation Analysis (0)] |
| 13. | Caron KM, Clark BJ, Ikeda Y, Parker KL. Steroidogenic factor 1 acts at all levels of the reproductive axis. Steroids. 1997;62:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Liu H, Yu Y, Guo N, Wang X, Han B, Xiang X. Application of Physiologically Based Pharmacokinetic Modeling to Evaluate the Drug-Drug and Drug-Disease Interactions of Apatinib. Front Pharmacol. 2021;12:780937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Niwa T, Narita K, Okamoto A, Murayama N, Yamazaki H. Comparison of Steroid Hormone Hydroxylations by and Docking to Human Cytochromes P450 3A4 and 3A5. J Pharm Pharm Sci. 2019;22:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kang S, Park M, Cho JY, Ahn SJ, Yoon C, Kim SG, Cho SJ. Tumorigenic mechanisms of estrogen and Helicobacter pylori cytotoxin-associated gene A in estrogen receptor α-positive diffuse-type gastric adenocarcinoma. Gastric Cancer. 2022;25:678-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lütjohann D, Stellaard F, Kerksiek A, Lötsch J, Oertel BG. Serum 4β-hydroxycholesterol increases during fluconazole treatment. Eur J Clin Pharmacol. 2021;77:659-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Rebelo A, Kleeff J, Sunami Y. Cholesterol Metabolism in Pancreatic Cancer. Cancers (Basel). 2023;15:5177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Chen WC, Boursi B, Mamtani R, Yang YX. Total Serum Cholesterol and Pancreatic Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2019;28:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Zheng S, Lin J, Pang Z, Zhang H, Wang Y, Ma L, Zhang H, Zhang X, Chen M, Zhang X, Zhao C, Qi J, Cao L, Wang M, He X, Sheng R. Aberrant Cholesterol Metabolism and Wnt/β-Catenin Signaling Coalesce via Frizzled5 in Supporting Cancer Growth. Adv Sci (Weinh). 2022;9:e2200750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Valencia-Cervantes J, Huerta-Yepez S, Aquino-Jarquín G, Rodríguez-Enríquez S, Martínez-Fong D, Arias-Montaño JA, Dávila-Borja VM. Hypoxia increases chemoresistance in human medulloblastoma DAOY cells via hypoxiainducible factor 1αmediated downregulation of the CYP2B6, CYP3A4 and CYP3A5 enzymes and inhibition of cell proliferation. Oncol Rep. 2019;41:178-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Trujillo-Paolillo A, Tesser-Gamba F, Petrilli AS, de Seixas Alves MT, Garcia Filho RJ, de Oliveira R, de Toledo SRC. CYP genes in osteosarcoma: Their role in tumorigenesis, pulmonary metastatic microenvironment and treatment response. Oncotarget. 2017;8:38530-38540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Hu B, Zhang X, Zhu S, Wang C, Deng Z, Wang T, Wu Y. Identification and validation of an individualized metabolic prognostic signature for predicting the biochemical recurrence of prostate cancer based on the immune microenvironment. Eur J Med Res. 2024;29:92. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Wang F, Zhang X, Wang Y, Chen Y, Lu H, Meng X, Ye X, Chen W. Activation/Inactivation of Anticancer Drugs by CYP3A4: Influencing Factors for Personalized Cancer Therapy. Drug Metab Dispos. 2023;51:543-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Ghiaur G, Valkenburg KC, Esteb C, Ambinder A, Imus PH, Pienta KJ, Jones RJ. Bone marrow niche chemoprotection of metastatic solid tumors mediated by CYP3A4. Cancer. 2023;129:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Chen X, Li H. Bruceine D and Narclasine inhibit the proliferation of breast cancer cells and the prediction of potential drug targets. PLoS One. 2024;19:e0297203. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Sugiyama M, Fujita K, Murayama N, Akiyama Y, Yamazaki H, Sasaki Y. Sorafenib and sunitinib, two anticancer drugs, inhibit CYP3A4-mediated and activate CY3A5-mediated midazolam 1'-hydroxylation. Drug Metab Dispos. 2011;39:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kim A, Balis FM, Widemann BC. Sorafenib and sunitinib. Oncologist. 2009;14:800-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Lolodi O, Wang YM, Wright WC, Chen T. Differential Regulation of CYP3A4 and CYP3A5 and its Implication in Drug Discovery. Curr Drug Metab. 