Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101274
Revised: November 14, 2024
Accepted: December 9, 2024
Published online: March 24, 2025
Processing time: 133 Days and 19.7 Hours
Hepatic arterial infusion (HAI) chemotherapy, first introduced in the 1980s, has gained recognition as an effective locoregional treatment for colorectal liver metastasis (CRLM). Initially used for unresectable liver metastases, HAI’s app
Core Tip: This letter to the editor highlights the evolving role of hepatic arterial infusion (HAI) chemotherapy in the treat
- Citation: Messaoudi N, Vanlander A, Benhadda M, Makarian R, Kortbeek K, De Haar-Holleman A, Gumbs AA. Hepatic arterial infusion pump chemotherapy for colorectal liver metastases: Revisiting traditional techniques to explore new frontiers. World J Clin Oncol 2025; 16(3): 101274
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101274.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101274
Colorectal cancer (CRC) continues to represent a significant global health burden, with around 25% of patients presenting with metastases at the time of initial diagnosis, and over half eventually developing metastases to the liver throughout their disease progression[1,2]. Metastatic CRC confined to the liver is associated with a poor prognosis, with five-year overall survival (OS) rates hovering around 20%[3]. However, in carefully selected patients undergoing complete re
For patients with initially unresectable CRLM, regional therapies have gained increasing interest due to the limited efficacy of systemic chemotherapy, which offers a median survival of around 20 months[10]. Standard first-line systemic regimens include a combination of fluoropyrimidines with oxaliplatin or irinotecan, and targeted agents such as anti-epidermal growth factor receptor or anti-vascular endothelial growth factor therapies[1,3]. While these treatments yield response rates in two thirds of patients in first-line settings, effectiveness declines in second-line therapies, with response rates dropping to 40%[11]. Furthermore, progression-free survival (PFS) decreases from 13 months in first-line treatment to a maximum of 7 months with second-line agents[12]. In light of these challenges, hepatic arterial infusion (HAI) che
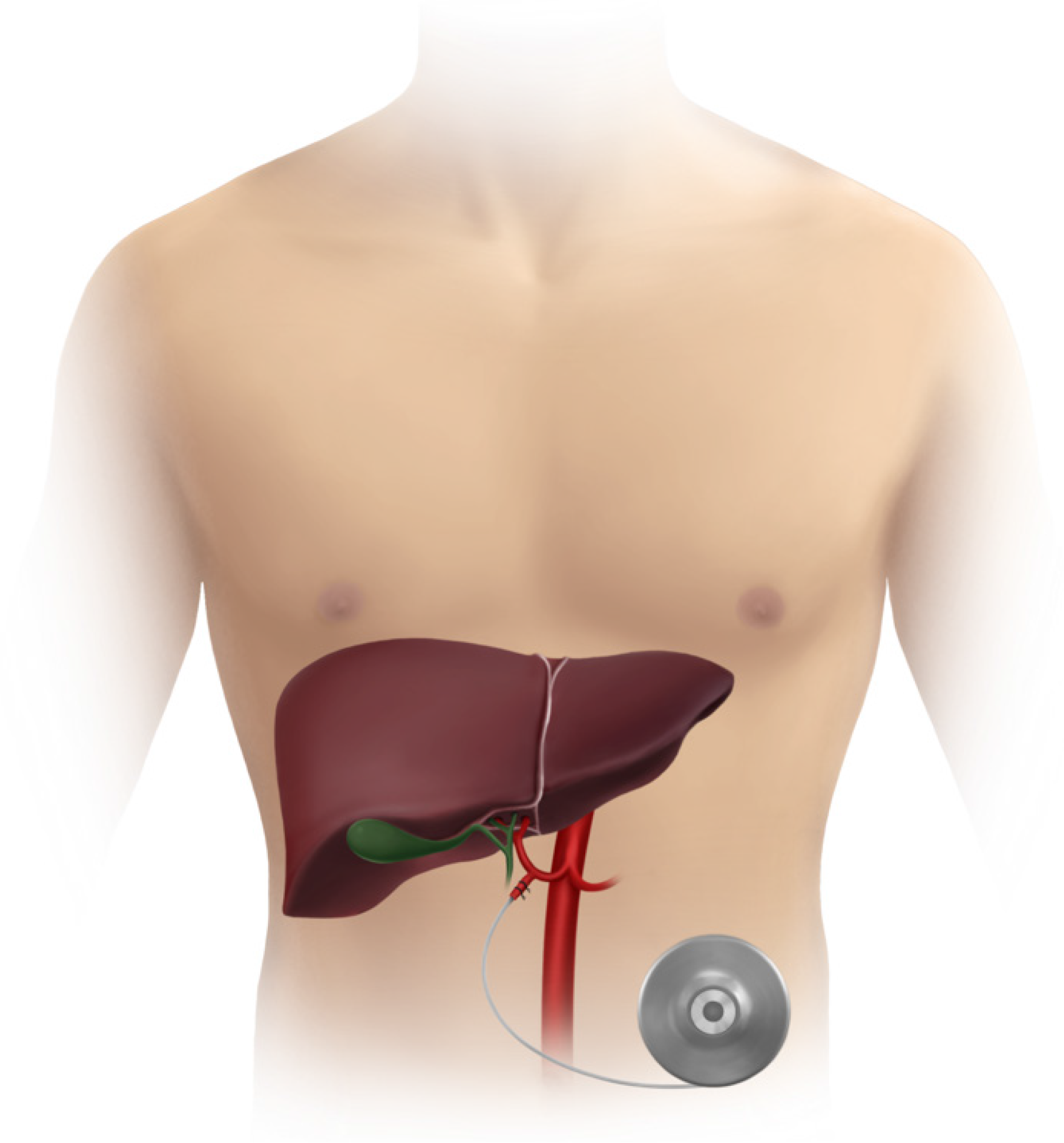
HAI chemotherapy leverages the distinct vascular supply of the liver to deliver high concentrations of chemotherapeutic agents directly to metastatic liver lesions, minimizing systemic toxicity (Figure 1). Unlike normal liver paren
