Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.95910
Revised: September 28, 2024
Accepted: November 20, 2024
Published online: February 24, 2025
Processing time: 233 Days and 7 Hours
Ampullary adenocarcinoma is a rare malignant tumor of the gastrointestinal tract. Currently, only a few cases have been reported, resulting in limited information on survival.
To develop a dynamic nomogram using internal and external validation to predict survival in patients with ampullary adenocarcinoma.
Data were sourced from the surveillance, epidemiology, and end results stat database. The patients in the database were randomized in a 7:3 ratio into training and validation groups. Using Cox regression univariate and multivariate analyses in the training group, we identified independent risk factors for overall survival and cancer-specific survival to develop the nomogram. The nomogram was validated with a cohort of patients from the First Affiliated Hospital of the Army Medical University.
For overall and cancer-specific survival, 12 (sex, age, race, lymph node ratio, tumor size, chemotherapy, surgical modality, T stage, tumor differentiation, brain metastasis, lung metastasis, and extension) and 6 (age; surveillance, epide
The dynamic nomogram offers robust predictive efficacy for the overall and cancer-specific survival of ampullary adenocarcinoma.
Core Tip: In this study, we analyzed the prognosis of patients with ampullary adenocarcinoma by incorporating data from the surveillance, epidemiology, and end results stat and data from Chinese medical centers. Different from other studies, we found that chemotherapy can improve the prognosis of patients.
- Citation: Yang J, Wang ZY, Chen J, Zhang Y, Chen L. Nomogram for overall survival in ampullary adenocarcinoma using the surveillance, epidemiology, and end results database and external validation. World J Clin Oncol 2025; 16(2): 95910
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/95910.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.95910
Ampullary tumors are rare cancers with an incidence of approximately five to nine cases per million individuals[1]. Ampullary adenocarcinoma is a malignant tumor derived from the epithelium of the pelvic glands of Vater[2]. As endoscopic diagnostics and other complementary diagnostic techniques have evolved, the annual incidence of ampullary tumors has increased[3]. Endoscopy and endoscopic retrograde cholangiopancreatography (ERCP) are currently the optimal methods for assessing ampullary tumors[2]; however, as ERCP is difficult to perform, it still only operates in some major tertiary hospitals. Furthermore, most patients have already presented obvious symptoms when they seek treatment, and their conditions have reached advanced stages. Symptoms that may present at an early stage owing to the special location of the ampullary tumor include jaundice and abdominal aching. Previous literature has reported a wide variation in 5-year survival rates for ampullary tumor cancer, varying from 20% to 88%[4]. Nonetheless, ampullary carcinoma has a more favorable prognosis than other periampullary malignancies[5].
Currently, treatment includes endoscopic papillectomy, Whipple resection, and pylorus-preserving pancreatoduodenectomy. After diagnosis, patients are generally confronted with the choice of treatment modality. However, the choice of treatment significantly determines patient prognosis. A valuable tool for evaluating diagnosis and prognosis, nomograms allow for individualized prediction and improve the accuracy of prognostic prediction compared with the traditional tumor node metastasis (TNM) staging system[6].
Given its low prevalence and the absence of large-sample studies, this study developed a prognostic nomogram for ampullary adenocarcinoma based on the surveillance, epidemiology, and end results (SEER) database, aimed at re
In the SEER queue, data were derived from SEER stat (version 8.4.2). The inclusion criteria for the study population were as follows: (1) Diagnosis between 2000 and 2020; (2) Primary tumor site of the ampulla of Vater; and (3) International classification of diseases of oncology, malignant, and adenocarcinoma. The exclusion criteria were as follows: (1) Unknown survival status or failure of visitation; and (2) Uncertain operative modality. Ultimately, we included 1858 patients. As the SEER database is publicly available for research purposes, access is not subject to ethics committee approval or informed consent.
For the Chinese cohort, data were obtained from the First Affiliated Hospital of the Army Medical University. The inclusion criteria for the study population were as follows: (1) Diagnosis between 2016 and 2023; (2) The primary tumor site was the ampulla of Vater; and (3) The pathological type was indicative of an adenocarcinoma. The exclusion criteria were as follows: (1) Incomplete clinical data; and (2) Missed visits. Ultimately, 257 patients were included in this cohort. This study was approved by the Ethics Committee of the First Affiliated Hospital of the Army Medical University, No. (B) KY2023175. The ethics review board exempted this study from the requirement of informed consent.
Clinical data included: Patients’ sex; age; race; marital status at diagnosis; surgical modality; lymph node ratio (LNR): regional nodes positive/examined; tumor size; tumor differentiation; American Joint Committee on Cancer (AJCC) TNM stage; radiotherapy; chemotherapy; SEER stage; distant metastatic sites (bone, liver, brain, lungs); status; monthly survival rate. The overall survival (OS) and cancer-specific survival (CSS) were the primary endpoints.
Statistical product and service solutions 26.0 (IBM Corp, Armonk, NY, United States) and R version 4.1.3 were used for statistical analysis. Differences between the cohorts were compared using the χ2 test. The 2009 patients collected from the SEER database were randomized in a 7:3 ratio into training (n = 1406) and validation groups (n = 603) using R software. The training set in the SEER cohort was screened for variables significantly associated with survival using Cox regression univariate and multivariate analyses, and the “Survival” package in R was used to develop the prognostic column plots. The internal validation set and Chinese cohort (external validation set) were used to assess the constructed column line graphs, and consistency index (C-index) and receiver operating characteristic (ROC) curves with the calculated area under the curve (AUC) were used to assess the discrimination and performance of the nomogram. A dynamic network-based nomogram based on the nomogram was developed using the “DynNom” software package. The package “rms” was used to plot calibration curves, and “gg decision curve analysis” was used to plot clinical decision curves. The risk score for each individual was obtained from the nomogram, and the threshold for risk stratification was calculated by the “surv_cutpoint” function of the R package “survminer.” The cohort was categorized into low-risk and high-risk groups based on the median risk score. Kaplan-Meier survival analyses were performed to determine whether a significant difference in the incidence of OS and CSS existed between the two risk groups.