2017;18:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Šemeláková M, Maliničová L, Macejová M, Pristaš P. CYP3A gene variability and cancer cells response to the treatment. Gen Physiol Biophys. 2021;40:49-59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Li Q, Wang J, Wang ZL, Shen Y, Zhou Q, Liu YN, Hu GX, Cai JP, Xu RA. The impacts of CYP3A4 genetic polymorphism and drug interactions on the metabolism of lurasidone. Biomed Pharmacother. 2023;168:115833. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Dagenais R, Wilby KJ, Elewa H, Ensom MHH. Impact of Genetic Polymorphisms on Phenytoin Pharmacokinetics and Clinical Outcomes in the Middle East and North Africa Region. Drugs R D. 2017;17:341-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Zhou LP, Yao F, Luan H, Wang YL, Dong XH, Zhou WW, Wang QH. CYP3A4*1B polymorphism and cancer risk: a HuGE review and meta-analysis. Tumour Biol. 2013;34:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Wang BS, Liu Z, Xu WX, Sun SL. CYP3A5*3 polymorphism and cancer risk: a meta-analysis and meta-regression. Tumour Biol. 2013;34:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Wang Z, Wang Y, Jin W, Zhang Z, Jin L, Qian J, Zheng L. CYP3A4 and CYP3A5: the crucial roles in clinical drug metabolism and the significant implications of genetic polymorphisms. PeerJ. 2024;12:e18636. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Junnila S, Kokkola A, Karjalainen-Lindsberg ML, Puolakkainen P, Monni O. Genome-wide gene copy number and expression analysis of primary gastric tumors and gastric cancer cell lines. BMC Cancer. 2010;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Gervasini G, García-Martín E, Ladero JM, Pizarro R, Sastre J, Martínez C, García M, Diaz-Rubio M, Agúndez JA. Genetic variability in CYP3A4 and CYP3A5 in primary liver, gastric and colorectal cancer patients. BMC Cancer. 2007;7:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Zhang F, Wang F, Chen C, Wang T, Hu J, Su R, Li X, Gu B, Tang S, Chen H, Li Y. Prediction of progression of chronic atrophic gastritis with Helicobacter pylori and poor prognosis of gastric cancer by CYP3A4. J Gastroenterol Hepatol. 2020;35:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 486] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 40. | Fanni D, Ambu R, Gerosa C, Nemolato S, Castagnola M, Van Eyken P, Faa G, Fanos V. Cytochrome P450 genetic polymorphism in neonatal drug metabolism: role and practical consequences towards a new drug culture in neonatology. Int J Immunopathol Pharmacol. 2014;27:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Phillips A, Hood SR, Gibson GG, Plant NJ. Impact of transcription factor profile and chromatin conformation on human hepatocyte CYP3A gene expression. Drug Metab Dispos. 2005;33:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Vences-Mejía A, Caballero-Ortega H, Dorado-González V, Gamboa-Domínguez A, Gómez-Ruiz C, Camacho-Carranza R, Espinosa-Aguirre JJ. Cytochrome P450 expression in rat gastric epithelium with intestinal metaplasia induced by high dietary NaCl levels. Environ Toxicol Pharmacol. 2005;20:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Spruiell K, Jones DZ, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA. Role of human pregnane X receptor in high fat diet-induced obesity in pre-menopausal female mice. Biochem Pharmacol. 2014;89:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 445] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 45. | Booth Depaz IM, Toselli F, Wilce PA, Gillam EM. Differential expression of human cytochrome P450 enzymes from the CYP3A subfamily in the brains of alcoholic subjects and drug-free controls. Drug Metab Dispos. 2013;41:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Anderson GD, Chan LN. Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clin Pharmacokinet. 