Over the past 30 years, advancements in both surgical techniques and systemic agents have significantly refined HAI therapy. New developments, such as robotic-assisted pump placement, have improved the precision and safety of the procedure, further enhancing its role in managing unresectable CRLM. Although HAI has proven effective as both a first-line treatment for unresectable CRLM and as an adjuvant therapy in specific cases, its widespread adoption remains hindered by factors such as the technical demands of pump implantation, the need for expertise across a multidisciplinary care team, and the continued preference for systemic chemotherapy as the standard first-line treatment[20].
Successfully launching a HAI program requires securing buy-in from both internal teams and external referrers, particularly in the context of positive trial results that have demonstrated significant OS benefits with adjuvant HAI. These results have reignited interest in such programs. To ensure success, it is critical to effectively communicate these benefits to key stakeholders, including surgeons, oncologists, radiologists, and gastroenterologists, while also securing institutional support for acquiring the necessary devices, drugs, and operating room resources. Surgical expertise is central to the success of HAI programs, with essential skills such as navigating variations in the gastroduodenal artery and performing catheter insertions requiring advanced surgical planning. Mentorship from established centers can greatly benefit new programs, and careful patient selection—especially for those with unresectable disease—is crucial. For example, performing pump insertions without concurrent resections can help mitigate complications.
After implantation, medical oncologists play a critical role in managing HAI pump therapy and systemic chemotherapy, ensuring proper dosing and addressing potential complications, such as biliary sclerosis, which affects approximately 5% of patients. Adherence to established dosing protocols is essential to prevent these complications. Building a strong multidisciplinary team-comprising surgeons, pharmacists, nurses, radiologists, and interventional radiologists-is vital to navigate the complexities of HAI. Effective treatment and complication management depend on the coordination and communication among these specialists. Therefore, the success of an HAI program depends on the creation of a skilled, multidisciplinary team, securing institutional and external support, leveraging mentorship from experienced centers, and ensuring close collaboration with meticulous patient selection.