Based on the inclusion criteria, 2009 patients were included in the SEER cohort (Table 1). Of these, 1109 were men and 900 were women, with most patients (91.8%) older than 50 years. The median survival duration was 33 months. A total of 257 patients (147 men and 116 women) were included in the Chinese cohort (Table 1). The 1-, 3-, and 5-year OS of the SEER cohort were 71.7%, 47.3%, and 38.1%, respectively, whereas those of the Chinese cohort were 86.9%, 66.4%, and 56.5%, respectively.
| Characteristics | Training cohort (n = 1406) | Internal validation cohort (n = 603) | External validation cohort (n = 257) |
| Age | |||
| < 50 | 109 (7.8) | 56 (9.3) | 24 (9.3) |
| 50-59 | 267 (19.0) | 127 (21.1) | 80 (31.1) |
| 60-69 | 381 (27.1) | 153 (25.4) | 78 (30.4) |
| 70-79 | 358 (25.5) | 172 (28.5) | 61 (23.7) |
| ≥ 80 | 291 (20.7) | 95 (15.8) | 14 (5.4) |
| Sex | |||
| Female | 626 (44.5) | 274 (45.4) | 110 (42.8) |
| Male | 780 (55.5) | 329 (54.6) | 147 (57.2) |
| Race | |||
| White | 1075 (76.5) | 465 (77.1) | 0 (0) |
| Black | 107 (7.6) | 53 (8.8) | 0 (0) |
| Unknown | 224 (15.9) | 85 (14.1) | 257 (100) |
| SEER stage | |||
| Localized | 207 (14.7) | 79 (13.1) | 91 (35.4) |
| Regional | 795 (56.5) | 371 (61.5) | 57 (22.2) |
| Distant | 357 (25.4) | 130 (21.6) | 84 (32.7) |
| Unknown | 47 (3.3) | 23 (3.8) | 25 (9.7) |
| Surgery | |||
| No | 373 (26.5) | 155 (25.7) | 38 (14.8) |
| Surgical removal of primary site | 412 (29.3) | 201 (33.3) | 154 (59.9) |
| Radical surgery | 621 (44.2) | 247 (41.0) | 65 (25.3) |
| Radiation | |||
| No | 1123 (79.8) | 478 (79.3) | 249 (96.9) |
| Yes | 283 (20.1) | 125 (20.7) | 8 (3.1) |
| Chemotherapy | |||
| No | 762 (54.2) | 325 (53.9) | 167 (65.0) |
| Yes | 644 (45.8) | 278 (46.1) | 90 (35.0) |
| Bone metastasis | |||
| No | 1404 (99.9) | 598 (99.2) | 254 (98.8) |
| Yes | 2 (0.1) | 5 (0.8) | 3 (1.2) |
| Brain metastasis | |||
| No | 1402 (99.7) | 601 (99.7) | 257 (100) |
| Yes | 4 (0.3) | 2 (0.3) | 0 (0) |
| Liver metastasis | |||
| No | 1309 (93.1) | 557 (92.4) | 226 (87.9) |
| Yes | 97 (6.9) | 46 (7.6) | 31 (12.1) |
| Lung metastasis | |||
| No | 1378 (98.0) | 591 (98.0) | 250 (97.3) |
| Yes | 28 (2.0) | 12 (2.0) | 7 (2.7) |
| Tumor size | |||
| < 2 cm | 452 (32.1) | 185 (30.7) | 82 (31.9) |
| > 2 cm | 698 (49.6) | 322 (53.4) | 117 (45.5) |
| Unknown | 256 (18.2) | 96 (15.9) | 58 (22.6) |
| LNR | |||
| 0 | 468 (33.3) | 198 (32.8) | 175 (68.1) |
| 0-0.5 | 468 (33.3) | 220 (36.5) | 14 (5.4) |
| 0.5-1 | 61 (4.3) | 20 (3.3) | 25 (9.7) |
| Unknown | 409 (29.1) | 165 (27.4) | 43 (16.7) |
| Marital status | |||
| Single | 235 (16.7) | 110 (18.2) | 3 (1.2) |
| Divorced | 127 (9.0) | 48 (8.0) | 6 (2.3) |
| Married | 769 (54.7) | 341 (56.6) | 237 (92.2) |
| Widowed | 220 (15.6) | 71 (11.8) | 11 (4.3) |
| Unknow | 55 (3.9) | 33 (5.5) | 0 (0) |
| Stage T | |||
| T1 | 276 (19.6) | 107 (17.7) | 27 (10.5) |
| T2 | 366 (26.0) | 171 (28.4) | 96 (37.4) |
| T3 | 334 (23.8) | 152 (25.2) | 37 (14.4) |
| T4 | 351 (25.0) | 140 (23.2) | 57 (22.2) |
| Unknown | 79 (5.6) | 33 (5.5) | 40 (15.6) |
| Stage N | |||
| N0 | 775 (55.1) | 322 (53.4) | 135 (52.5) |
| N1 | 583 (41.5) | 264 (43.8) | 50 (19.5) |
| Unknown | 48 (3.4) | 17 (2.8) | 72 (28.0) |
| Stage M | |||
| M0 | 1251 (89.0) | 543 (90.0) | 223 (86.8) |
| M1 | 155 (11.0) | 60 (10.0) | 34 (13.2) |
| Extension | |||
| Localized | 131 (9.3) | 54 (9.0) | 40 (15.6) |