2016;55:1353-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Darakjian L, Deodhar M, Turgeon J, Michaud V. Chronic Inflammatory Status Observed in Patients with Type 2 Diabetes Induces Modulation of Cytochrome P450 Expression and Activity. Int J Mol Sci. 2021;22:4967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Liu C, Chen J, Liu B, Yuan S, Shou D, Wen L, Wu X, Gong W. Role of IL-18 in transplant biology. Eur Cytokine Netw. 2018;29:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Wu Y, Zhao J, Wang Z, Liu D, Tian C, Ye B, Sun Y, Li H, Wang X. Association of systemic inflammatory markers and tertiary lymphoid structure with pathological complete response in gastric cancer patients receiving preoperative treatment: a retrospective cohort study. Int J Surg. 2023;109:4151-4161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 50. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 837] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 51. | Shu N, Hu M, Ling Z, Liu P, Wang F, Xu P, Zhong Z, Sun B, Zhang M, Li F, Xie Q, Liu X, Liu L. The enhanced atorvastatin hepatotoxicity in diabetic rats was partly attributed to the upregulated hepatic CYP3A and SLCO1B1. Sci Rep. 2016;6:33072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Yamashita F, Sasa Y, Yoshida S, Hisaka A, Asai Y, Kitano H, Hashida M, Suzuki H. Modeling of rifampicin-induced CYP3A4 activation dynamics for the prediction of clinical drug-drug interactions from in vitro data. PLoS One. 2013;8:e70330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Molenaar-Kuijsten L, Van Balen DEM, Beijnen JH, Steeghs N, Huitema ADR. A Review of CYP3A Drug-Drug Interaction Studies: Practical Guidelines for Patients Using Targeted Oral Anticancer Drugs. Front Pharmacol. 2021;12:670862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Chakraborty A, Panda AK, Ghosh R, Roy I, Biswas A. Depicting the DNA binding and photo-nuclease ability of anti-mycobacterial drug rifampicin: A biophysical and molecular docking perspective. Int J Biol Macromol. 2019;127:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Salman DM, Mohammad TAM. siRNA-based therapy for gastric adenocarcinoma: what's next step? Pathol Res Pract. 2024;258:155328. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Zhang H, McCarty N. CRISPR-Cas9 technology and its application in haematological disorders. Br J Haematol. 2016;175:208-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Johnson AM, Kleczko EK, Nemenoff RA. Eicosanoids in Cancer: New Roles in Immunoregulation. Front Pharmacol. 2020;11:595498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 58. | Kasarla SS, Garikapati V, Kumar Y, Dodoala S. Interplay of Vitamin D and CYP3A4 Polymorphisms in Endocrine Disorders and Cancer. Endocrinol Metab (Seoul). 2022;37:392-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 59. | Zhou Q, Yu X, Shu C, Cai Y, Gong W, Wang X, Wang DM, Hu S. Analysis of CYP3A4 genetic polymorphisms in Han Chinese. J Hum Genet. 2011;56:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Ruzilawati AB, Gan SH. CYP3A4 genetic polymorphism influences repaglinide's pharmacokinetics. Pharmacology. 2010;85:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Lloberas N, Elens L, Llaudó I, Padullés A, van Gelder T, Hesselink DA, Colom H, Andreu F, Torras J, Bestard O, Cruzado JM, Gil-Vernet S, van Schaik R, Grinyó JM. The combination of CYP3A4*22 and CYP3A5*3 single-nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet Genomics. 2017;27:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Ren Y, Ma Q, Li F, Zeng X, Tan S, Fu X, Zheng C, You F, Li X. [Analysis of Salivary Microbiota Characteristics in Patients With Pulmonary Nodules: A Prospective Nonrandomized Concurrent Controlled Trial]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023;54:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 63. | Jiang J, Luan J. Effect of CYP3A5 Gene Polymorphisms on Tacrolimus Blood Concentrations and Adverse Events in Allogeneic Hematopoietic Stem Cell Transplant Patients. Transplant Proc. 2024;56:1678-1682. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Zong H, Zhang Y, Liu F, Zhang X, Yang Y, Cao X, Li Y, Li A, Zhou P, Gao R, Li Y. Interaction between tacrolimus and calcium channel blockers based on CYP3A5 genotype in Chinese renal transplant recipients. Front Pharmacol. 2024;15:1458838. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |