Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.102301
Revised: November 19, 2024
Accepted: December 9, 2024
Published online: March 24, 2025
Processing time: 99 Days and 2.3 Hours
Despite advancements in early detection and treatment, the prognosis and his
This case report presents a rare occurrence of residual GC featuring a combination of small cell neuroendocrine carcinoma (SCNEC) and squamous cell carcinoma (SCC) in a 60-year-old male patient. The patient, with a history of Billroth II gas
In this case report, we detail a rare instance of residual GC with mixed SCNEC and SCC, emphasizing the complexity of diagnosis and treatment, and the need for ongoing research.
Core Tip: This case report presents a unique instance of residual gastric cancer with a combination of small cell neuroendocrine carcinoma and squamous cell carcinoma, highlighting the emergence of such rare histological types post-gastrectomy underscores the need for vigilant surveillance and further research into their management. The complexity of diagnosing and treating this mixed histology gastric cancer is further emphasized by the current trends in gastric cancer management. The pursuit of integrated, resource-sensitive approaches to surveillance and treatment is crucial for enhancing outcomes in this patient population.
- Citation: Wang T, Cheng Y, Hu F, Wang Q. Residual gastric cancer with a mixed small cell neuroendocrine and keratinizing squamous cell carcinoma: A case report. World J Clin Oncol 2025; 16(3): 102301
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/102301.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.102301
Gastric cancer (GC) ranks among the most prevalent malignant neoplasms worldwide. Previous statistical data indicated that GC accounted for 5.6% of new cancer cases and 7.7% of cancer-related deaths[1]. The disease continues to be a significant oncological challenge worldwide. A concerning trend is the increasing incidence of GC among younger adults that has been observed over the past decade[2]. In terms of GC treatment, early-stage GC is usually resected via en
Melena for three days.
On November 19, 2021, a 60 years old male patient was admitted to the hospital due to melena that had lasted for three days.
The patient had a history of Billroth II gastrectomy for bleeding from a duodenal ulcer approximately 40 years prior and had not undergone follow-up gastroscopy post-surgery. Additionally, the patient had as a history of appendectomy and hepatitis B virus infection.
The patient had a 40-year history of smoking and alcohol consumption.
Only surgical scars on the abdominal skin were found during the admission examination, with no other positive findings.
Laboratory tests revealed a haemoglobin level of 100 g/L, with no abnormal findings for the other blood counts. Coagulation function, liver and kidney function, electrolytes, and lipid profiles were normal. A stool occult blood test was positive. The levels of tumor markers were as follows: 2.2 ng/mL alpha-fetoprotein, 1.24 ng/mL carcinoembryonic antigen, 7.53 U/mL cancer antigen 199, 1.530 ng/mL prostate-specific antigen, and 0.139 ng/mL free prostate-specific antigen; all of these test results were within the normal range. The hepatitis virus panel was positive for hepatitis B surface antigen, negative for hepatitis B e antigen, positive for hepatitis B e antibody, positive for hepatitis B core antibody, negative for hepatitis B pre-S1 antigen, and negative for other hepatitis viruses.
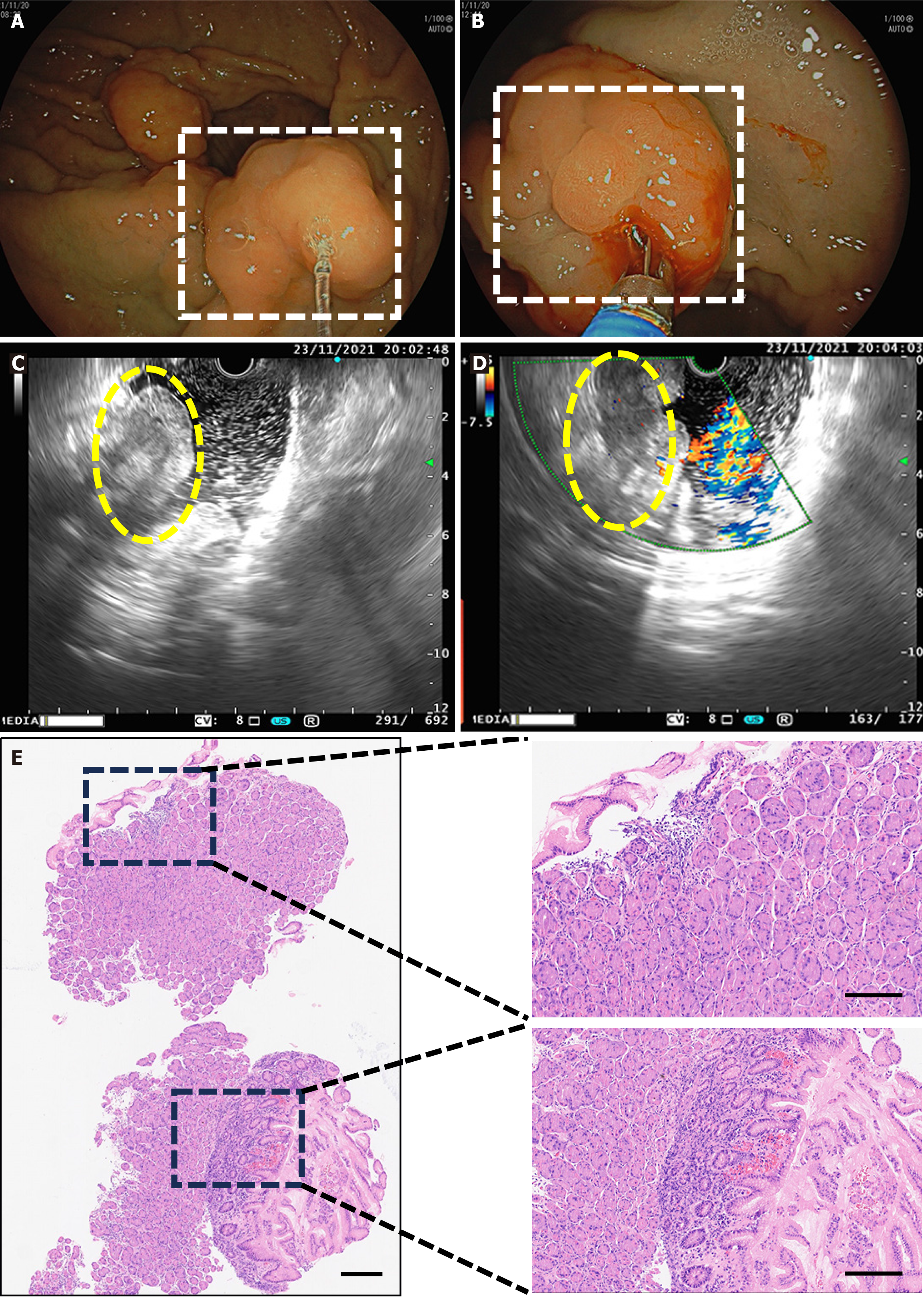
Electronic gastroduodenoscopy examination: Postgastrectomy: (Billroth II style) with residual gastritis and bile reflux; anastomosis inflammation with a raised mucosal lesion at the anastomosis (nature to be determined); and gastric xanthoma (Figure 1A and B).
Endoscopic ultrasound: Endoscopic ultrasound revealed a raised mucosal lesion at the anastomosis site (suspected anastomotic cancer) and postgastrectomy (Billroth II style) with residual gastritis, bile reflux, and anastomotic inflammation (Figure 1C and D).
Histopathological examination (biopsy from the anastomosis): Examination revealed moderate chronic nonatrophic gastritis with acute activity, Helicobacter pylori (+), neutrophils (+), lymphocytes (++), atrophy (-), and intestinal me
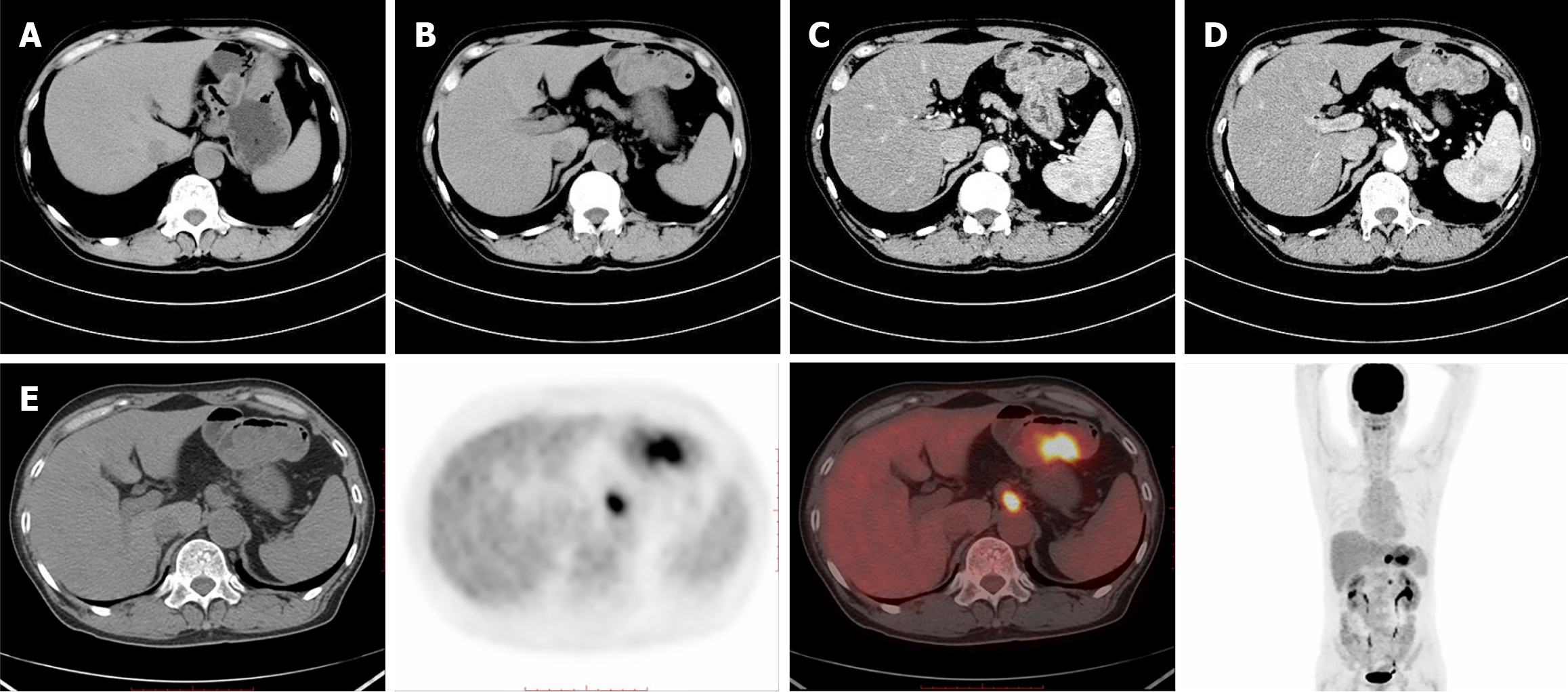
Abdominal computed tomography scan with contrast: Scan revealed changes after gastrectomy, with significant thickening and elevation of the residual gastric wall and enlarged lymph nodes in the hepatogastric space. This suggests a high possibility of postoperative recurrence and lymph node metastasis in the hepatogastric space (Figure 2A-D).
Positron emission tomography-computed tomography: Results revealed changes after gastrectomy, with nodular thickening of the residual gastric wall and increased metabolism, suggesting residual GC. Enlarged lymph nodes beside the abdominal aorta (at the T12 level) and in the middle abdomen (at the L2 level) with increased metabolism suggested metastasis (Figure 2E).
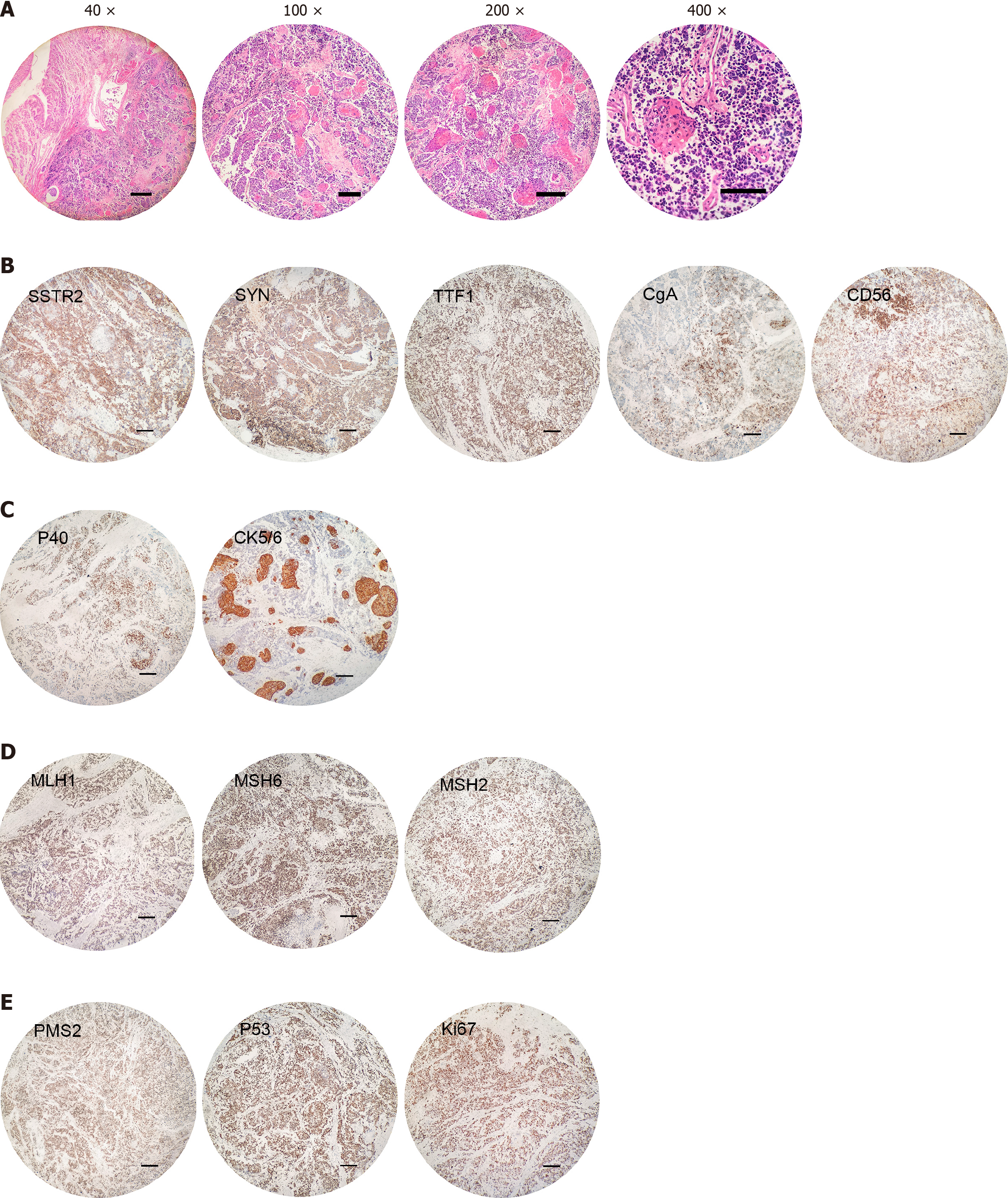
In March 2022, the patient experienced a severe episode of gastrointestinal bleeding, which prompted an urgent referral to another hospital for surgical intervention. Upon arrival, a repeat gastroscopy was conducted at the secondary facility, and subsequent pathological examination revealed the presence of residual gastric, small cell carcinoma and SCC. To address the life-threatening haemorrhage, the patient underwent an emergent surgical procedure that included resection of the remaining stomach, the creation of an oesophagojejunal anastomosis to restore gastrointestinal continuity, and the release of abdominal adhesions to facilitate surgery. During the operation, the source of the massive gastrointestinal bleeding was identified and traced back to the anastomotic site, likely due to a rupture of vessels within the cancerous lesion. Histological typing suggested a mixed neuroendocrine tumor [70% small cell NEC (SCNEC) and 30% keratinizing SCC (KSCC)]; the depth of invasion reached the subserosa; there were vascular tumor emboli and nerve invasion; the resection margins were not involved; and one of the thirteen peri-gastric lymph nodes showed cancer metastasis (1/13), with the cancer components being SCNEC - KSCC (T4aN2M1, according to the Eighth Edition American Joint Committee on Cancer staging)[10]. Two of the two pericolic lymph nodes showed cancer metastasis (2/2), with the cancer com
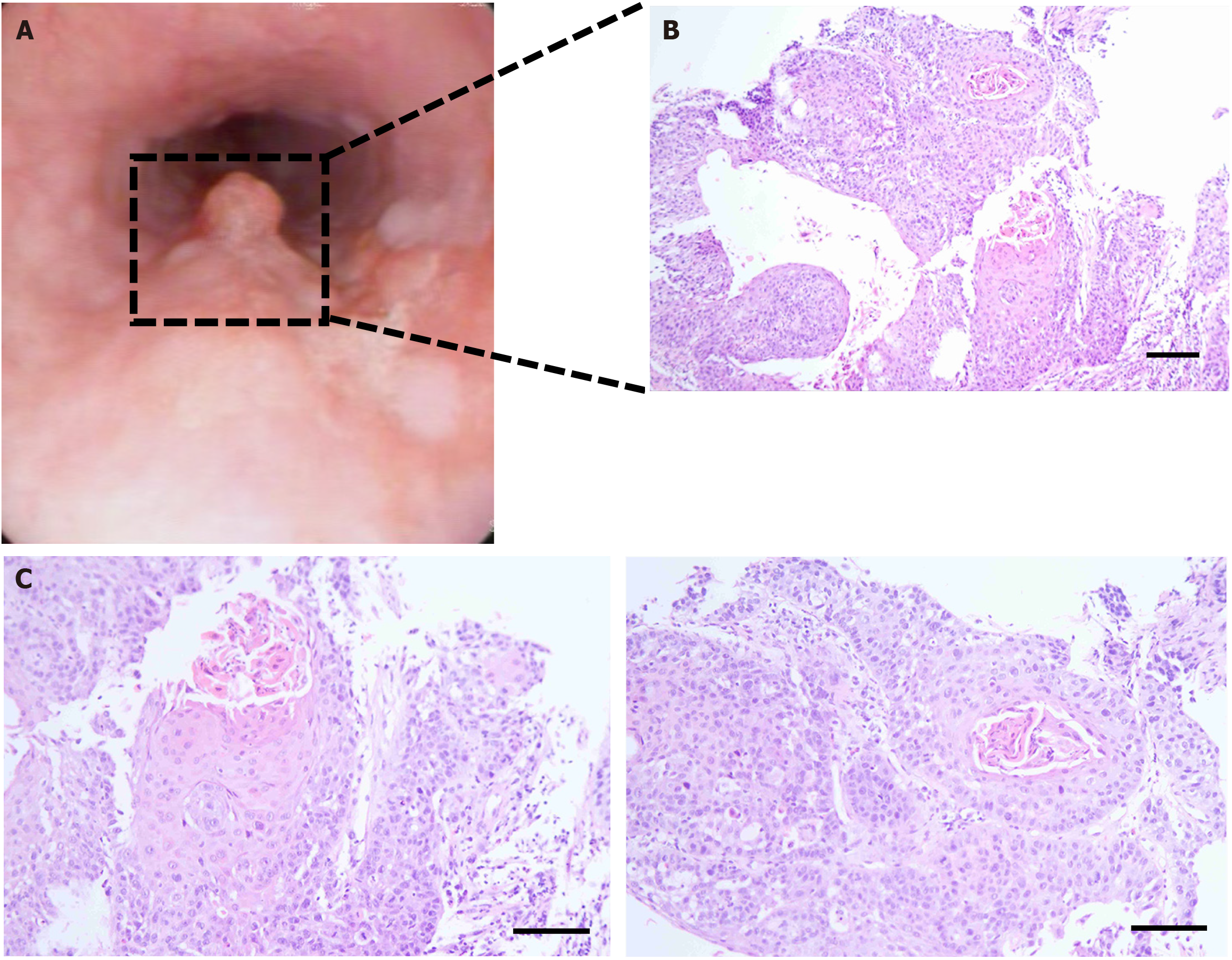
Following the surgical procedure, the patient received chemotherapy on four occasions: April 25, 2022, May 20, 2022, June 13, 2022, and July 8, 2022. The chemotherapeutic drugs that were used were 100 mg of etoposide and 30 mg of cisplatin. The patient was in good condition and underwent a follow-up examination in May 2024. An endoscopic examination at an external hospital revealed a protruding lesion in the oesophagus, of which a biopsy was collected. Pathology revealed oesophageal cancer (Figure 4).
The patient was subsequently transferred to another thoracic hospital for a small bowel stoma and radical oeso
This case report presents a unique case of a 60-year-old male who developed mixed SCNEC and KSCC in the gastric remnant after distal gastrectomy for duodenal ulcer bleeding. GSC is a rare and aggressive malignancy that can arise in the gastric remnant after gastrectomy for benign or malignant conditions. Surgical outcomes for patients with GSC vary significantly across studies, with reported 5-year survival rates ranging widely from 10%-70%[11]. The uniqueness of this case lies in the development of a mixed SCNEC and KSCC in the gastric remnant of a patient with a history of distal gastrectomy.
Peptic ulcer disease, once predominantly a surgical issue, especially when there is no endoscopic technology, is now predominantly managed medically for most patients[5]. Surgical intervention for ulcer disease is now strictly limited to life-threatening complications, such as free perforation, refractory bleeding, and gastric outlet obstruction, that cannot be controlled with medical therapy[12]. The development of GSC is often attributed to the persistence of risk factors such as Helicobacter pylori infection, chronic inflammation, and genetic predisposition. In this case, the patient’s history of smoking and alcohol consumption may have contributed to the multifaceted carcinogenesis process. According to the World Health Organization’s classification criteria for digestive system tumors[13], a tumor is considered truly mixed when both constituent elements are prominent, with a minimum of 30% of each element discernible within the tumor. Therefore, the tumor could be classified as having a mixed histology of SCNEC and KSCC in this case.
Primary SCC of the stomach is an exceedingly rare malignant neoplasm, constituting approximately 0.04% to 0.07% of GC cases[14]. According to the literature, NEC may arise from a GC precursor at the anastomotic site in the gastric remnant. For genomic alterations, a previous study provided additional support that NECs often have genomic alterations in
The diagnosis of GSC is challenging due to nonspecific symptoms and the limitations of endoscopic biopsies in capturing the full extent of the tumor. In this case, the initial endoscopic biopsy did not yield positive findings. However, the use of endoscopic ultrasound, computed tomography and positron emission tomography-computed tomography was instrumental in identifying the tumor and its metastatic potential[19]. The histological confirmation of SCNEC and KSCC was crucial for determining the therapeutic approach, as this histotype has distinct treatment implications and prognostic significance[19]. The treatment of GSC with mixed histology requires a multimodal approach, often involving surgery, chemotherapy, and radiation therapy[14]. In this case, the patient underwent total gastrectomy and lymph node di
The prognosis for GSCs with mixed SCNEC and KSCC is generally poor because of the advanced stage at presentation and the aggressive biological behavior of these tumors. Despite aggressive treatment, the risk of recurrence and me
In conclusion, this case highlights the complexity of GSC with mixed histology and the importance of a comprehensive diagnostic and therapeutic approach. These findings highlight the need for further research into the molecular mechanisms underlying the development of GSC, particularly in the context of mixed histology.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64645] [Article Influence: 16161.3] [Reference Citation Analysis (176)] |
| 2. | Tan N, Wu H, Cao M, Yang F, Yan X, He S, Cao M, Zhang S, Teng Y, Li Q, Wang J, Xia C, Chen W. Global, regional, and national burden of early-onset gastric cancer. Cancer Biol Med. 2024;21:667-678. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Jeong SH, Lee JK, Seo KW, Min JS. Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer. J Clin Med. 2023;12:3880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Umeki Y, Shibasaki S, Suzuki K, Serizawa A, Akimoto S, Nakauchi M, Tanaka T, Inaba K, Uyama I, Suda K. Laparoscopic gastrectomy for remnant gastric cancer: A single-center retrospective study. Surg Oncol. 2023;51:101988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Khalayleh H, Kim YW, Yoon HM, Ryu KW. Assessment of Lymph Node Metastasis in Patients With Gastric Cancer to Identify Those Suitable for Middle Segmental Gastrectomy. JAMA Netw Open. 2021;4:e211840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 6. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 7. | Ruck P, Wehrmann M, Campbell M, Horny HP, Breucha G, Kaiserling E. Squamous cell carcinoma of the gastric stump. A case report and review of the literature. Am J Surg Pathol. 1989;13:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ma FH, Xue LY, Chen YT, Xie YB, Zhong YX, Xu Q, Tian YT. Neuroendocrine carcinoma of the gastric stump: A case report and literature review. World J Gastroenterol. 2018;24:543-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Cazzo E, de Saito HP. Mixed adenoneuroendocrine carcinoma of the gastric stump following Billroth II gastrectomy: case report and review of the literature. Sao Paulo Med J. 2016;134:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4402] [Article Influence: 550.3] [Reference Citation Analysis (4)] |
| 11. | Shimada H, Fukagawa T, Haga Y, Oba K. Does remnant gastric cancer really differ from primary gastric cancer? A systematic review of the literature by the Task Force of Japanese Gastric Cancer Association. Gastric Cancer. 2016;19:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Toka B, Eminler AT, Karacaer C, Uslan MI, Koksal AS, Parlak E. Comparison of monopolar hemostatic forceps with soft coagulation versus hemoclip for peptic ulcer bleeding: a randomized trial (with video). Gastrointest Endosc. 2019;89:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 14. | Wakabayashi H, Matsutani T, Fujita I, Kanazawa Y, Nomura T, Hagiwara N, Hosone M, Katayama H, Uchida E. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Farooq F, Zarrabi K, Sweeney K, Kim J, Bandovic J, Patel C, Choi M. Multiregion Comprehensive Genomic Profiling of a Gastric Mixed Neuroendocrine-Nonneuroendocrine Neoplasm with Trilineage Differentiation. J Gastric Cancer. 2018;18:200-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | de Back TR, van Hooff SR, Sommeijer DW, Vermeulen L. Transcriptomic subtyping of gastrointestinal malignancies. Trends Cancer. 2024;10:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 595] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 18. | Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Okuda K, Ishihara S, Fujita Y, Yamamoto N, Kishimoto M, Konishi E, Kato Y, Yanagisawa A. Simple pathological examination technique for detection of cancer located at the surgical margin of the stomach. Gastric Cancer. 2014;17:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Haag GM, Czink E, Ahadova A, Schmidt T, Sisic L, Blank S, Heger U, Apostolidis L, Berger AK, Springfeld C, Lasitschka F, Jäger D, von Knebel Doeberitz M, Kloor M. Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer. 2019;144:1697-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1166] [Article Influence: 166.6] [Reference Citation Analysis (0)] |