| Duodenal wall | 554 (39.4) | 244 (40.5) | 95 (40.0) |
| Pancreas | 346 (24.6) | 157 (26.0) | 52 (20.2) |
| Common bile duct | 88 (6.3) | 43 (7.1) | 27 (10.5) |
| Invasive tumor confined | 208 (14.8) | 72 (11.9) | 33 (12.8) |
| Extension to other adjacent organs | 79 (5.6) | 33 (5.5) | 10 (3.9) |
| Tumor differentiation | |||
| Well differentiated | 138 (9.8) | 65 (10.8) | 20 (7.8) |
| Moderately differentiated | 668 (47.5) | 288 (47.8) | 105 (40.9) |
| Poorly differentiated | 377 (26.8) | 160 (26.5) | 77 (30.0) |
| Undifferentiated | 16 (1.1) | 2 (0.3) | 27 (10.5) |
| Unknown | 207 (14.7) | 88 (14.6) | 28 (10.9) |
Age, race, marital status at diagnosis, SEER stage, surgical modality, tumor size, chemotherapy, brain metastasis, liver metastasis, lung metastasis, tumor differentiation, LNR, T stage, N stage, M stage, extension, and history of lymph node surgery were significantly correlated with OS in univariate Cox regression analyses (all P < 0.05). All of the above statistically significant factors were included in the multivariate Cox analysis, which identified sex, age, race, LNR, tumor size, chemotherapy, surgical modality, T stage, tumor differentiation, brain metastasis, and lung metastasis as independent risk factors for OS (Table 2) and age, SEER stage, LNR, chemotherapy, surgical modality, and tumor differentiation as independent risk factors for CSS (Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | ||||||
| < 50 | Reference | |||||
| 50-59 | 1.25 | 0.91-1.71 | 0.17 | 1.19 | 0.86-1.64 | 0.30 |
| 60-69 | 1.44 | 1.07-1.953 | < 0.05 | 1.10 | 0.81-1.50 | 0.55 |
| 70-79 | 1.93 | 1.43-2.61 | < 0.05 | 1.62 | 1.19-2.20 | < 0.05 |
| ≥ 80 | 3.35 | 2.48-4.53 | < 0.05 | 1.83 | 1.31-2.56 | < 0.05 |
| Sex | ||||||
| Female | Reference | |||||
| Male | 1.08 | 0.95-1.23 | 0.23 | 1.20 | 1.03-1.38 | < 0.05 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.09 | 0.86-1.38 | 0.46 | 1.28 | 1.00-1.65 | < 0.05 |
| Unknown | 0.71 | 0.59-0.86 | < 0.05 | 0.90 | 0.74-1.09 | 0.29 |
| SEER stage | ||||||
| Localized | Reference | |||||
| Regional | 0.98 | 0.80-1.19 | 0.81 | 1.30 | 0.89-1.90 | 0.18 |
| Distant | 2.17 | 1.76-2.68 | < 0.05 | 1.80 | 0.82-3.94 | 0.14 |
| Unknown | 3.01 | 2.12-4.28 | < 0.05 | 1.49 | 0.82-2.71 | 0.19 |
| Surgical modality | ||||||
| None | Reference | |||||
| Surgical removal of primary site | 0.21 | 0.18-0.25 | < 0.05 | 0.35 | 0.25-0.50 | < 0.05 |
| Radical surgery | 0.22 | 0.19-0.26 | < 0.05 | 0.38 | 0.26-0.54 | < 0.05 |
| Radiation | ||||||
| No | Reference | |||||
| Yes | 0.89 | 0.76-1.05 | 0.16 | |||
| Chemotherapy | ||||||
| No | Reference | |||||
| Yes | 0.81 | 0.71-0.92 | < 0.05 | 0.61 | 0.52-0.71 | < 0.05 |
| Bone metastasis | ||||||
| No | Reference | |||||
| Yes | 2.86 | 0.71-11.46 | 0.14 | |||
| Brain metastasis | ||||||
| No | Reference | |||||
| Yes | 6.57 | 2.46-17.58 | < 0.05 | 4.18 | 1.42-12.33 | < 0.05 |
| Liver metastasis | ||||||
| No | Reference | |||||
| Yes | 3.55 | 2.86-4.42 | < 0.05 | 1.01 | 0.68-1.51 | 0.96 |
| Lung metastasis | ||||||
| No | Reference | |||||
| Yes | 3.27 | 2.23-4.80 | < 0.05 | 0.63 | 0.38-1.06 | 0.08 |
| Tumor size | ||||||
| < 2 cm | Reference | |||||
| > 2 cm | 1.40 | 1.20-1.63 | < 0.05 | 1.04 | 0.88-1.23 | 0.63 |
| Unknown | 3.12 | 2.61-3.74 | < 0.05 | 1.28 | 1.02-1.63 | < 0.05 |
| LNR | ||||||
| 0 | Reference | |||||
| 0-0.5 | 2.04 | 1.71-2.42 | < 0.05 | 1.49 | 1.02-2.19 | < 0.05 |
| 0.5-1 | 5.11 | 3.79-6.89 | < 0.05 | 2.99 | 1.90-4.72 | < 0.05 |
| Unknown | 6.02 | 5.05-7.18 | < 0.05 | 2.22 | 1.55-3.17 | < 0.05 |
| Marital status | ||||||
| Single | Reference | |||||
| Divorced | 1.15 | 0.89-1.50 | 0.28 | 1.03 | 0.79-1.34 | 0.85 |
| Married | 0.96 | 0.80-1.15 | 0.66 | 0.97 | 0.80-1.17 | 0.74 |
| Widowed | 1.66 | 1.33-2.06 | < 0.05 | 1.08 | 0.84-1.38 | 0.55 |
| Unknown | 1.02 | 0.70-1.48 | 0.91 | 0.88 | 0.60-1.29 | 0.52 |
| T stage | ||||||
| T1 | Reference | |||||
| T2 | 0.64 | 0.52-0.78 | < 0.05 | 0.97 | 0.67-1.39 | 0.87 |
| T3 | 0.98 | 0.80-1.19 | 0.83 | 1.72 | 0.79-3.73 | 0.17 |
| T4 | 1.44 | 1.19-1.74 | < 0.05 | 1.77 | 1.11-2.81 | < 0.05 |
| Unknown | 2.65 | 2.01-3.49 | < 0.05 | 0.73 | 0.44-1.22 | 0.23 |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 1.25 | 1.10-1.43 | < 0.05 | 1.23 | 0.87-1.74 | 0.24 |
| Unknown | 3.14 | 2.30-4.29 | < 0.05 | 1.12 | 0.79-1.59 | 0.53 |
| M stage | ||||||
| M0 | Reference | |||||
| M1 | 3.89 | 3.24-4.66 | < 0.05 | 1.46 | 0.71-3.00 | 0.31 |
| Extension | ||||||
| Localized | Reference | |||||
| Duodenal wall | 0.36 | 0.29-0.44 | < 0.05 | 1.02 | 0.74-1.40 | 0.92 |
| Pancreas | 0.53 | 0.42-0.66 | < 0.05 | 0.87 | 0.45-1.69 | 0.68 |
| Common bile duct | 0.78 | 0.58-1.07 | 0.12 | 0.53 | 0.36-0.80 | < 0.05 |
| Invasive tumor confined | 0.66 | 0.52-0.84 | < 0.05 | 0.83 | 0.39-1.77 | 0.63 |
| Extension to other adjacent organs | 1.39 | 1.03-1.87 | < 0.05 | |||
| Tumor differentiation | ||||||
| Well-differentiated | Reference | |||||
| Moderately differentiated | 1.19 | 0.93-1.52 | 0.16 | 1.07 | 0.83-1.38 | 0.59 |
| Poorly differentiated | 1.81 | 1.41-2.34 | < 0.05 | 1.65 | 1.26-2.15 | < 0.05 |
| Undifferentiated | 1.62 | 0.86-3.04 | 0.14 | 0.98 | 0.51-1.86 | 0.94 |
| Unknown | 2.54 | 1.94-3.33 | < 0.05 | 1.03 | 0.77-1.38 | 0.83 |
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | ||||||
| < 50 | Reference | |||||
| 50-59 | 1.37 | 1.02-1.85 | < 0.05 | 1.19 | 0.88-1.61 | 0.26 |
| 60-69 | 1.49 | 1.12-1.98 | < 0.05 | 1.52 | 0.86-1.55 | 0.35 |
| 70-79 | 2.11 | 1.59-2.80 | < 0.05 | 1.60 | 1.19-2.14 | < 0.05 |
| ≥ 80 | 3.57 | 2.68-4.75 | < 0.05 | 1.98 | 1.43-2.73 | < 0.05 |
| Sex | ||||||
| Female | Reference | |||||
| Male | 1.08 | 0.95-1.23 | 0.23 | 1.20 | 1.03-1.38 | < 0.02 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.09 | 0.86-1.38 | 0.46 | 1.28 | 1.00-1.65 | < 0.05 |
| Unknown | 0.71 | 0.59-0.86 | < 0.05 | 0.90 | 0.74-1.09 | 0.29 |
| SEER stage | ||||||
| Localized | Reference | |||||
| Regional | 0.98 | 0.80-1.19 | 0.81 | 1.30 | 0.89-1.90 | 0.18 |
| Distant | 2.17 | 1.76-2.68 | < 0.05 | 1.80 | 0.82-3.94 | 0.14 |
| Unknown | 3.01 | 2.12-4.28 | < 0.05 | 1.49 | 0.82-2.71 | 0.19 |
| Surgical modality | ||||||
| None | Reference | |||||
| surgical removal of primary site | 0.21 | 0.18-0.25 | < 0.05 | 0.35 | 0.25-0.50 | < 0.05 |
| Radical surgery | 0.22 | 0.19-0.26 | < 0.05 | 0.38 | 0.26-0.54 | < 0.05 |
| Radiation | ||||||
| No | Reference | |||||
| Yes | 0.89 | 0.76-1.05 | 0.16 | |||
| Chemotherapy | ||||||
| No | Reference | |||||
| Yes | 0.81 | 0.71-0.92 | < 0.05 | 0.61 | 0.52-0.71 | < 0.05 |
| Bone metastasis | ||||||
| No | Reference | |||||
| Yes | 2.86 | 0.71-11.46 | 0.14 | |||
| Brain metastasis | ||||||
| No | Reference | |||||
| Yes | 6.57 | 2.46-17.58 | < 0.05 | 4.18 | 1.42-12.33 | < 0.05 |
| Liver metastasis | ||||||
| No | Reference | |||||
| Yes | 3.55 | 2.86-4.42 | < 0.05 | 1.01 | 0.68-1.51 | 0.96 |
| Lung metastasis | ||||||
| No | Reference | |||||
| Yes | 3.27 | 2.23-4.80 | < 0.05 | 0.63 | 0.38-1.06 | 0.08 |
| Tumor size | ||||||
| < 2 cm | Reference | |||||
| > 2 cm | 1.40 | 1.20-1.63 | < 0.05 | 1.04 | 0.88-1.23 | 0.63 |
| Unknown | 3.12 | 2.61-3.74 | < 0.05 | 1.28 | 1.02-1.61 | < 0.05 |
| LNR | ||||||
| 0 | Reference | |||||
| 0-0.5 | 2.04 | 1.71-2.43 | < 0.05 | 1.49 | 1.02-2.19 | < 0.05 |
| 0.5-1 | 5.11 | 3.79-6.89 | < 0.05 | 2.99 | 1.89-4.72 | < 0.05 |
| Unknown | 6.02 | 5.048-7.182 | < 0.05 | 2.22 | 1.55-3.17 | < 0.05 |
| Marital status | ||||||
| Single | Reference | |||||
| Divorced | 1.15 | 0.89-1.50 | 0.28 | 1.03 | 0.77-1.34 | 0.85 |
| Married | 0.96 | 0.80-1.15 | 0.66 | 0.97 | 0.80-1.17 | 0.74 |
| Widowed | 1.66 | 1.33-2.06 | < 0.05 | 1.08 | 0.84-1.38 | 0.55 |
| Unknown | 1.02 | 0.70-1.48 | 0.91 | 0.88 | 0.60-1.29 | 0.52 |
| T stage | ||||||
| T1 | Reference | |||||
| T2 | 0.64 | 0.52-0.78 | < 0.05 | 0.97 | 0.67-1.39 | 0.87 |
| T3 | 0.98 | 0.80-1.19 | < 0.05 | 1.72 | 0.79-3.73 | 0.17 |
| T4 | 1.44 | 1.19-1.74 | < 0.05 | 1.77 | 1.11-2.81 | < 0.05 |
| Unknown | 2.65 | 2.01-3.49 | < 0.05 | 0.73 | 0.44-1.22 | 0.23 |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 1.25 | 1.10-1.43 | < 0.05 | 1.23 | 0.87-1.74 | 0.24 |
| Unknown | 3.14 | 2.30-4.29 | < 0.05 | 1.12 | 0.79-1.59 | 0.53 |
| M stage | ||||||
| M0 | Reference | |||||
| M1 | 3.89 | 3.24-4.66 | < 0.05 | 1.46 | 0.71-3.00 | 0.31 |
| Extension | ||||||
| Localized | Reference | |||||
| Duodenal wall | 0.36 | 0.29-0.44 | < 0.05 | 1.02 | 0.74-1.40 | 0.92 |
| Pancreas | 0.53 | 0.42-0.66 | < 0.05 | 0.87 | 0.45-1.69 | 0.68 |
| Common bile duct | 0.78 | 0.58-1.07 | 0.12 | 0.53 | 0.36-0.80 | < 0.05 |
| Invasive tumor confined | 0.66 | 0.52-0.84 | < 0.05 | 0.83 | 0.39-1.77 | 0.63 |
| Extension to other adjacent organs | 1.39 | 1.03-1.87 | < 0.05 | |||
| Tumor differentiation | ||||||
| Well differentiated | Reference | |||||
| Moderately differentiated | 1.19 | 0.93-1.52 | 0.16 | 1.07 | 0.83-1.38 | 0.59 |
| Poorly differentiated | 1.81 | 1.41-2.34 | < 0.05 | 1.65 | 1.26-2.15 | < 0.05 |
| Undifferentiated | 1.62 | 0.86-3.04 | 0.14 | 0.98 | 0.51-1.86 | 0.94 |
| Unknown | 2.54 | 1.94-3.33 | < 0.05 | 1.03 | 0.77-1.38 | 0.83 |
We developed a nomogram based on the results of univariate and multivariate Cox regression analyses. In the nomogram (Figure 1), the scores corresponding to the different categories of these risk factors can be read. All scores added together correspond to a certain probability of survival. Furthermore, we generated an online dynamic nomogram to promote its clinical application (https://yangjia-1998.shinyapps.io/DynNomapp/;https://zhailei1997.shinyapps.io/DynNomapp/).
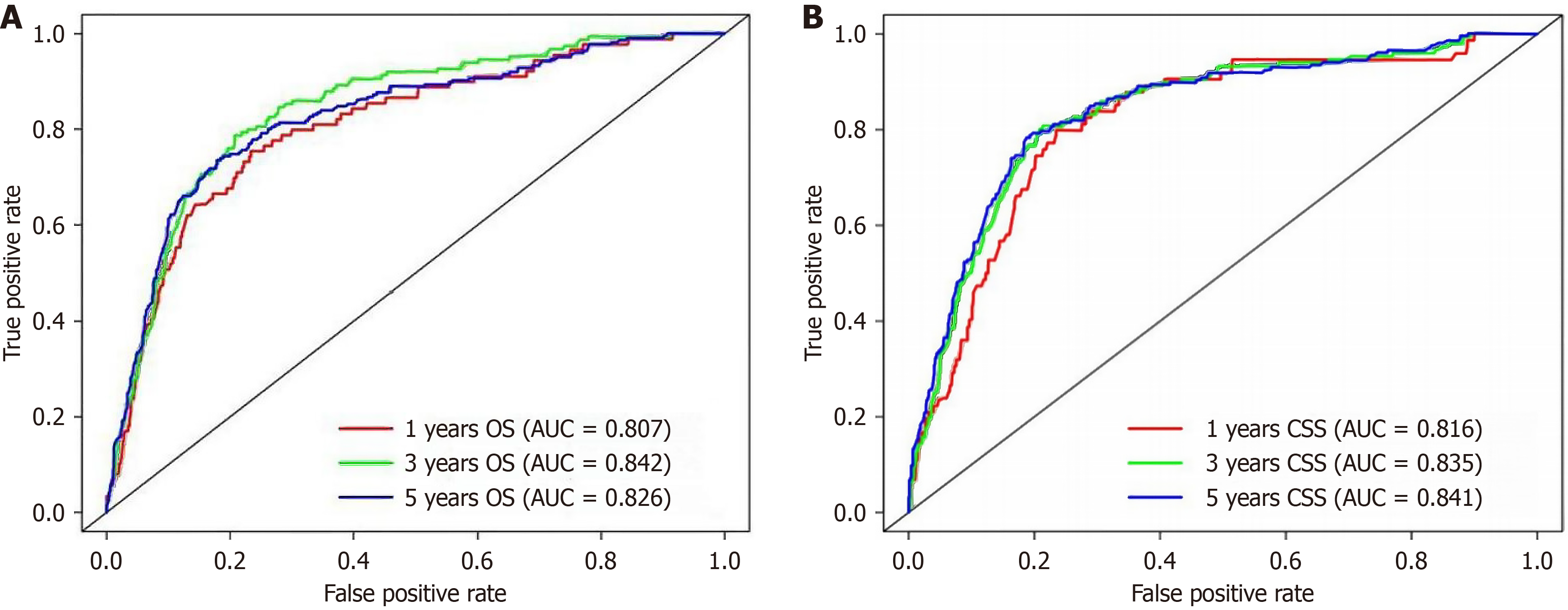
We plotted the AUC of the nomogram and TNM staging system, and the nomogram outperformed the TNM staging system. Figure 2 present the accuracy of the prognostic model for 1-, 3-, and 5-year OS and CSS in the SEER training cohort. The AUC values were 0.807, 0.842, and 0.826 for OS and 0.816, 0.835, and 0.841 for CSS, respectively. The C-indices for OS and CSS in the internal validation set were 0.778 and 0.736, respectively, and the areas under the OS and CSS curves were 0.805 and 0.747, respectively. The C-index for OS in the Chinese cohort was 0.790, and the AUC value was 0.747.