HAI chemotherapy has been studied extensively as an adjuvant therapy for CRLM to reduce the high rate of hepatic recurrences following resection, which can exceed 50% within two years of surgery[21,22]. Historically, the role of HAI was focused on eradicating micro-metastatic disease within the liver, where systemic chemotherapy alone showed limited efficacy. However, clinical evidence regarding the survival benefit of HAI has been mixed, with early studies yielding conflicting results. One of the initial randomized controlled trials (RCTs) by Lorenz et al[23] in 1998 demon
However, data from several other trials and retrospective studies have suggested that HAI could indeed confer a survival benefit, particularly when combined with modern systemic chemotherapy. A seminal study from Memorial Sloan Kettering Cancer Center (MSKCC) found a significant improvement in both PFS and OS in patients treated with adjuvant FUDR via HAI combined with systemic therapy (OS = 72 months vs OS = 59 months), compared to systemic therapy alone[25]. Similarly, a European Cooperative Group trial in 2002 reported improved median OS (64 months vs 49 months) and higher 4-year hepatic disease-free survival rates (67% vs 43%) in patients receiving HAI with FUDR and systemic therapy[26].
A major retrospective study from MSKCC in 2017 further validated the potential benefits of HAI. This 21-year analysis of 2368 patients who underwent resection of colorectal liver metastases found prolonged 5-year OS in those treated with HAI (53% vs 38%) and a significantly greater 10-year OS (38% vs 24%) compared to patients who did not receive HAI[18]. Notably, subgroup analyses demonstrated a consistent OS benefit from HAI regardless of whether patients received historic or modern systemic chemotherapy, preoperatively or in adjuvant setting. This study reinforced earlier findings, showing that HAI offers additional survival advantages even in the context of contemporary systemic therapies like FOLFOX and FOLFIRI combined with new targeted therapies which were introduced in the early 2000s[25].
Furthermore, genetic factors such as RAS mutation status have also been evaluated in conjunction with HAI therapy. An MSKCC follow-up study revealed that even among patients with KRAS-mutated tumors, HAI with systemic therapy improved 5-year OS rates compared to those who did not receive HAI (59% vs 40%)[17]. This evidence suggests that HAI may mitigate the adverse prognostic effects of certain genetic mutations, offering a more tailored approach to post-surgical treatment.
Despite these encouraging results, skepticism remains about the generalizability of HAI, particularly due to the fact that these results have been predominantly generated in a limited number of high-volume centers with extensive expertise. Current guidelines, such as those from the National Comprehensive Cancer Network (NCCN), remain equi
Ongoing prospective trials may provide further clarity on the role of adjuvant HAI. The PUMP trial, currently underway in the Netherlands, is evaluating the efficacy of adjuvant HAI FUDR in patients at low risk of recurrence, using PFS as the primary endpoint[9]. The PACHA-01 trial in France is another phase II/III study focused on high-risk patients, comparing adjuvant oxaliplatin HAI with systemic FOLFOX chemotherapy[27]. These trials are expected to elucidate whether HAI confers long-term survival benefits when used in conjunction with modern systemic therapies.
While HAI has shown promise in reducing hepatic recurrences and improving survival in select patients with resectable colorectal liver metastases, its use remains largely limited to specialized centers with extensive experience. Further large-scale RCTs are necessary to determine whether HAI can become a standardized component of adjuvant therapy, particularly in the context of modern systemic chemotherapy.
The primary goal of HAI therapy and systemic chemotherapy in patients with unresectable CRLM is conversion to resection, as surgical resection is independently linked with improved survival[6,7]. However, resectability remains highly dependent on the expertise of the surgeon and institution, making reported conversion rates variable across studies. Early systemic treatments for CRLM were limited to 5-FU, which had a modest response rate of 20% and average survival of 11 months[28]. Later developments, such as irinotecan and oxaliplatin, improved response rates to 45% with a median survival of up to 20 months[29]. Modern combination regimens, like FOLFOXIRI with bevacizumab, have signi
Historically, comparisons of HAI alone to systemic chemotherapy for unresectable CRLM showed higher response rates with HAI, but these studies did not demonstrate consistent improvements in OS[33-36]. Later, a multi-institutional RCT (CALGB 9481) prospectively revealed that adding HAI using FUDR to systemic 5-FU monotherapy significantly improved survival compared to systemic therapy only (24 months vs 20 months)[37]. However, up to 70% of patients treated with HAI later developed extrahepatic disease, highlighting the importance of combining HAI with systemic chemotherapy to manage both intrahepatic and extrahepatic metastases[21].
The advent of modern agents like oxaliplatin and irinotecan further expanded the scope of HAI therapy. A single-arm phase I study by Kemeny et al[38] at MSKCC demonstrated a 92% response rate and 47% conversion to resection using HAI combined with systemic oxaliplatin and irinotecan in patients with adverse prognostic features. Similar findings were reported by Goéré et al[39], where 24% of patients treated with HAI combined with systemic 5-FU and leucovorin underwent resection, with a 5-year survival of 56%. Recent studies continue to report high response rates of up to 76% and conversion to resection rates as high as 52% when combining HAI with modern systemic agents[40-43].
Particularly when combined with systemic chemotherapy, HAI therapy offers promising response rates and potential for resection in select patients with unresectable CRLM. However, careful patient selection based on biomarkers such as tumor burden remains critical for optimizing outcomes. As such, a combination therapy involving HAI with fruquintinib and tislelizumab has shown efficacy in microsatellite-stable CRLM cases that failed multiple treatments, achieving significant disease control rates with manageable toxicity[13]. Additionally, circulating tumor cell (CTC) monitoring has proven valuable in assessing post-HAI chemotherapy outcomes, where decreases in CTC levels correlated with improved PFS[14].
Furthermore, dose adjustments in HAI protocols are becoming crucial. For instance, a modified FUDR regimen re
These findings underscore the growing shift toward personalizing HAI treatments, incorporating factors such as tumor growth patterns and microsatellite instability status to optimize outcomes. This stratified approach not only seeks to maximize efficacy but also aims to minimize adverse effects and enhance surgical conversion rates, laying the ground
Finally, recent efforts have explored combining HAI with other treatment modalities, such as transarterial chemoembolization (TACE)[44]. Unlike HAI, which relies on continuous infusion to deliver high local drug concentrations without interrupting blood flow, TACE combines chemotherapy with embolic agents that block the tumor’s blood supply, creating ischemia and promoting tumor necrosis. Although HAI and TACE operate through different mechanisms, they may function as complementary rather than competing therapies. For instance, a recent report on the combination of irinotecan-eluting HepaSphere TACE with HAI for unresectable CRLM indicates potential synergistic benefits[44]. However, further research is necessary to establish optimal combinations and criteria for patient selection.
Over the past three decades, the role of HAI chemotherapy has evolved significantly. Initially introduced in the 1980s for patients with unresectable liver metastases, HAI has since expanded to include adjuvant therapy following hepatic resection, reflecting a growing recognition of its potential benefits in CRC management. This evolution highlights the shift from HAI as a niche treatment to a more integrated approach for patients with liver-dominant metastatic disease. Our study contributes to this progress by emphasizing the clinical value of HAI, especially when combined with systemic therapies, and by addressing patient selection criteria critical to optimizing outcomes in CRLM. Future research should focus on refining HAI protocols in combination with emerging systemic therapies, as well as overcoming technical challenges that currently limit broader adoption of this promising treatment. Through these efforts, HAI may continue to solidify its role as a valuable component of care for selected patients with metastatic CRC.
| 1. | Van Cutsem E, Cervantes A, Nordlinger B, Arnold D; ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 799] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 2. | Van Cutsem E, Oliveira J; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 818] [Article Influence: 409.0] [Reference Citation Analysis (34)] |
| 4. | Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 895] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 5. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1444] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 6. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 625] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 7. | Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 269] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez MJ. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 459] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Buisman FE, Homs MYV, Grünhagen DJ, Filipe WF, Bennink RJ, Besselink MGH, Borel Rinkes IHM, Bruijnen RCG, Cercek A, D'Angelica MI, van Delden OM, Donswijk ML, van Doorn L, Doornebosch PG, Emmering J, Erdmann JI, IJzerman NS, Grootscholten C, Hagendoorn J, Kemeny NE, Kingham TP, Klompenhouwer EG, Kok NFM, Koolen S, Kuhlmann KFD, Kuiper MC, Lam MGE, Mathijssen RHJ, Moelker A, Oomen-de Hoop E, Punt CJA, Te Riele WW, Roodhart JML, Swijnenburg RJ, Prevoo W, Tanis PJ, Vermaas M, Versleijen MWJ, Veuger FP, Weterman MJ, Verhoef C, Groot Koerkamp B. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC Cancer. 2019;19:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Goldberg RM. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721-5727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 12. | Holch J, Stintzing S, Heinemann V. Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives. Visc Med. 2016;32:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Zheng K, Zhu X, Xu L, Cao G, Niu C, Yan X, Xu D, Liu W, Bao Q, Wang L, Wang K, Xing B, Wang X. Efficacy and safety of hepatic arterial infusion chemotherapy combined with fruquintinib and tislelizumab for patients with microsatellite stable colorectal cancer liver metastasis following failure of multiple-line therapy. Front Oncol. 2024;14:1420956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 14. | Zhang E, Li H, Liu C, Zhou H, Liu B, Feng C. Clinical value of circulating tumour cells in evaluating the efficacy of continuous hepatic arterial infusion among colorectal cancer patients. J Chemother. 2024;1-9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Schwantes IR, Patel RK, Kardosh A, Paxton J, Eil R, Chen EY, Rocha FG, Latour E, Pegna G, Lopez CD, Mayo SC. A Modified Floxuridine Reduced-Dose Protocol for Patients with Unresectable Colorectal Liver Metastases Treated with Hepatic Arterial Infusion. Ann Surg Oncol. 2024;31:6537-6545. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Filipe WF, Meyer YM, Buisman FE, van den Braak RRJC, Galjart B, Höppener DJ, Jarnagin WR, Kemeny NE, Kingham TP, Nierop PMH, van der Stok EP, Grünhagen DJ, Vermeulen PB, Groot Koerkamp B, Verhoef C, D'Angelica MI. The Effect of Histopathological Growth Patterns of Colorectal Liver Metastases on the Survival Benefit of Adjuvant Hepatic Arterial Infusion Pump Chemotherapy. Ann Surg Oncol. 2023;30:7996-8005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Gholami S, Kemeny NE, Boucher TM, Gönen M, Cercek A, Kingham TP, Balachandran V, Allen P, DeMatteo R, Drebin J, Jarnagin W, D'Angelica M. Adjuvant Hepatic Artery Infusion Chemotherapy is Associated With Improved Survival Regardless of KRAS Mutation Status in Patients With Resected Colorectal Liver Metastases: A Retrospective Analysis of 674 Patients. Ann Surg. 2020;272:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Groot Koerkamp B, Sadot E, Kemeny NE, Gönen M, Leal JN, Allen PJ, Cercek A, DeMatteo RP, Kingham TP, Jarnagin WR, D'Angelica MI. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol. 2017;35:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Dizon DS, Schwartz J, Kemeny N. Regional chemotherapy: a focus on hepatic artery infusion for colorectal cancer liver metastases. Surg Oncol Clin N Am. 2008;17:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Karanicolas PJ, Metrakos P, Chan K, Asmis T, Chen E, Kingham TP, Kemeny N, Porter G, Fields RC, Pingpank J, Dixon E, Wei A, Cleary S, Zogopoulos G, Dey C, D'Angelica M, Fong Y, Dowden S, Ko YJ. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol. 2014;21:e129-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Chan KM, Wu TH, Cheng CH, Lee WC, Chiang JM, Chen JS, Wang JY. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J Surg Oncol. 2014;12:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Lorenz M, Müller HH, Schramm H, Gassel HJ, Rau HG, Ridwelski K, Hauss J, Stieger R, Jauch KW, Bechstein WO, Encke A. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg. 1998;228:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Nelson R, Freels S. Hepatic artery adjuvant chemotherapy for patients having resection or ablation of colorectal cancer metastatic to the liver. Cochrane Database Syst Rev. 2006;2006:CD003770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, Allen PJ, Fong YC, Cercek A, D'Angelica MI. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, Sigurdson ER, O'Dwyer PJ, Benson AB 3rd. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol. 2002;20:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 27. | Goéré D, Pignon JP, Gelli M, Elias D, Benhaim L, Deschamps F, Caramella C, Boige V, Ducreux M, de Baere T, Malka D. Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases-a randomized phase II/III trial - PACHA-01 (NCT02494973). BMC Cancer. 2018;18:787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | . Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 810] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 29. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2222] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 30. | Basso M, Dadduzio V, Ardito F, Lombardi P, Strippoli A, Vellone M, Orlandi A, Rossi S, Cerchiaro E, Cassano A, Giuliante F, Barone C. Conversion Chemotherapy for Technically Unresectable Colorectal Liver Metastases: A Retrospective, STROBE-Compliant, Single-Center Study Comparing Chemotherapy Alone and Combination Chemotherapy With Cetuximab or Bevacizumab. Medicine (Baltimore). 2016;95:e3722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 32. | Cho M, Gong J, Fakih M. The state of regional therapy in the management of metastatic colorectal cancer to the liver. Expert Rev Anticancer Ther. 2016;16:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Hohn DC, Stagg RJ, Friedman MA, Hannigan JF Jr, Rayner A, Ignoffo RJ, Acord P, Lewis BJ. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 302] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 431] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Kerr DJ, McArdle CS, Ledermann J, Taylor I, Sherlock DJ, Schlag PM, Buckels J, Mayer D, Cain D, Stephens RJ; Medical Research Council's colorectal cancer study group; European Organisation for Research and Treatment of Cancer colorectal cancer study group. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Martin JK Jr, O'Connell MJ, Wieand HS, Fitzgibbons RJ Jr, Mailliard JA, Rubin J, Nagorney DM, Tschetter LK, Krook JE. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg. 1990;125:1022-1027. [PubMed] [DOI] [Full Text] |
| 37. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE 2nd, Zhang C, Mayer RJ. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 38. | Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, Jarnagin WR, Patel D, D'Angelica M. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465-3471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Goéré D, Deshaies I, de Baere T, Boige V, Malka D, Dumont F, Dromain C, Ducreux M, Elias D. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg. 2010;251:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | D’Angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, Capanu M, Kingham TP, Fong Y, DeMatteo RP, Allen PJ, Jarnagin WR, Kemeny N. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Lévi FA, Boige V, Hebbar M, Smith D, Lepère C, Focan C, Karaboué A, Guimbaud R, Carvalho C, Tumolo S, Innominato P, Ajavon Y, Truant S, Castaing D, De Baere T, Kunstlinger F, Bouchahda M, Afshar M, Rougier P, Adam R, Ducreux M; Association Internationale pour Recherche sur Temps Biologique et Chronothérapie (ARTBC International). Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016;27:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Lim A, Le Sourd S, Senellart H, Luet D, Douane F, Perret C, Bouvier A, Métairie S, Cauchin E, Rougier P, Matysiak-Budnik T, Touchefeu Y. Hepatic Arterial Infusion Chemotherapy for Unresectable Liver Metastases of Colorectal Cancer: A Multicenter Retrospective Study. Clin Colorectal Cancer. 2017;16:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Pak LM, Kemeny NE, Capanu M, Chou JF, Boucher T, Cercek A, Balachandran VP, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, D'Angelica MI. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol. 2018;117:634-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Feng A, Gao S, Guo J, Kou F, Liu S, Zhang X, Liu B, Wang X, Chen H, Xu H, Liu P, Cao G, Gao Q, Zhu X. Efficacy and safety of irinotecan-eluting HepaSphere transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for unresectable colorectal liver metastases. JCO. 2023;41:3585-3585. [DOI] [Full Text] |