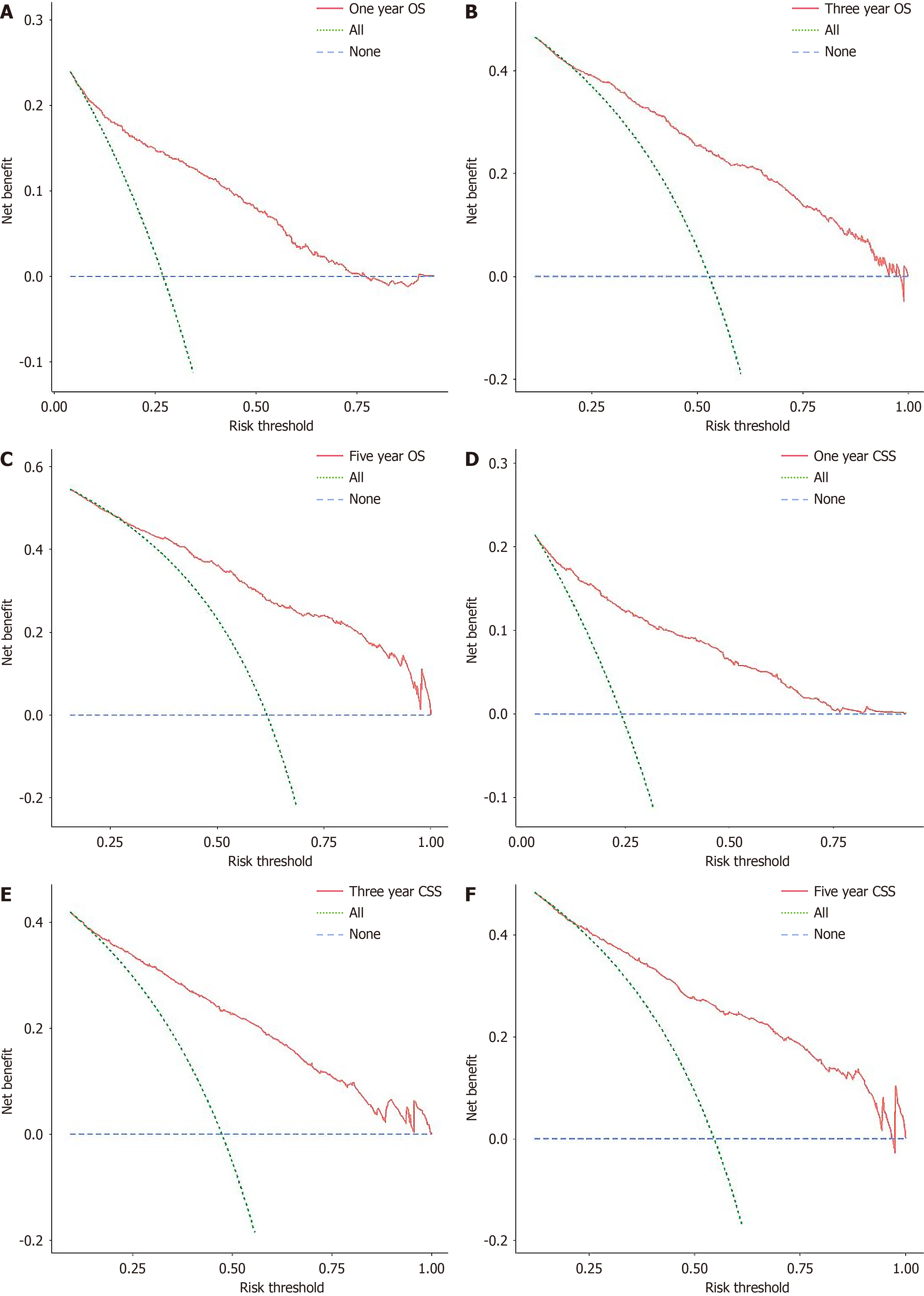
Whether within the training, internal validation, or external validation cohorts, the calibration curves for all cohorts showed predictions consistent with the actual observations (Figure 3), indicating good consistency of the nomogram. Furthermore, the decision curve analysis indicated higher clinical applicability of our nomogram via evaluation of the clinical applicability of our nomogram vs the conventional AJCC TNM staging system (Figure 4). ROC and decision curve analysis showed that the nomogram had robust calibration capabilities and clinical value in external validation. This benefit was further validated in the Chinese cohort, affirming the clinical utility of our nomogram.
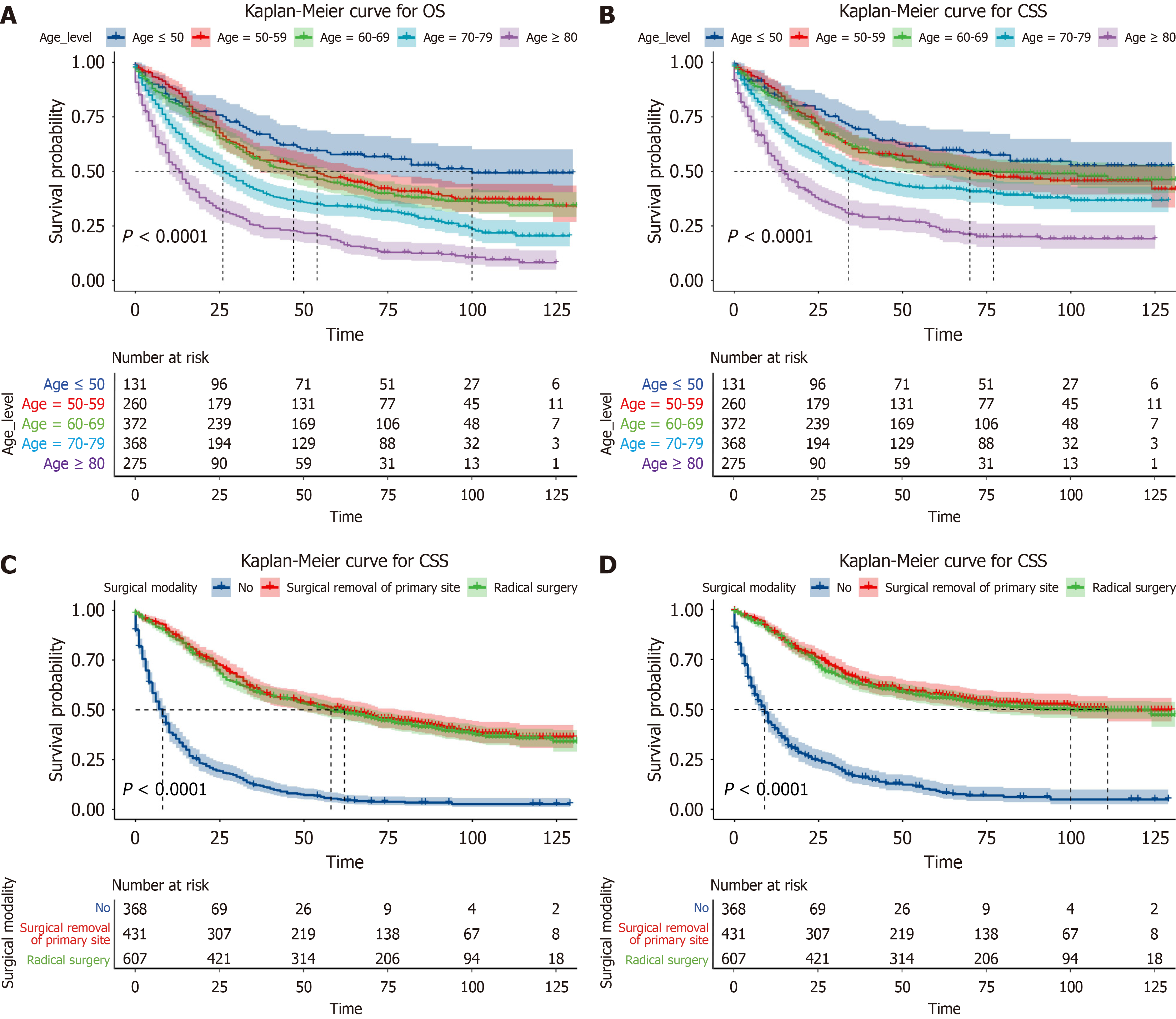
To better predict OS and CSS in patients with ampullary adenocarcinoma and assess the effects of age and surgical modality on OS and CSS, we analyzed Kaplan-Meier survival curves stratified by age and surgical modality. The entire cohort was categorized into low-risk and high-risk groups according to the median risk score calculated for all patients with ampullary adenocarcinoma using the established nomogram. Kaplan-Meier curves were plotted to compare the difference in survival between the two subgroups for further validation of the prognostic value of our nomogram (Figure 5).
The incidence and prevalence of ampullary adenocarcinoma are relatively low, and it accounts for 0.2% to 0.5% of malignant tumors of the gastrointestinal tract[8]. Owing to its rarity, relatively few studies have been conducted on this cancer type. In this study, we analyzed patients’ data from the SEER database and Chinese medical institutions to develop a nomogram based on nine factors, including patient sex, age, race, LNR, tumor size, surgical modality, T stage, tumor differentiation, and metastatic sites (brain and lung). This nomogram demonstrated excellent clinical utility and was superior to previously reported nomograms.
Risk scores increase gradually with age, especially in patients aged > 80 years who often experience organ dysfunction[9]. Kaplan-Meier analysis showed that the median survival of patients > 80 years was 15 months, with a 5-year survival rate of 25%, whereas the median survival of patients < 50 years was 90 months, with a 5-year survival rate of 60% (P < 0.0001). Large racial and ethnic discrepancies persist in cancer treatment and survival[10]. For most tumors, black patients tend to have lower 5-year relative survival rates than white patients[10]. Similar results were obtained in this study.
Similar to other studies[7,11-14], our nomogram incorporated surgery and tumor stage as the valuable indicators. Our study revealed that undergoing surgery and the surgical modality were independent risk factors for OS and CSS in patients with ampullary adenocarcinoma. This may be attributed to the early occurrence of suggestive symptoms; therefore, early detection, diagnosis, and treatment can achieve a better prognosis. LNR has been demonstrated to be negatively associated with long-term survival in many malignancies[15]. Similarly, the present study showed that LNR was a significant independent risk factor for OS and CSS in adenocarcinoma of the ampulla of Vater.
In contrast to other studies[7,14,16], our study found that chemotherapy was statistically significant in the univariate analysis, indicating that it can improve the prognosis of patients. This could be because systemic treatment can reduce the tumor burden and prolong the patient’s lifespan. Furthermore, tumor size and differentiation appeared to significantly affect patient survival. Our study also found that marital status at diagnosis was not an independent risk factor for OS and CSS and may have a minor influence on patient survival.
To apply this method, we developed two online predictive forecasting models. Clinicians can obtain the 1-, 3-, and 5-year OS and CSS quickly and easily by directly entering the patient’s clinical information. As the AJCC staging system does not incorporate significant predictive factors such as age and race, our nomogram compensates for the deficiency of the traditional TNM staging system in predicting the prognosis of tumors and improves the prediction accuracy of the model. Moreover, further validation of the predictive value of the model was performed using internal and external validation sets.
Our study has some limitations. Firstly, not all potential predictor variables were included in the nomogram. For example, increased carbohydrate antigen (CA) 199 has been reported to be a significant predictor of low survival after surgery in patients with ampullary adenocarcinoma[14,17]. However, the SEER cohort of patients with ampullary adenocarcinoma lacked laboratory-related data and did not incorporate important indicators such as CA199, which could not be included in the final model. Secondly, the SEER cohort consisted primarily of white and black populations, with fewer Asian populations, which somewhat limits the utility of the established nomogram for Asian populations. Furthermore, caution must be exercised when applying the nomogram to other populations, as it was validated based on a population from China only. Therefore, the next step may be to validate the nomogram in a different population. In the future, we will collect data from multiple medical institutions in China to develop a column chart suitable and optimized for the Chinese population.
This nomogram may be used to effectively predict survival in patients with ampullary adenocarcinoma, which may guide clinical decision making to help improve treatment outcomes and patient prognosis.
The authors are profoundly grateful to the patients who participated in the study.
| 1. | Walter D, Schnitzbauer AA, Schulze F, Trojan J. The Diagnosis and Treatment of Ampullary Carcinoma. Dtsch Arztebl Int. 2023;120:729-735. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Chiorean EG, Chiaro MD, Tempero MA, Malafa MP, Benson AB, Cardin DB, Christensen JA, Chung V, Czito B, Dillhoff M, Donahue TR, Dotan E, Fountzilas C, Glazer ES, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Masood A, Moravek C, Nakakura EK, Narang AK, Nardo L, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Truty MJ, Vollmer C, Wolff RA, Wolpin BM, Rn BM, Lubin S, Darlow SD. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:753-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Ramai D, Ofosu A, Singh J, John F, Reddy M, Adler DG. Demographics, tumor characteristics, treatment, and clinical outcomes of patients with ampullary cancer: a Surveillance, Epidemiology, and End Results (SEER) cohort study. Minerva Gastroenterol Dietol. 2019;65:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Patel MA, Kratz JD, Carlson AS, Ascencio YO, Kelley BS, LoConte NK. Molecular Targets and Therapies for Ampullary Cancer. J Natl Compr Canc Netw. 2024;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Zhou Y, Li D, Wu L, Si X. The histopathologic type predicts survival of patients with ampullary carcinoma after resection: A meta-analysis. Pancreatology. 2017;17:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Nie D, Yang J, Zheng H, Lai G, Wang F, Cao J, Gong C. Survival analysis and individualized prediction of survival benefit for pancreatic signet ring cell carcinoma: a population study based on the SEER database. BMC Gastroenterol. 2023;23:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Tang N, Chen ZY, Yang Z, Shang HZ, Shi GJ. Development and verification of prognostic nomogram for ampullary carcinoma based on the SEER database. Front Oncol. 2023;13:1197626. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Chen R, Zhu L, Zhang Y, Cui D, Chen R, Guo H, Peng L, Xiao C. Predicting the unpredictable: a robust nomogram for predicting recurrence in patients with ampullary carcinoma. BMC Cancer. 2024;24:212. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Burdett N, Vincent AD, O'Callaghan M, Kichenadasse G. Competing Risks in Older Patients With Cancer: A Systematic Review of Geriatric Oncology Trials. J Natl Cancer Inst. 2018;110:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 3074] [Article Influence: 512.3] [Reference Citation Analysis (0)] |
| 11. | Moekotte AL, Lof S, Van Roessel S, Fontana M, Dreyer S, Shablak A, Casciani F, Mavroeidis VK, Robinson S, Khalil K, Gradinariu G, Mowbray N, Al-Sarireh B, Fusai GK, Roberts K, White S, Soonawalla Z, Jamieson NB, Salvia R, Besselink MG, Abu Hilal M. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann Surg. 2020;272:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Li Y, Hua R, He J, Zhang H. Clinicopathological Features and Prognosis Analysis of Primary Bile Duct and Ampullary Neuroendocrine Neoplasms: A Population-Based Study from 1975 to 2016. Curr Oncol. 2022;30:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Moekotte AL, van Roessel S, Malleo G, Rajak R, Ecker BL, Fontana M, Han HS, Rabie M, Roberts KJ, Khalil K, White SA, Robinson S, Halimi A, Zarantonello L, Fusai GK, Gradinariu G, Alseidi A, Bonds M, Dreyer S, Jamieson NB, Mowbray N, Al-Sarireh B, Mavroeidis VK, Soonawalla Z, Napoli N, Boggi U, Kent TS, Fisher WE, Tang CN, Bolm L, House MG, Dillhoff ME, Behrman SW, Nakamura M, Ball CG, Berger AC, Christein JD, Zureikat AH, Salem RR, Vollmer CM, Salvia R, Besselink MG, Abu Hilal M; International Study Group on Ampullary Cancer (ISGACA) Collaborators, Aljarrah R, Barrows C, Cagigas MN, Lai ECH, Wellner U, Aversa J, Dickson PV, Ohtsuka T, Dixon E, Zheng R, Kowalski S, Freedman-Weiss M. Development and external validation of a prediction model for survival in patients with resected ampullary adenocarcinoma. Eur J Surg Oncol. 2020;46:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Klein F, Jacob D, Bahra M, Pelzer U, Puhl G, Krannich A, Andreou A, Gül S, Guckelberger O. Prognostic factors for long-term survival in patients with ampullary carcinoma: the results of a 15-year observation period after pancreaticoduodenectomy. HPB Surg. 2014;2014:970234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Hurtuk MG, Hughes C, Shoup M, Aranha GV. Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg. 2009;197:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ecker BL, Vollmer CM Jr, Behrman SW, Allegrini V, Aversa J, Ball CG, Barrows CE, Berger AC, Cagigas MN, Christein JD, Dixon E, Fisher WE, Freedman-Weiss M, Guzman-Pruneda F, Hollis RH, House MG, Kent TS, Kowalsky SJ, Malleo G, Salem RR, Salvia R, Schmidt CR, Seykora TF, Zheng R, Zureikat AH, Dickson PV. Role of Adjuvant Multimodality Therapy After Curative-Intent Resection of Ampullary Carcinoma. JAMA Surg. 2019;154:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Yoen H, Kim JH, Hur BY, Ahn SJ, Jeon SK, Choi SY, Lee KB, Han JK. Prediction of tumor recurrence and poor survival of ampullary adenocarcinoma using preoperative clinical and CT findings. Eur Radiol. 2021;31:2433-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |