Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1136
Revised: June 11, 2024
Accepted: July 29, 2024
Published online: September 24, 2024
Processing time: 245 Days and 11.5 Hours
Colorectal cancer (CRC) is the third most common cancer worldwide, and the second most common cause of cancer-related death. In 2020, the estimated number of deaths due to CRC was approximately 930000, accounting for 10% of all cancer deaths worldwide. Accordingly, there is a vast amount of ongoing research aiming to find new and improved treatment modalities for CRC that can potentially increase survival and decrease overall morbidity and mortality. Current management strategies for CRC include surgical procedures for resectable cases, and radiotherapy, chemotherapy, and immunotherapy, in addition to their combination, for non-resectable tumors. Despite these options, CRC remains incurable in 50% of cases. Nonetheless, significant improvements in research techniques have allowed for treatment approaches for CRC to be frequently updated, leading to the availability of new drugs and therapeutic strategies. This review summarizes the most recent therapeutic approaches for CRC, with special emphasis on new strategies that are currently being studied and have great potential to improve the prognosis and lifespan of patients with CRC.
Core Tip: As one of the most prevalent cancers worldwide, research efforts have focused on finding new treatment modalities for colorectal cancer (CRC), with higher efficiency and better overall survival rates. Current management options include surgery, chemotherapy, immunotherapy, radiation therapy, and targeted therapy. Despite all efforts, CRC is still highly incurable, necessitating additional research in this field. This review summarizes recent advances in therapeutic approaches targeting CRC.
- Citation: Fadlallah H, El Masri J, Fakhereddine H, Youssef J, Chemaly C, Doughan S, Abou-Kheir W. Colorectal cancer: Recent advances in management and treatment. World J Clin Oncol 2024; 15(9): 1136-1156
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1136.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1136
Colorectal cancer (CRC) is defined as any cancer that occurs in the last two parts of the large intestine: namely, the colon and rectum. CRC is the second leading cause of cancer-related death and the third most common malignancy worldwide[1]. It accounts for approximately 10% of all cancer cases, with most cases affecting older individuals aged 50 years and above[2]. According to previous studies, CRC cases will rise by 71.5% in males and by 60.0% in females by 2035[3]. This makes CRC a major public health issue as a result of the great economic burden it has on countries worldwide.
CRC is typically screened using fecal occult blood testing or by endoscopic screening (either sigmoidoscopy or colonoscopy). It is diagnosed using a combination of colonoscopy, biopsy, and imaging tests[4]. Other diagnostic procedures include the use of biomarkers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9, although their use is restricted to monitoring of disease progression rather than disease development[5].
Regarding treatment, surgical resection is considered the primary modality for resectable CRC, and standard therapies including chemotherapy, radiotherapy, and immunotherapy are utilized for non-resectable cases. Nevertheless, these therapies are not without disadvantages. Some drawbacks to these therapies include non-specificity and cytotoxicity to normal cells, leading to secondary complications.
Although considerable progress has been made regarding screening, diagnosis, and therapy for CRC, the prognosis remains poor and major challenges still exist. Biomarkers have recently been investigated for their usefulness in individualization of therapy, and were found to positively impact patient survival. However, no parameters are currently available that reveal continuing pathological processes without posing a risk to the patient. Moreover, there is still a need for parameters that are generally accessible, simple, and easy to perform. There is also a need for more sensitive and specific biomarkers that are useful in CRC diagnosis and are less expensive and invasive than current methods[6].
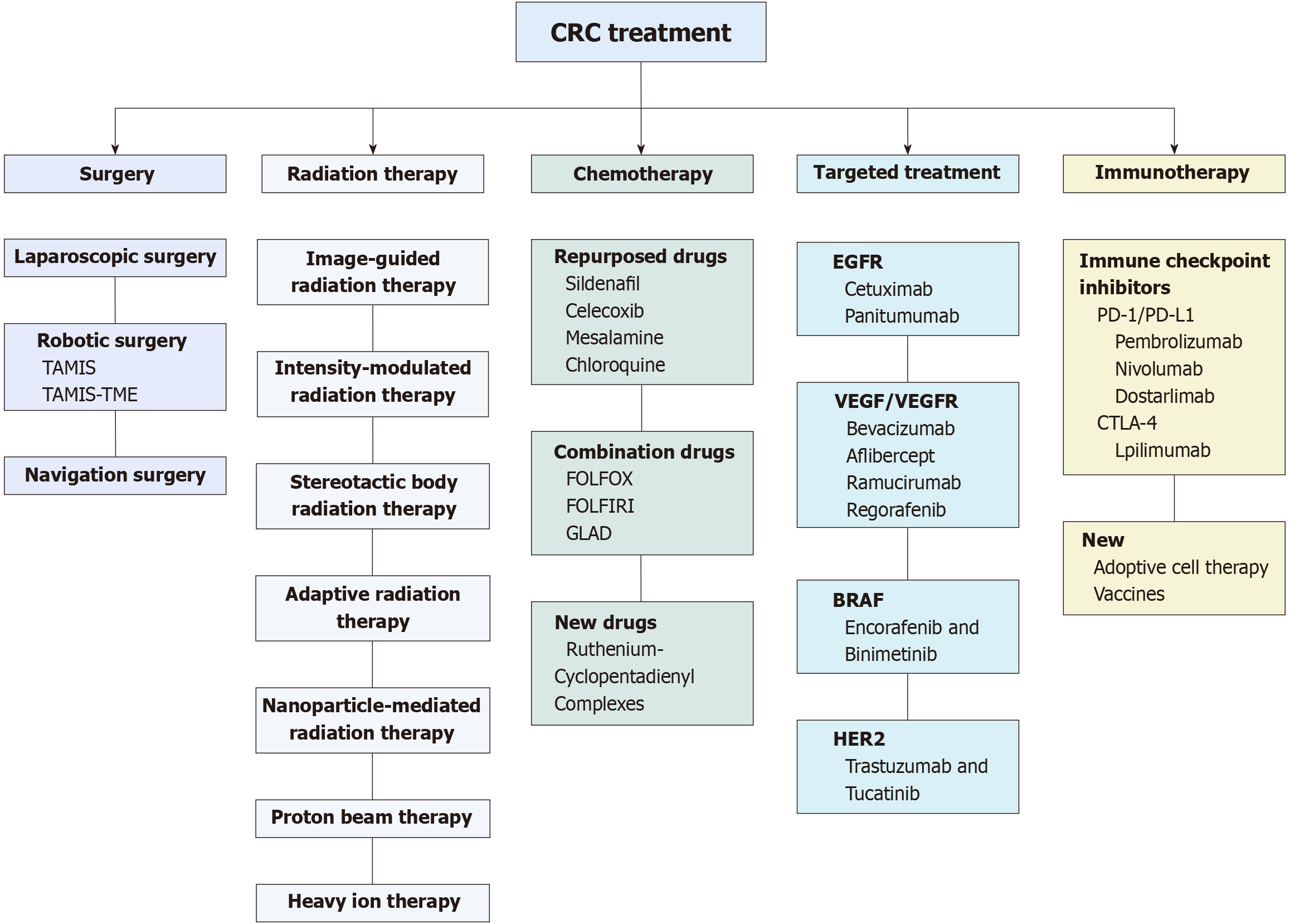
Current treatment strategies for early stage CRC involve endoscopic mucosal resection and endoscopic submucosal dissection. Considering the high incidence of lymph node metastasis, lymph node dissection is also an essential component of treatment for advanced-stage cancer. Recently, laparoscopic and robotic surgery have been used instead of conventional open surgery where possible. However, pertinent treatment models for rectal and transverse colon cancer need to be evaluated and validated. Moreover, complete cure of CRC requires multidisciplinary treatment[5]. In this review, we aimed to provide the status quo of CRC treatments and explore the latest developments and novel therapeutics to manage the disease. These treatments are summarized in Figure 1.
Although there has been rapid development in other treatment modalities, surgery is still the only method by which cure is achievable in advanced-stage CRC[7]. Thus, improving and optimizing surgical treatment modalities is imperative.
For localized resectable colon cancer, the American Society of Colon and Rectal surgeons practice guidelines for CRC treatment recommend colectomy as primary therapy[8]. Additional considerations include the extent of resection, which should correspond to the lymphovascular drainage of the site of colon cancer. Moreover, the lymphadenectomy should be complete and en bloc with the involved segment of bowel. Biopsy is indicated for clinically positive lymph nodes located outside the standard resection field, and in which there is suspicion of metastatic disease[8]. Involved adjacent organs should also be resected en bloc. Colectomy with en bloc removal of regional lymph nodes is also recommended by the National Comprehensive Cancer Network guidelines for the treatment of resectable, nonobstructing colon cancer. Moreover, neoadjuvant chemotherapy may be considered for clinical T4b disease.
Alternatively, in patients with rectal cancer, the ASCRS 2013 revised management guidelines recommend primary surgical treatment for patients with low-risk early stage cancer. Conversely, neoadjuvant radiation or chemoradiation may be indicated prior to surgery in the treatment of locally advanced or high-risk disease[9]. Herein, we discuss the recent advances pertaining to the various surgical techniques currently in use for CRC treatment.
Laparoscopic surgery for colon cancer: Laparoscopic surgery was first suggested as a possible alternative to open surgery for colonic and rectal lesions in the 1990s[10]. Previous studies reported several short-term benefits of laparoscopic surgery over open surgery, but no long-term benefits were established[11]. Following some concerns regarding the safety of laparoscopic surgery for CRC, it quickly became one of the main modalities for treatment, and is now routinely performed worldwide. Laparoscopy has been shown to be superior to open surgery in that it carries significantly lower costs and decreased risk of surgical site infection than open surgery[12]. However, a subset of patients may require cautious selection of the surgical method, as some patients have shown decreased survival with laparoscopic surgery. These patients include those with a high body mass index, a tumor size of ≥ 6 cm, and clinical N2 and T4 disease[13].
While laparoscopic surgery is safe and effective for CRC, its use for transverse colon cancer has been questioned due to anatomical challenges. The complex anatomy of the transverse colon and its surrounding structures, along with variations in vascular anatomy and the lack of standardized lymph node dissection guidelines, make transverse colon cancer surgery more demanding. However, studies have demonstrated the feasibility and benefits of laparoscopic surgery for transverse colon cancer. Meta-analyses have shown that laparoscopic transverse colectomy offers similar survival rates, quicker recovery, and shorter hospital stays than open surgery[14]. Additionally, a randomized controlled trial found that laparoscopic transverse colectomy had comparable short- and long-term outcomes to open surgery[15]. For mid-transverse colon cancer, both laparoscopic extended right hemicolectomy and transverse colectomy provide similar oncological outcomes[16].
Laparoscopic surgery in advanced rectal cancer: Although laparoscopic surgery has been shown to be safe and effective for rectal cancer, there is still some debate about whether it is as good as open surgery in terms of short-term oncologic outcomes. Several randomized and observational studies found that laparoscopic surgery yielded similar long-term oncologic outcomes to open resection, as well as comparable oncologic margins and lymph node harvest[17]. Two exceptions to this were the ACOSOG Z6051 and AlaCaRT randomized trials, which were not able to prove that the short-term oncologic outcomes of laparoscopic and open surgery were equivalent, even when the procedure was performed by surgeons with laparoscopic expertise[17]. Thus, caution should be exercised when making a decision regarding the surgical options for rectal cancer.
Robotic-assisted colon cancer surgery was first introduced in 2002. It offers several advantages over traditional laparoscopic surgery, including improved dexterity, precision, stereoscopic vision, and more precise lymph node dissection and intracorporeal anastomoses. Studies suggest that robotic-assisted colectomy may lead to better long-term survival for patients with stage I-III colon cancer compared to laparoscopic approaches[18]. However, robotic surgery has some disadvantages. It requires longer operating times, higher costs, and a steeper learning curve. Its role in colon cancer surgery is still being investigated.
In contrast, transanal minimally invasive surgery (TAMIS) and transanal total mesorectal excision (TaTME) have shown promise in improving outcomes for mid-rectal and distal rectal cancer. TAMIS is a new technique for rectal cancer surgery that is performed through the anus. This approach can be used to remove early stage rectal cancers without the need for an abdominal incision. However, it is mainly utilized in the setting of benign disease, specifically villous and tubulovillous adenomas in the lower and mid-rectum. TAMIS played a significant role as a stepping stone for TaTME, which is a critical surgical procedure for rectal cancer that involves removing the entire lymph node-containing tissue around the rectum. Since its establishment in 2010, numerous publications have revealed equivocal or superior results with TaTME when compared to standard or open laparoscopic surgery[19]. Nevertheless, TaTME has its disadvantages. These include a non-standardized operative technique, risk of contamination of the surrounding space and the peritoneal cavity with bacteria or malignant cells, risk of urethral injury, anastomotic leaks, bowel injuries, urinary dysfunction, and bleeding[20]. TAMIS can be used to perform TME, which can improve outcomes for patients with rectal cancer. TAMIS-TME offers a significant advantage over traditional transanal rectal cancer surgery methods by providing superior visibility of the lower mesorectum during the procedure[21]. This enhanced visualization enables a more precise and thorough resection of the tumor, leading to improved specimen quality and a reduced risk of adverse complications. Both TAMIS and TAMIS-TME are minimally invasive CRC surgery procedures that do not require an abdominal incision. These procedures offer several advantages over traditional open surgery, including shorter hospital stays, less pain, and a quicker recovery[21]. A meta-analysis suggests that robotic-assisted surgery for rectal cancer may have better therapeutic effects and similar oncological outcomes compared to laparoscopic surgery. Additionally, a prospective study indicates that robotic surgery may offer advantages in protecting pelvic autonomic nerves and reducing postoperative urinary and sexual dysfunction[22].
Navigation technology has greatly enhanced the safety and accuracy of colorectal surgery. Injecting fluorescence and indocyanine green (ICG) into the artery allows for real-time visualization of blood flow in the colon and rectum, which can be used to plan reconstructions and assess the perfusion of a colorectal anastomosis[23]. Artificial intelligence (AI)-based real-time microcirculation analysis has been shown to be more accurate than conventional parameter-based methods in assessing perfusion.
ICG is also used for lymph node mapping. Sentinel lymph node mapping is a technique used to identify the first to fourth lymph nodes in the lymphatic pathway, which are the most likely to harbor micrometastases[24]. However, the fluorescent signal only indicates lymph node presence; therefore, tumor-specific targeting by CEA-targeted fluorescent imaging using SGM-101 is expected to be a tool to detect the presence of lymph node metastasis[25].
A complete understanding of the anatomy of blood vessels is vital for lymph node dissection and vascular resection for the treatment of colon cancer[26]. Three-dimensional computed tomographic angiography with computed tomographic colonography may contribute to laparoscopic colorectal surgery by providing surgeons with reliable preoperative vascular anatomy. Preoperative information on vessel anatomy can help manipulate the blood vessels, prevent intraoperative vascular injury, and determine the appropriate extent of lymph node dissection[26].
A crucial component in the treatment of CRC is radiation therapy (RT). The use of high energy X-rays or gamma rays to halt the growth of tumors has been shown to be effective in local control and improving quality of life. However, the side effects, local recurrence, and metastasis associated with RT remain a concern. This prompts the search for technological advancement aiming at reducing such adverse events and improving patient quality of life[27,28].
According to the American Society for Radiation Oncology Clinical Practice Guidelines, neoadjuvant RT is reco
In a study carried out on patients with locally advanced stage 4 CRC patients undergoing curative surgery, local recurrence was shown in 14% of patients who did not receive RT, while no recurrence was observed in those who received neoadjuvant radiotherapy[36]. Preoperative RT was shown to be more beneficial and less toxic than post-op RT[37].
Patients with metastatic CRC (mCRC) are usually treated with chemotherapy alone, aimed at symptom management and improving quality of life[38,39]. Multimodal therapy, including surgery, ablation, and RT, was associated with an increment in survival; those who received RT had a statistically significant increase in median overall survival compared to those who did not, and were more likely to have their disease controlled and less likely to experience disease progression[40].
Image-guided RT (IGRT) is an advanced medical technique that involves the use of high-resolution imaging, such as computed tomography (CT) scans, X-rays, cone beam CT (CBCT), or magnetic resonance imaging (MRI), to precisely locate and target tumors within the body; with CBRT being the preferred imaging modality in rectal cancer. Unlike conventional radiotherapy, which relies on external landmarks for targeting, IGRT allows for real-time monitoring and adjustments, ensuring that the RT is delivered with pinpoint accuracy[41]. This not only maximizes the destruction of cancerous cells, but also minimizes the exposure of nearby healthy tissues to radiation. IGRT is often used in tumors that are prone to movement, much like the rectum and mesorectum, and require accurate target volume delineation[42]. IGRT is useful in patients receiving intensity-modulated RT (IMRT)[43], and specifically, stereotactic body RT (SBRT)[44], to ensure the radiation beams accurately target the tumor.
IMRT is an advanced radiation treatment method in oncology. It utilizes cutting-edge technology to precisely deliver radiation to tumors, optimizing the balance between tumor eradication and the protection of healthy tissues. IMRT achieves this precision by dynamically adjusting the intensity and shape of radiation beams, tailoring the treatment to match the tumor’s unique contours by using various imaging techniques such as CT, MRI, and positron emission tomography scans[45]. In contrast to conventional RT, which uses uniform doses, IMRT creates a highly precise, three-dimensional dose distribution. This personalized approach enhances the therapeutic impact on cancer cells while minimizing radiation exposure to adjacent healthy tissues and, therefore, has the potential to reduce RT associated side effects on bladder, large bowel, and small bowel[29].
SBRT is an innovative and highly precise radiation treatment modality used in the field of radiation oncology, distinguished by its remarkable accuracy in delivering high doses of radiation to tumor sites while minimizing exposure to nearby healthy tissues[46]. This precision is achieved through sophisticated technology and image-guidance systems that allow clinicians to precisely locate the tumor in three-dimensional space[47]. SBRT has revolutionized the approach to RT, ushering in a new era of non-invasive, high-impact cancer treatment. SBRT is not routinely recommended for the treatment of rectal cancer, but may be considered for those with early stage rectal cancer, who do not qualify for surgery, or who have recurrent rectal cancer[29].
Adaptive RT: Adaptive RT (ART) is a type of RT that uses imaging data to adjust the radiation treatment plan through
AI, specifically deep learning models, has been implicated in radiation oncology, especially ART. Various aspects of adaptive radiotherapy, like contouring, registration, planning, quality assurance, and decision-making, can benefit from AI algorithms. AI-based auto-segmentation algorithms are proving to be more accurate than earlier methods. The use of AI has the potential to turn once thought hard to achieve ideas into real possibilities, like targeting tissue in real-time, quickly adjusting patient plans, and calculating doses in real-time. This technology opens up the opportunity to explore new adaptive radiotherapy strategies that were never considered before[49]. The advantages of ART were shown in the case of a 76-year-old man with locally advanced sigmoid colon cancer as it addressed the challenges posed by tumor movement and organ motion. The MRIdian system enabled daily monitoring of tumor location and real-time adjustments to the treatment plan. This ensured that the tumor received the prescribed dose while minimizing exposure to the surrounding organs. As a result, there was a significant reduction in tumor volume without causing any side effects, along with a reduction of tumor volume by 62%. The successful implementation of ART in this case demonstrates its potential to improve treatment outcomes for patients with CRC[50].
Nanoparticle-mediated RT: This technique uses nanoparticles to deliver radiation directly to the tumor. The nano
Proton beam therapy: Proton beam therapy (PBT) uses positively charged particles to deliver radiation to the tumor. Protons have the advantage of being able to deposit their energy more precisely in the tumor than traditional X-ray radiation. This can help to reduce the dose to surrounding healthy tissues. PBT is already in clinical use for CRC, but it is not yet widely available[54]. PBT is gaining attention in cancer treatment due to its superior dosimetric parameters. Its potential benefits include patient survival by enhancing local tumor treatment rates while minimizing radiation-induced effects on normal organs[55]. The challenges posed by the proximity of gastrointestinal cancers to radiosensitive organs highlight the need for more targeted and tolerable therapies. PBT’s unique physical and dosimetric characteristics offer a promising path to treat both primary and recurrent CRC. The application of advanced delivery techniques such as volumetric modulated arc therapy and IMRT further refine the precision of dose delivery, stabilizing local control rates while reducing complications. The potential to spare organs at risk, such as the bone marrow, small and large bowel, and skin, presents an opportunity to improve the side-effect profile. Moreover, PBT holds the promise of enhancing patient compliance, minimizing treatment breaks, and enabling dose escalation or hypofractionation, which means mitigating long-term morbidity[52]. A study focused on PBT’s application for local recurrence of rectal cancer. Results from a retrospective study of 12 patients show encouraging 3-year local control rates (80.2%), progression-free survival (PFS) (12.1%), and overall survival (71.3%) without severe events. This highlights PBT’s potential as a safe and effective treatment for challenging cases of recurrent rectal cancer[56].
Heavy ion therapy: Heavy ion therapy (HIT) uses heavy ions, such as carbon ions, to deliver radiation to the tumor. Heavy ions have the advantage of being more effective at killing cancer cells than traditional X-ray radiation or protons. This is because heavy ions deposit their energy more densely in the tumor. HIT is still in the early stages of development for CRC, but has the potential to significantly improve the efficacy of RT[57]. Unlike traditional photon methods, carbon-ion radiotherapy (CIRT) offers unique advantages such as being able to deliver substantial doses to tumors with minimal damage to the surrounding tissues. CIRT, specifically effective in radio-resistant cancers like CRCs, induces DNA damage more effectively than protons or photons. Based on this study, there are promising results in treating local recurrent rectal cancer, lung metastasis, liver metastasis, and lymph node metastasis[58].
The concept of the abscopal effect is characterized by a phenomenon involving the remission of distant, unirradiated tumors following local irradiation. The abscopal effect has been traditionally associated with conventional photon irradiation, particle irradiation, particularly heavy ion irradiation like carbon ions, and offers unique dose distributive benefits. Two case reports illustrate the abscopal effect response following carbon-ion irradiation for metastatic recurrent CRC. These cases show a disease remission of 46 months in one patient and remission/stable disease of 92 months in another, providing evidence of the potential efficacy of HIT in eliciting systemic responses. Digging deeper into the details of the case reports, the first patient with recurrent CRC experienced a reduction in the size of the non-irradiated tumor 1 month post-irradiation, preceding the administration of chemotherapy. The second patient, following carbon-ion irradiation for isolated para-aortic lymph node recurrences, showed a noticeable reduction in the treated lymph node and untreated right subclavian node, with stable mediastinal nodes. Both patients demonstrated abscopal effects downstream of the treated site, highlighting the potential of HIT to induce systemic responses[59].
HIT, especially CIRT, highlights precision and unique benefits, presenting a promising avenue to treat challenging cancers like CRCs and inducing systemic responses.
Current chemotherapy regimens in CRC consist mainly of fluoropyrimidine (5-FU, or capecitabine) combined with oxaliplatin and/or irinotecan and capecitabine. Adding leucovorin to this combination is also recommended, depending on the patient and the treatment regimen chosen[60-62]. The drug regimen used depends on several factors such as the disease stage (adjuvant chemotherapy is not recommended in stage 0 and 1), availability of drugs, and the patient’s tolerability of chemotherapy[60]. Bevacizumab, a monoclonal antibody (mAb), is currently used as first-line treatment along with chemotherapy[62-64]. The American Society of Clinical Oncologists’ guidelines recommend against routine use of adjuvant therapy in patients with stage II colon cancer with low recurrence risk. However, adjuvant therapy should be offered to patients with stage IIB and stage IIC colon cancer. It may also be offered to patients with stage IIA colon cancer with high-risk features[65]. The significance of adjuvant therapy in the treatment of stage II colon cancer is debatable, but is still considered in patients with high-risk disease[65].
Repurposing of drugs: This compilation features repurposed drugs that have successfully undergone clinical trials, substantiating their safety and efficacy in treating CRC. The inclusion of these drugs underscores their proven performance within the structured and controlled environment of clinical studies and thus have passed the in vitro/in vivo phases. It is important to note, however, that the selection presented here represents only a fraction of the potential candidates within the realm of repurposed drugs.
Sildenafil: Sildenafil had several uses such as treating hypertension and erectile dysfunction, and continues to prove its efficacy in other fields such as cancer. In fact, this PDE5 inhibitor is a potent chemotherapeutic drug that exhibits varying effects depending on the specific drug it is combined with. For instance, with exisulind, it can induce apoptosis[66]: PDE5 inhibitors inhibit the degradation of cyclic guanosine 3', 5'-monophosphate and elevate its levels, restrain the progression of intestinal cancer, and promote the functionality of the epithelial barrier[67].
On the other hand, sildenafil with curcumin increases the efficacy of the first-line treatment 5-FU by modifying the expression of multiple proteins, such as BCL-XL, and thus changes the levels of reactive oxygen species (ROS), which induces apoptosis; thus sildenafil can be used as an antitumor agent[68,69].
Celecoxib: Other drugs such as celecoxib were also repurposed to be used for CRC. In fact, celecoxib is a non-steroidal anti-inflammatory drug (NSAID) used for pain and inflammation, but is now also considered to reduce the occurrence of colorectal adenomas after polypectomy[70]. Moreover, when combined with cetuximab in CRC, it improves its antitumor effect. The combination of celecoxib enhances the effectiveness of cetuximab in treating CRC by disrupting the epidermal growth factor receptor (EGFR)-RAS-FOXM1-β-catenin signaling pathway[71-73].
Mesalamine: Mesalamine, also used as an NSAID, was repurposed as a drug to treat CRC. In fact, without being combined, it encourages the anchorage-independent CRC cell death[74-76]. Also known as 5-Aminosalicylic acid, a new study showed the ability of this drug to inhibit COX enzymes through pathways independent of COX. Moreover, it plays an inhibitory influence on colon cancer stem cells responsible for cancer recurrence[77].
Chloroquine: Chloroquine is an antimalarial drug repurposed to be used for the treatment of CRC[78]. In fact, some studies show that the resistance to the 5-FU treatment may be resolved using chloroquine, by the inhibition of autophagy, which enhances sensitivity to other drugs and provides a better tumor control[79-82].
Combination drugs: In fact, 5-FU was the first-line chemotherapy effective treatment used for metastatic colon cancer. Other drugs, such as Cetuximab and Leucovorin, are also known for their efficacy toward CRC. New regimens include the combination of several drugs for a better outcome, such as the combination of Leucovorin with 5-FU and irinotecan, fluorouracil, and leucovorin (FOLFIRI) or oxaliplatin (FOLFOX). 5-FU and Leucovorin as a combination improve progression-free results by 20% and advanced survival rates by 6%. This combination is now used as a first and second-line treatment option for CRC stage II and III patients[83-85].
However, while comparing 5-FU and Leucovorin combinations with 5-FU and oxaliplatin, it was demonstrated that the second regimen causes 41.7% of neutropenia, 11.9% of diarrhea, and 18.2% of toxicity, compared to 5.3%, 5.3%, and 0%, respectively, in the first combination[86]. Another combination of drugs, called GLAD (gefitinib, licofelone, atorvastatin), and α-difluoromethylornithine has been proven to be effective toward stopping the metastasis of CRC. In fact, it works by activating the autophagy signaling pathways and changing the expression of inflammatory cytokines. Moreover, it was tested to be used as a preventive approach with very low doses by suppressing tumorigenesis with minimal undesirable side effects[87].
Ruthenium-cyclopentadienyl complexes: New emerging drugs are being tested for a potential therapeutic effect, such as Ruthenium-cyclopentadienyl complex. This metallodrug has a high selectivity to cancer cells, and has been shown to promote cell cycle arrest and diminish the ability of cells to clone. Some leads (PMC79, LCR220, and LCR134) have also demonstrated apoptotic characteristics and inhibition of cellular motility through ROS production and mitochondrial dysfunction. Moreover, characteristics such as inducing actin cytoskeleton changes are considered important factors in the treatment of CRC. The goal in new therapies is to target only cancerous cells. Ru compounds (those containing a transition metal element named ruthenium), in contrast to cisplatin used in current regimens, target the mitochondria and not the DNA, and eliminate cancerous cells with no effect on normal cells[88-90].
Targeted therapy is a type of cancer treatment that targets specific biological molecules to stop tumor cell growth and proliferation[91,92]. Thus, by understanding the principal pathways involved in the development and spread of CRC, it is possible to design drugs that would, in theory, selectively kill these tumor cells. Targeted therapy relies on the usage of small molecule drugs that can easily enter cells and monoclonal antibodies[92]. When it comes to CRC, several targeted therapy drugs have been developed and received Food and Drug Administration (FDA) approval since 2004.
The EGFR is a transmembrane receptor tyrosine kinase member of the ErbB family[93]. Upon binding of its ligand, the EGFR homodimerizes or heterodimerizes with other members of the ErbB receptor tyrosine kinases, leading to the activation of downstream pathways, including the RAS-RAF-MAPK and the PI3K-Akt pathways[94,95]. Mutations affecting the EGFR and its downstream signaling cascade have been shown to play a pivotal role in many different cancers, including CRC. In fact, these mutations lead to abnormal cell proliferation, resistance to apoptosis, and invasion[94,95].
Cetuximab: Cetuximab was the first mAb to be FDA approved in 2004 for the treatment of mCRC following the BOND trial[96]. It is a chimeric immunoglobulin G (IgG) mAb that targets the extracellular domain of EGFR, competitively inhibiting the binding of EGF. Cetuximab thus prevents downstream phosphorylation and activation of kinases, leading to decreased cell proliferation and migration, as well as induction of apoptosis[97]. At the time of the BOND study, patients with mCRC in whom treatment with the combination of irinotecan, oxaliplatin, and fluoropyrimidine failed, had no other treatment options. The use of cetuximab/irinotecan dual therapy in these patients showed promising results, with a 22.9% overall response rate[96]. Later, the CRYSTAL III trial showed that the combination of cetuximab with FOLFIRI decreased the risk of progression of the disease by 15% in patients with mCRC compared to FOLFIRI therapy alone [hazard ratio (HR) = 0.85; P = 0.048][98]. Subgroup analysis showed that KRAS mutant patients did not significantly benefit from cetuximab/FOLFIRI therapy compared to FOLFIRI alone [median overall survival (OS): 16.2 months vs 16.7 months], whereas KRAS wild-type patients showed a significant improvement in overall survival with cetuximab/FOLFIRI therapy (median OS: 23.5 months vs 20 months)[98,99]. Interestingly, the use of cetuximab in addition to the combination of folinic acid, FOLFOX, compared to FOLFOX therapy alone did not show any added benefit in term of overall survival or PFS, independent of the KRAS mutation status[100]. Current evidence recommends the use of cetuximab only in patients with no detected KRAS mutations[101]. Furthermore, the American Society of Clinical Oncology (ASCO) recommends the use of cetuximab along with encorafenib for patients with BRAF-V600E mutant mCRC that have progressed despite a previous line of therapy[102]. Common adverse side effects that follow the use of cetuximab include an acneiform rash in more than 75% of patients, in addition to electrolyte imbalance, nausea, malaise, and weakness[103].
Panitumumab: Panitumumab is a humanized mAb that was developed to reduce the hypersensitivity reaction caused by the chimeric mAb cetuximab, while maintaining therapeutic effects for mCRC patients[104]. Panitumumab exerts its therapeutic action by inhibiting the binding of EGFR to its ligands, thus leading to inhibition of proliferation and induction of apoptosis, as well as inhibition of angiogenesis. Additionally, it was shown to trigger antibody-mediated cellular cytotoxicity by myeloid lineage immune cells, which might also explain its in vivo effects[104]. The PRIME trial demonstrated that use of panitumumab in conjunction with FOLFOX resulted in higher median PFS compared to FOLFOX alone in wild-type KRAS patients with mCRC (PFS: 9.6 months vs 8 months; P = 0.02). In contrast, no significant effect was reported for mutant KRAS patients[105]. A randomized phase III study later compared the use of panitumumab along with best supportive care (BSC) vs BSC alone, showing improved overall survival (HR = 0.72; P = 0.015) and PFS (HR = 0.45; P < 0.0001) with the first arm. This study further validated RAS as a predictor of response to anti-EGFR therapy[106]. Furthermore, a recent meta-analysis highlighted a differential response to treatment regarding tumor side, strongly recommending the use of anti-EGFR mAb for left sided tumors, and bevacizumab [anti-vascular endothelial growth factor (VEGF)] for right-sided tumors[107]. Finally, both cetuximab and panitumumab displayed no significant difference in terms of overall survival, PFS, and response rate in the treatment of wild-type KRAS mCRC patients. This finding further extends to adverse effects, with both anti-EGFR agents causing similar acneiform rashes and diarrhea. However, panitumumab was shown to have a reduced incidence of paronychia, while cetuximab had a decreased risk of hypomagnesemia[108].
Fruquintinib: Fruquintinib is an oral tyrosine kinase inhibitor that targets all three subtypes of VEGF receptor (VEGFR)[109]. It was FDA approved in November 2023 for the treatment of mCRC following the results of the FRESCO-2 phase III trial. This study included patients with medically documented mCRC that have been treated with current standard therapy. Patients treated with fruquintinib had a higher median overall survival rate compared to matched placebo plus BSC patients (mOS: 7.4 months vs 4.8 months; HR = 0.66; P < 0.0001). Common side effects encountered with fruquintinib included hypotension and asthenia[110].
The VEGF refers to a family of five proteins (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor) that bind with different affinities to three different VEGF receptors (VEGFR1, VEGFR2, and VEGFR3), mediating angiogenesis and lymphangionesis[111]. The interaction of VEGF with VEGFR leads to downstream activation of RAS-RAF-MAPK, PI3K-Akt, and PLC-PKC pathways. Angiogenesis is an essential component of tumor growth and metastasis; thus, targeting this pathway seems very promising in targeting mCRC. Interestingly, it was recently shown that VEGF mainly acts in an intracrine manner in mCRC, with little involvement of paracrine and autocrine signaling, shifting the paradigm to targeting intracellular VEGF with siRNA[112,113].
Bevacizumab: Bevacizumab is a humanized mAB that binds to all VEGF-A isoforms, resulting in regression of newly formed vessels and inhibition of angiogenesis. It also has a direct cytotoxic effect on tumor cells[114]. Bevacizumab was approved by the FDA approved in 2004 for the treatment of metastatic cancer. This came after a phase III trial demonstrated that the combination of bevacizumab with irinotecan, fluorouracil, and leucovorin (IFL) compared to IFL alone yielded a higher overall survival (median OS: 20.3 months vs 15.6 months; P < 0.001) and longer PFS (PFS: 10.6 months vs 6.2 months; P < 0.001) in mCRC patients[115]. A recent systematic review compared the efficacy and side effects of chemotherapy associated with anti-EGFR (cetuximab or panitumumab) vs bevacizumab in the treatment of wild-type KRAS mCRC. Anti-EGFR therapy was characterized by a prolonged overall survival (HR = 0.83; P = 0.003), and higher overall response rate [risk ratio (RR) = 1.11; P = 0.0003], but higher incidence of adverse events (RR = 1.12; P < 0.0001) compared to bevacizumab when both were used in conjunction to first-line chemotherapy[116]. Another sys
Aflibercept: Aflibercept is a fusion recombinant protein that consists of the extracellular domains of VEGFR1 and VEGFR2 combined to the Fc portion of human IgG1, acting as a decoy receptor[118]. It was FDA approved in 2012 as a second-line therapy for mCRC, after the VELOUR trial showed an improved overall survival of FOLFIRI with aflibercept compared to FOLFIRI alone (HR = 0.857; P = 0.0032)[118]. However, it should be noted that aflibercept is associated with high incidence side effects such as hypertension, venous thromboembolic events, diarrhea, and neutropenia[119].
Ramucirumab: Ramucirumab is a fully human mAb that targets the VEGFR-2 protein with high affinity, effectively blocking the binding of VEGF ligands and preventing receptor activation[120]. It is used as second-line therapy for the treatment of mCRC. Indeed, the phase III RAISE trial demonstrated that patients treated with FOLFIRI combined with ramucirumab, as compared to FOLFIRI alone, had a longer overall survival (HR = 0.844; P = 0.0219)[120]. Common adverse effects associated with ramucirumab include neutropenia, hypertension, diarrhea, and fatigue[120].
Regorafenib: Regorafenib is a small molecule multitargeted tyrosine kinase inhibitor that blocks signaling through VEGFR1, VEGFR2, VEGFR3, RET, c-KIT, PDGFR, FGFR1, and TIE2[121]. It is an agent administered orally in the treatment of mCRC after second-line therapy[122]. This small molecule inhibits angiogenesis, and limits tumor oncogenesis and metastasis[122]. The phase III clinical trial CORRECT showed that the combination of BSC with regorafenib resulted in a longer median overall survival (HR = 0.77; P = 0.0052) as compared to BSC alone in patients with mCRC. A recent systematic review that performed indirect pairwise comparisons of regorafenib with other therapeutic agents concluded that regorafenib is superior to aflibercept, panitumumab, and ramucirumab in term of PFS, with results comparable to cetuximab and bevacizumab[121].
BRAF is a serine/threonine kinase that plays an integral role in the activation of the RAF/MEK/ERK signaling cascade for cell proliferation. It is estimated that 8%-12% of CRCs harbor the BRAF-V600E mutation[123].
Encorafenib and Binimetinib: Encorafenib is a BRAF inhibitor characterized by a prolonged activity compared to previous generations of BRAF inhibitors; on the other hand, binimetinib is a MEK inhibitor[124]. The phase III trial BEACON compared the effect of the triple therapy (encorafenib/binimetinib/cetuximab), double therapy (encorafenib/cetuximab), and controls (chemotherapy/cetuximab) in patients with mCRC with the BRAF-V600E mutation with disease progression after one or two prior line treatments. The study showed a superior median overall survival for both triplet and doublet therapy as compared to controls [mOS: 9.3 months vs 5.9 months; HR = 0.60, 95% confidence interval (CI): 0.47-0.75][124]. Thus, the combination of cetuximab and encorafenib (FDA approved in 2020) is recommended by ASCO to be given to patients with BRAF-V600E mCRC that has progressed despite at least one line of prior treatment[102].
Human EGFR-2 (HER2) belongs to the family of ErbB tyrosine kinase receptors. When HER2 is overexpressed, it tends to heterodimerize with other ErbB family members. Consequently, tyrosine residues within the cytoplasmic domain of the heterodimer undergo autophosphorylation. This initiates a cascade of signaling pathways that drive cellular proliferation and play a role in the development of tumors[125]. It is estimated that approximately 5% of patients with mCRC harbor a HER2 mutation[126].
Trastuzumab and tucatinib: Trastuzumab is a recombinant humanized mAb that targets the extracellular domain of HER2[127]. Similarly, Tucatinib is an oral small molecule tyrosine kinase inhibitor that is selective for HER2[128]. The combination of tucatinib plus trastuzumab recently received an accelerated FDA approval (January 2023) for the treatment of HER2 + mCRC that has failed to respond to irinotecan, oxaliplatin, and fluoropyrimidine[126]. Indeed, the phase II MOUNTAINEER trial indicated that HER2 + mCRC treated with tucatinib and trastuzumab had an objective response rate of 38.2% (95%CI: 27.7-49.3) and a median duration of response of 12.4 months (95%CI: 8.3-25.5)[128].
Cancer immunotherapy is an approach that boosts the host immune system to attack cancer cells without any toxic effects on normal cells[129]. Immunotherapy alone or in combination with conventional treatments has achieved considerable success as a standard treatment in several cancers[130]. Lately, several immune treatment methods have been designed and therapeutically used to overcome immune evasion by tumor cells, such as immune checkpoint (IC) inhibition, adoptive cell transfer (ACT), cytokine therapy, and dendritic cell vaccines[131].
ICs are ligand–receptor complexes that lead to a co-stimulatory or inhibitory signal during the immune response[132]. Dysregulation of the IC is an important mechanism by which the tumor escapes the immune response. For instance, some of the inhibitory checkpoints are overexpressed within the tumor microenvironment (TME), causing downregulation of the immune response[133]. Therefore, inhibitory IC molecules can be blocked to trigger immunity against the tumor[134]. Programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) are the more extensively studied IC molecules in patients with several types of cancer, including CRC, proving the reactivation of T cells by ICIs to be therapeutically effective[135].
PD-1 is an inhibitory IC receptor belonging to the immunoglobulin superfamily expressed on different immune cells such as activated T cells, NK cells, and dendritic cells[136]. PD-1 binds to PD-L1 present in TME and stimulates Treg cell signaling, which together lead to the suppression of the immune response[137]. Therefore, blocking of the PD-1/PD-L1 pathway is used to boost the activity of the immune system against tumor cells[138]. Until recently, only three PD-1 inhibitors were approved for the treatment of CRC [Pembrolizumab (Keytruda), nivolumab (Opdivo), and dostarlimab (Jemperli)][139].
Pembrolizumab: Pembrolizumab is a mAb against PD-1 on the surface of T cells. It was approved by the FDA on May 23, 2017, for those who have been treated prior, and on June 29, 2020, for patients with unresectable or metastatic high microsatellite instability (MSI-H)/deficient mismatch-repair (dMMR) CRC not treated systemically before[140]. Compared to chemotherapy, pembrolizumab showed durable antitumor activity, with no significant difference in overall survival; however, higher levels of safety were shown (diarrhea, fatigue/asthenia, and nausea were the most prevalent adverse effects). Therefore, pembrolizumab can be considered as a first-line treatment for MSI-H/dMMR mCRC[141].
Nivolumab: Nivolumab is a humanized monoclonal anti-PD-1 immunoglobulin G4 that promotes T-cell proliferation and cytokine production by blocking the binding of PD-L1 to PD-1[142]. It was approved for the treatment of dMMR/MSI-H mCRC in July 2017; however, it had disappointing results when used broadly[143]. No difference in response to nivolumab was observed between tumors expressing more or less than 1% PD-L1[144]. This drug is generally considered safe, as the most common adverse events are rash and pruritus. However, clinically important adverse events occur in low frequency (< 10%), including ventricular arrhythmia, neuropathy, iridocyclitis, and infusion-related reactions[144].
The combination of nivolumab with other treatments, such as chemotherapy, seems promising in terms of long-term survival and outcomes. For instance, those who received two or fewer chemotherapy treatments showed better responses to nivolumab compared to those who received three[145].
Dostarlimab: Dostarlimab is an antibody against PD-1, used for the treatment of adults with recurrent or advanced dMMR solid tumors[146]. FDA approval was granted in January 2023 for dMMR-MSI-H for locally advanced rectal cancer, for which the current standard of care is neoadjuvant chemoradiotherapy followed by surgery and adjuvant chemotherapy[147].
A prospective phase 2 study including patients with stage II or III dMMR rectal adenocarcinomas was performed, and single-agent anti-PD-1 mAB dostarlimab was administered every 3 weeks for 6 months. All 12 patients that successfully completed treatment with dostarlimab had a complete clinical response, and, until 6 months of follow-up, no form of tumor progression or recurrence was noticed. Moreover, the drug was safe, as no adverse events were reported. Therefore, positive results are predicted, yet longer follow-up is required for validation[148].
CTLA-4 is an IC molecule expressed on the surface of activated T cells that plays a role in downregulating immune responses. Having a similar structure to CD28, CTLA-4 binds to CD80 and CD86, yet with higher affinity. In contrast to activated T cells, CTLA-4 is constantly expressed on Tregs, where it plays a suppressive role in immunity[149]. Therefore, the high expression of CTLA-4 in CRC was studied, and found to affect the prognosis negatively[150], leading to an improvement in antitumor immunity after its blockade in animal models.
Ipilimumab: Ipilimumab is a mAb against CTLA-4 that was approved by the FDA in July 2018, to be used in previously treated dMMR/MSI-H CRC patients. Based on its mechanism of action, adverse effects result from excess activation of T cells, affecting any organ system. These adverse effects are usually manageable, yet they can also be life-threatening[151].
In patients with relapsing or refractory disease, the use of low-dose ipilimumab with nivolumab has been supported. This combination showed a high response rate, low rate of disease progression, and long-term survival benefit[152].
Table 1 summarizes and describes the immunotherapies that have gained FDA approval for treatment of CRC. Despite the impressive success of ICIs in the treatment of dMMR/MSI-H mCRC, the use thereof in patients with non-dMMR/MSI-H mCRC, who constitute approximately 95% of patients with mCRC, has failed to show any clinical benefit or improvement of survival. Lately, dostarlimab was found to be effective in the treatment of local tumors, becoming the first approved immunotherapy for non-mCRC[153].
| Trade name | Generic name | Strategy | Date of FDA approval | Type of cancer | Local/metastatic |
| Jemperli | Dostarlimab | PD-1 inhibitor | January 2023 | dMMR/MSI-H | Local |
| Yervoy | Ipilimumab | CTLA-4 inhibitor | July 2018 | dMMR/MSI-H | Metastatic |
| Opdivo | Nivolumab | PD-1 inhibitor | July 2017 | dMMR/MSI-H | Metastatic |
| Keytruda | Pembrolizumab | PD-1 inhibitor | June 2020 (first-line treatment) | dMMR/MSI-H | Metastatic |
| May 2017 (second-line treatment) |
ACT: ACT, representing intrinsic molecular pathway-based immunotherapy, is a passive approach that uses ex vivo stimulated autologous lymphocytes to promote tumor eradication. This type of therapy can be divided into two groups based on their mechanism of action, being: (1) ACT with tumor-infiltrating lymphocytes; and (2) ACT using T-cell receptor gene therapy, such as chimeric antigen receptor (CAR) modified T cells[154]. As of 2023, there are six FDA-approved CAR-T cell therapies, yet none of them treat CRC (four treat B cell leukemia and lymphoma, and two treat myeloma)[155].
Recently, the use of cytokine-induced killer (CIK) cells with DCs in patients with CRC showed a better overall survival and PFS. Moreover, the use of CIK cell therapy postoperatively, combined with chemotherapy, prevented the recurrence of the disease, and prolonged the survival in patients with advanced CRC[156].
Moreover, several studies targeted CEA, since it is a common antigen in CRC. Clinical studies found a 70% disease control rate with no major adverse events when using CEA-targeting CAR-T cells. However, a more recent study found that the selection of the appropriate single-chain variable fragment had a significant effect on efficacy of CAR-T cell therapy in tumors expressing CEA[157].
Despite the advancements in studying ACT in CRC, several points need to be resolved before considering ACT as a first-line treatment of CRC. For instance, the limited infiltration of transferred T cells to the solid tumor’s microenvironment and the possibility of systemic cytokine release are main limitations. Moreover, the assessment of the safety of ACT by clinical trials showed that most patients had no severe adverse events, yet it is still being optimized[158].
Cancer vaccines represent a new therapeutic form of active immunotherapy that fights against tumors by stimulating the host’s immune cells to recognize and react against specific antigens of the tumor cells. Cancer vaccines can be divided into preventive and therapeutic vaccines, where the latter is the most studied, and includes those targeting CRC[159].
Tumor-associated antigens targeted in studies on CRC vaccines include CEA, mucin 1, Her2, Survivin-2B, and SART3, among others[159]. Despite many trials, no vaccine for CRC has been able to overcome the numerous challenges, such as immune-resistance, delivery systems, and antigen forms[160]. Recent research on therapeutic vaccines has found that cancer patients with minimal lesion residue or at advanced stages has shown promising results[161]. For this, the combination of vaccines with ICIs or ACT is being explored in clinical trials[162].
Several IC proteins have been studied recently. For example, HLA-E is an immune-suppressive feature that binds CD94/NKG2A on NK and T cells[163]. Moreover, NKG2A blockade recruits CD8 + T cells and activates NK cells[164]. Also, adenosine A2A and A2B receptors, CD47-SIRPα, TIM-3, LAG-3, TIGIT, PARPs, mARTs, B7-H3, and VISTA are considered for clinical trials due to their antitumor immunosuppressive role[164,165]. miRNA, mRNA, and CRISPR-Cas9 are also being considered in recent immunotherapeutic studies[165,166]. Table 2 offers a summary of all treatment modalities, highlighting their main uses, advantages, and disadvantages.
| Treatment | Modalities | Uses | Advantages | Disadvantages |
| Surgery | Laparoscopic surgery | Can be used in all stages of colon and rectal cancer | Laparoscopic surgery is safe and effective for CRC | Decreased survival with laparoscopic surgery in certain subgroups |
| Laparoscopic surgery carries significantly lower costs than open surgery and a decreased risk of surgical site infection | ||||
| Robotic surgery | Robotic surgery offers improved dexterity, precision, stereoscopic vision, and more precise lymph node dissection and intracorporeal anastomoses | Robotic surgery requires longer operating times, higher costs, and a steeper learning curve | ||
| Robotic-assisted colectomy may lead to better long-term survival for patients with stage I-III colon cancer | ||||
| Navigation surgery | Navigation surgery allows for real-time visualization of blood flow in the colon and rectum | Transanal total mesorectal excision involves a non-standardized operative technique, risk of contamination of the surrounding space and the peritoneal cavity with bacteria or malignant cells, risk of urethral injury, anastomotic leaks, bowel injuries, urinary dysfunction, and bleeding | ||
| Navigation surgery is useful for lymph node mapping | ||||
| Radiation therapy | Image-guided radiation therapy | Mainly used in stages III and IV colon and rectal cancers | Neoadjuvant-Shrink tumors to facilitate easier surgical removal (mainly for rectal cancer) | Causes skin irritation (redness, blistering, peeling) |
| Intensity-modulated radiation therapy | Adjuvant-Cancer cell eradication to reduce recurrence risk | Gastrointestinal complications (nausea, diarrhea, painful bowel movements, blood in stool) | ||
| Stereotactic body radiation therapy | Non-surgical candidates-Control cancer and alleviate symptoms in patients not fit for surgery | Bowel incontinence | ||
| Adaptive radiation therapy | Can be used to prevent or relieve symptoms such as pain from advanced colorectal cancer. It may also treat metastatic areas like the lungs or bones, shrinking tumors temporarily, but not likely curing the cancer | Bladder irritation (frequency, burning, pain, hematuria) | ||
| Nanoparticle-mediated radiation therapy | Fatigue | |||
| Proton beam therapy | Sexual adverse events (erection issues, vaginal irritation) | |||
| Heavy ion therapy | Tissue damage (scarring, fibrosis, adhesions) | |||
| Chemotherapy | Alkylating agents | Used in stage II, III and IV in colorectal cancer | Wide range of use | Common toxicity to cancer and normal cells |
| Antimetabolites | May stop or slow cancer growth | Several side effects | ||
| Antimicrotubular agents | Variety of available agents | Decreased immunity by harming to immune cells | ||
| Antibiotics | Can be used as a combination therapy | Individual variations | ||
| Miscellaneous | Needs frequent monitoring and hospital visits | |||
| Targeted therapy | Targeting EGFR pathway | Used in stage IV colon and rectal cancers | Can be used when chemotherapy fails | Expensive drugs |
| Targeting VEGF/VEGFR pathway | Synergy with chemotherapy: Improved survival compared to chemotherapy alone | Genetic testing often needed: Drugs do not work on all patients | ||
| Targeting BRAF | Targeted mechanism of action: Fewer side effects compared to chemotherapy | Limited long-term clinical experience | ||
| Targeting HER2 | Side effects include hypertension, skin rashes, cardiotoxicity, and diarrhea | |||
| Immunotherapy | Immune checkpoint inhibitors | Can be used in stage II, III and IV in colorectal cancer | Potential to treat many cancer types | Dependent on immune status of patient |
| Adoptive cell therapy | May be nonspecific to cancer type | Several side effects | ||
| Vaccines | May provide lifelong protection from cancer | Possible resistance | ||
| Possible adverse immunological events |
Both targeted therapy and immunotherapy have some unique challenges in treating cancer, where resistance remains the major challenge in enhancing their therapeutic effects. Resistance to targeted therapy can be acquired via several mechanisms: (1) Alteration of the driver oncogene; (2) activation of a critical signaling pathway in a parallel or down
Regarding immunotherapies in CRC, the main causes of resistance are: (1) The low tumor mutational burden of pMMR/MSS tumors; (2) the immunosuppressive shift caused by the TME; (3) the downregulation of MHC molecules on the tumor cells; and (4) the abnormal vasculature with its resultant low density of TIL in the tumor body[171]. For this, chemo-immunotherapies, fecal microbiota transplantation, immune-adjuvants, oncolytic viruses, and dendritic cell proliferation assays are being tested in an attempt to overcome resistance to immunotherapies in CRC.
New developments were made in CRC, mainly regarding targeted and immuno-therapy, aiming to overcome resistance and yield a higher effectiveness with lower side effects. One of the main advancements in targeted therapy was through targeting RAS mutant CRC by: (1) Using RAS direct inhibitors; (2) targeting the downstream pathways, such as the MAPK pathway; (3) using immunotherapy combinations; and (4) targeting RAS through metabolic pathways[172,173]. Another approach was through targeting BRAF mutant CRC: (1) Using monotherapy strategies; (2) blocking BRAF and EGFR; and (3) blocking BRAF and MEK[172,173]. Regarding immunotherapy, most promising approaches under development are the therapeutic cancer vaccines, new ICIs and their combinations, bi-specific T-cell engagers, and ACT.
To improve the survival benefits and efficacy of treatments in patients with CRC, several inventions have been suggested, such as CRC 3D models, xenograft models, single-cell sequencing strategy, and applying new antibody-based therapies strategies[174]. There are also several barriers to translating the findings of new research into clinical practice. These include the limited ability to predict the efficacy and response in patients, in addition to the development of resistance[175]. Furthermore, due to the delayed effects of therapies relying on immune cells, clinical study designs need to focus on long-term follow-up, making some endpoints inadequate[176]. The high cost of some new therapies is also a barrier in reaching efficient results[177]. Finally, the lack of sufficient biomarkers to follow-up the progress of treatment, and to determine the most effective treatment option, is a major limitation facing new therapies[178].
Until now, there was no optimal biomarker to predict the response to immunotherapy in CRC. A large number of trials, in addition to the application of multi-methods and optimization of technology, could improve the accuracy of biomarkers for CRC immunotherapy, leading to a better prognosis and overall survival rate.
Despite the continuous efforts to reach better treatment options, only MSI has been approved to screen for the effectiveness of immunotherapy in CRC patients[179]. However, some clinical studies showed that this has a low efficacy as a predictive biomarker[180]. New predictive biomarkers have been developed in this regard, and can be divided into: (1) Biomarkers related to genetic alterations (such as MSI, TMB, and POLE/POLD1)[173,179]; (2) biomolecular markers related to TME, (such as TILs, PD-L1 expression, TLS, and CAF related genes)[181]; (3) the specific gut microbiome[182]; and (4) peripheral blood biomarkers (such as ctDNA, bTMB, circulating immune cells, and inflammatory cell related indicators)[183].
Through personalized medicine, patients can be classified into different groups based on the susceptibility to CRC, response to treatment types (such as targeted and immune therapies), progression, and possibility of side effects[184]. Despite the advancements in this field, minimal personalized therapeutic options resulted with the improved understanding of genomic and proteomic alterations in CRC[185]. Therefore, more studies are necessary to enhance treatment outcomes and reduce adverse reactions using individually adjusted drugs, based on each patient’s genetic and epigenetic features.
Surgery, in combination with neoadjuvant chemotherapy, adjuvant chemotherapy, and radiation, is currently the mainstay of treatment of CRC. With the recent advances in medical sciences, human genomics, transcriptional, and epigenetic information have become more accessible. Consequently, individualized treatment of diseases such as CRC is now feasible. In fact, due to the vast tissue heterogeneity between individuals, individualized therapy is considered necessary.
However, clinical trials are still needed to provide supporting data for the continuous improvement in the treatment of advanced CRC. These clinical trials will help provide evidence for best practice guidelines to be followed in the future. Further research should focus on the determination of the optimal approach to treatment, while taking into consideration the different therapy options as well as individual heterogeneity.
| 1. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9950] [Article Influence: 4975.0] [Reference Citation Analysis (2)] |
| 3. | Kumar A, Gautam V, Sandhu A, Rawat K, Sharma A, Saha L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J Gastrointest Surg. 2023;15:495-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (2)] |
| 4. | Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M, Holme Ø, Zauber AG, Pilonis ND, Mroz A, Kuipers EJ, Shi J, Hernán MA, Adami HO, Regula J, Hoff G, Kaminski MF; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 409] [Article Influence: 136.3] [Reference Citation Analysis (1)] |
| 5. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (1)] |
| 6. | Zajkowska M, Mroczko B. A Novel Approach to Staging and Detection of Colorectal Cancer in Early Stages. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 7. | Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, Nordlinger B, Adam R, Gruenberger T, Choti MA, Bilchik AJ, Van Cutsem EJ, Chiang JM, D'Angelica MI. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828-4833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Chang GJ, Kaiser AM, Mills S, Rafferty JF, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of colon cancer. Dis Colon Rectum. 2012;55:831-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Blackmore AE, Wong MT, Tang CL. Evolution of laparoscopy in colorectal surgery: an evidence-based review. World J Gastroenterol. 2014;20:4926-4933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 12. | Mar J, Anton-Ladislao A, Ibarrondo O, Arrospide A, Lázaro S, Gonzalez N, Bare M, Callejo D, Redondo M, Quintana JM; REDISSEC-CARESS/CCR group. Cost-effectiveness analysis of laparoscopic versus open surgery in colon cancer. Surg Endosc. 2018;32:4912-4922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 13. | Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, Saida Y, Hasegawa H, Akagi T, Sugihara K, Yamaguchi T, Masaki T, Fukunaga Y, Murata K, Okajima M, Moriya Y, Shimada Y. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 14. | Gavriilidis P, Katsanos K. Laparoscopic Versus Open Transverse Colectomy: A Systematic Review and Meta-Analysis. World J Surg. 2018;42:3008-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Toritani K, Watanabe J, Nakagawa K, Suwa Y, Suwa H, Ishibe A, Ota M, Fujii S, Kunisaki C, Endo I. Randomized controlled trial to evaluate laparoscopic versus open surgery in transverse and descending colon cancer patients. Int J Colorectal Dis. 2019;34:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Matsuda T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y. Optimal Surgery for Mid-Transverse Colon Cancer: Laparoscopic Extended Right Hemicolectomy Versus Laparoscopic Transverse Colectomy. World J Surg. 2018;42:3398-3404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Cleary RK, Morris AM, Chang GJ, Halverson AL. Controversies in Surgical Oncology: Does the Minimally Invasive Approach for Rectal Cancer Provide Equivalent Oncologic Outcomes Compared with the Open Approach? Ann Surg Oncol. 2018;25:3587-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc. 2018;32:2894-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 19. | Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, Larach S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014;18:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Bjørn MX, Perdawood SK. Transanal total mesorectal excision--a systematic review. Dan Med J. 2015;62. [PubMed] |
| 21. | Shen Z, Ye Y, Atallah S, Xie Q, Jiang K, Wang S. [Three procedures of transanal minimally invasive surgery (TAMIS) for advanced mid-low rectal tumor]. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:998-1001. [PubMed] |
| 22. | Wang G, Wang Z, Jiang Z, Liu J, Zhao J, Li J. Male urinary and sexual function after robotic pelvic autonomic nerve-preserving surgery for rectal cancer. Int J Med Robot. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Arezzo A, Bonino MA, Ris F, Boni L, Cassinotti E, Foo DCC, Shum NF, Brolese A, Ciarleglio F, Keller DS, Rosati R, De Nardi P, Elmore U, Fumagalli Romario U, Jafari MD, Pigazzi A, Rybakov E, Alekseev M, Watanabe J, Vettoretto N, Cirocchi R, Passera R, Forcignanò E, Morino M. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc. 2020;34:4281-4290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Park SH, Park HM, Baek KR, Ahn HM, Lee IY, Son GM. Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J Gastroenterol. 2020;26:6945-6962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Vuijk FA, Hilling DE, Mieog JSD, Vahrmeijer AL. Fluorescent-guided surgery for sentinel lymph node detection in gastric cancer and carcinoembryonic antigen targeted fluorescent-guided surgery in colorectal and pancreatic cancer. J Surg Oncol. 2018;118:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Hiroishi A, Yamada T, Morimoto T, Horikoshi K, Nakajima Y. Three-dimensional computed tomographic angiography with computed tomographic colonography for laparoscopic colorectal surgery. Jpn J Radiol. 2018;36:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Letschert JG, Lebesque JV, de Boer RW, Hart AA, Bartelink H. Dose-volume correlation in radiation-related late small-bowel complications: a clinical study. Radiother Oncol. 1990;18:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT, Dorth J, Garcia-Aguilar J, Goff D, Jacqmin D, Kelly P, Newman NB, Olsen J, Raldow AC, Ruiz-Garcia E, Stitzenberg KB, Thomas CR Jr, Wu QJ, Das P. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2021;11:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 30. | Paroder V, Fraum TJ, Nougaret S, Petkovska I, Rauch GM, Kaur H. Key clinical trials in rectal cancer shaping the current treatment paradigms: reference guide for radiologists. Abdom Radiol (NY). 2023;48:2825-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 31. | Bascoul-Mollevi C, Gourgou S, Borg C, Etienne PL, Rio E, Rullier E, Juzyna B, Castan F, Conroy T. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER PRODIGE 23): Health-related quality of life longitudinal analysis. Eur J Cancer. 2023;186:151-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 965] [Article Influence: 241.3] [Reference Citation Analysis (0)] |
| 33. | Goffredo P, Quezada-Diaz FF, Garcia-Aguilar J, Smith JJ. Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 35. | Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech JJ, Valverde A, de Chaisemartin C, Rivoire M, Trilling B, Jafari M, Portier G, Meunier B, Sieleznieff I, Bertrand M, Marchal F, Dubois A, Pocard M, Rullier A, Smith D, Frulio N, Frison E, Denost Q. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol. 2020;5:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 36. | Fossum CC, Alabbad JY, Romak LB, Hallemeier CL, Haddock MG, Huebner M, Dozois EJ, Larson DW. The role of neoadjuvant radiotherapy for locally-advanced rectal cancer with resectable synchronous metastasis. J Gastrointest Oncol. 2017;8:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Simpson J, Scholefield JH. Treatment of colorectal cancer: surgery, chemotherapy and radiotherapy. Surgery (Oxford). 2008;26:329-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Feo L, Polcino M, Nash GM. Resection of the Primary Tumor in Stage IV Colorectal Cancer: When Is It Necessary? Surg Clin North Am. 2017;97:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15 Suppl 4:iv103-iv106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Joharatnam-Hogan N, Wilson W, Shiu KK, Fusai GK, Davidson B, Hochhauser D, Bridgewater J, Khan K. Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes-The University College London Hospital (UCLH) Experience. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Luh JY, Albuquerque KV, Cheng C, Ermoian RP, Nabavizadeh N, Parsai H, Roeske JC, Weiss SE, Wynn RB, Yu Y, Rosenthal SA, Hartford A. ACR-ASTRO Practice Parameter for Image-guided Radiation Therapy (IGRT). Am J Clin Oncol. 2020;43:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Gwynne S, Webster R, Adams R, Mukherjee S, Coles B, Staffurth J. Image-guided radiotherapy for rectal cancer: a systematic review. Clin Oncol (R Coll Radiol). 2012;24:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Hartford AC, Galvin JM, Beyer DC, Eichler TJ, Ibbott GS, Kavanagh B, Schultz CJ, Rosenthal SA; American College of Radiology; American Society for Radiation Oncology. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for Intensity-modulated Radiation Therapy (IMRT). Am J Clin Oncol. 2012;35:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Chao ST, Dad LK, Dawson LA, Desai NB, Pacella M, Rengan R, Xiao Y, Yenice KM, Rosenthal SA, Hartford A. ACR-ASTRO Practice Parameter for the Performance of Stereotactic Body Radiation Therapy. Am J Clin Oncol. 2020;43:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): a new promising technology in radiation oncology. Oncologist. 1999;4:433-442. [PubMed] |
| 46. | Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 47. | Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park). 2015;29:331-340. [PubMed] |
| 48. | Lavrova E, Garrett MD, Wang YF, Chin C, Elliston C, Savacool M, Price M, Kachnic LA, Horowitz DP. Adaptive Radiation Therapy: A Review of CT-based Techniques. Radiol Imaging Cancer. 2023;5:e230011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Brock KK. Adaptive Radiotherapy: Moving Into the Future. Semin Radiat Oncol. 2019;29:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 50. | Chiang CH, Chao TY, Huang MY. Adaptive radiotherapy of locally advanced sigmoid colon cancer with intrafractional motion using the MRIdian system: A case report. Oncol Lett. 2023;26:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Haque M, Shakil MS, Mahmud KM. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Vaios EJ, Wo JY. Proton beam radiotherapy for anal and rectal cancers. J Gastrointest Oncol. 2020;11:176-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Yan J, Wang C, Jiang X, Wei Y, Wang Q, Cui K, Xu X, Wang F, Zhang L. Application of phototherapeutic-based nanoparticles in colorectal cancer. Int J Biol Sci. 2021;17:1361-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol. 2014;32:2855-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Tian X, Liu K, Hou Y, Cheng J, Zhang J. The evolution of proton beam therapy: Current and future status. Mol Clin Oncol. 2018;8:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Hiroshima Y, Ishikawa H, Murakami M, Nakamura M, Shimizu S, Enomoto T, Oda T, Mizumoto M, Nakai K, Okumura T, Sakurai H. Proton Beam Therapy for Local Recurrence of Rectal Cancer. Anticancer Res. 2021;41:3589-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Kraft G. [Heavy ion tumor therapy]. Med Monatsschr Pharm. 2009;32:328-334. [PubMed] |
| 58. | Yamada S, Takiyama H, Isozaki Y, Shinoto M, Makishima H, Yamamoto N, Tsuji H. Carbon-ion Radiotherapy for Colorectal Cancer. J Anus Rectum Colon. 2021;5:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Ebner DK, Kamada T, Yamada S. Abscopal effect in recurrent colorectal cancer treated with carbon-ion radiation therapy: 2 case reports. Adv Radiat Oncol. 2017;2:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, Croitoru AE, Grover S, Lohar PV, Odhiambo A, Park SH, Garcia ER, Teh C, Rose A, Zaki B, Chamberlin MD. Treatment of Patients With Late-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. JCO Glob Oncol. 2020;6:414-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 61. | Hernandez Dominguez O, Yilmaz S, Steele SR. Stage IV Colorectal Cancer Management and Treatment. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 62. | Duarte D, Vale N. Combining repurposed drugs to treat colorectal cancer. Drug Discov Today. 2022;27:165-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | You XH, Jiang YH, Fang Z, Sun F, Li Y, Wang W, Xia ZJ, Wang XZ, Ying HQ. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open. 2020;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Xiong L, Lou Y, Wang L. Effect of bevacizumab combined with first-line chemotherapy on metastatic colorectal cancer. Am J Transl Res. 2021;13:3609-3617. [PubMed] |
| 65. | Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, Mahmoud N, Morris AM, Ruiz-Garcia E, You YN, Meyerhardt JA. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40:892-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 66. | Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3',5'-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338-3342. [PubMed] |
| 67. | Islam BN, Browning DD. Phosphodiesterase-5 inhibitors for colon cancer chemoprevention. Aging (Albany NY). 2018;10:2216-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Cruz-Burgos M, Losada-Garcia A, Cruz-Hernández CD, Cortés-Ramírez SA, Camacho-Arroyo I, Gonzalez-Covarrubias V, Morales-Pacheco M, Trujillo-Bornios SI, Rodríguez-Dorantes M. New Approaches in Oncology for Repositioning Drugs: The Case of PDE5 Inhibitor Sildenafil. Front Oncol. 2021;11:627229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Dent P, Booth L, Roberts JL, Poklepovic A, Hancock JF. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J Cell Physiol. 2020;235:6862-6874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B; PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 725] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 71. | Valverde A, Peñarando J, Cañas A, López-Sánchez LM, Conde F, Guil-Luna S, Hernández V, Villar C, Morales-Estévez C, de la Haba-Rodríguez J, Aranda E, Rodríguez-Ariza A. The addition of celecoxib improves the antitumor effect of cetuximab in colorectal cancer: role of EGFR-RAS-FOXM1-β- catenin signaling axis. Oncotarget. 2017;8:21754-21769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L, Rodrigues-Bigas M, Su LK, Sherman J, Kelloff G, Levin B, Steinbach G; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Lynch PM, Burke CA, Phillips R, Morris JS, Slack R, Wang X, Liu J, Patterson S, Sinicrope FA, Rodriguez-Bigas MA, Half E, Bulow S, Latchford A, Clark S, Ross WA, Malone B, Hasson H, Richmond E, Hawk E. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 74. | Fina D, Franchi L, Caruso R, Peluso I, Naccari GC, Bellinvia S, Testi R, Pallone F, Monteleone G. 5-aminosalicylic acid enhances anchorage-independent colorectal cancer cell death. Eur J Cancer. 2006;42:2609-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Reinacher-Schick A, Schoeneck A, Graeven U, Schwarte-Waldhoff I, Schmiegel W. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis. 2003;24:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Stolfi C, Pallone F, Monteleone G. Colorectal cancer chemoprevention by mesalazine and its derivatives. J Biomed Biotechnol. 2012;2012:980458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Słoka J, Madej M, Strzalka-Mrozik B. Molecular Mechanisms of the Antitumor Effects of Mesalazine and Its Preventive Potential in Colorectal Cancer. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 78. | Slater AF. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993;57:203-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Choi JH, Yoon JS, Won YW, Park BB, Lee YY. Chloroquine enhances the chemotherapeutic activity of 5-fluorouracil in a colon cancer cell line via cell cycle alteration. APMIS. 2012;120:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M, Kaneko M, Murono K, Tada N, Nirei T, Takahashi K, Kitayama J. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs. 2012;23:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O'Dwyer PJ. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19:2995-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 82. | Anselmino LE, Baglioni MV, Reynoso G, Rozados VR, Scharovsky OG, Rico MJ, Menacho-Márquez M. Potential effect of chloroquine and propranolol combination to treat colorectal and triple-negative breast cancers. Sci Rep. 2023;13:7923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 83. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2733] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 84. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1645] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 85. | Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 86. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2950] [Cited by in RCA: 2825] [Article Influence: 113.0] [Reference Citation Analysis (1)] |
| 87. | Mohammed A, Janakiram NB, Brewer M, Vedala K, Steele VE, Rao CV. Multitargeted low-dose GLAD combination chemoprevention: a novel and promising approach to combat colon carcinogenesis. Neoplasia. 2013;15:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Brás AR, Fernandes P, Moreira T, Morales-Sanfrutos J, Sabidó E, Antunes AMM, Valente A, Preto A. New Ruthenium-Cyclopentadienyl Complexes Affect Colorectal Cancer Hallmarks Showing High Therapeutic Potential. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 89. | Teixeira-Guedes C, Brás AR, Teixeira RG, Valente A, Preto A. Ruthenium(II)-Cyclopentadienyl-Derived Complexes as New Emerging Anti-Colorectal Cancer Drugs. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Helena Garcia M, Morais TS, Florindo P, Piedade MF, Moreno V, Ciudad C, Noe V. Inhibition of cancer cell growth by ruthenium(II) cyclopentadienyl derivative complexes with heteroaromatic ligands. J Inorg Biochem. 2009;103:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 694] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 92. | Padma VV. An overview of targeted cancer therapy. Biomedicine (Taipei). 2015;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 93. | Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 255] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 94. | Khan K, Valeri N, Dearman C, Rao S, Watkins D, Starling N, Chau I, Cunningham D. Targeting EGFR pathway in metastatic colorectal cancer- tumour heterogeniety and convergent evolution. Crit Rev Oncol Hematol. 2019;143:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 95. | Neumann J, Wehweck L, Maatz S, Engel J, Kirchner T, Jung A. Alterations in the EGFR pathway coincide in colorectal cancer and impact on prognosis. Virchows Arch. 2013;463:509-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 97. | Fornasier G, Francescon S, Baldo P. An Update of Efficacy and Safety of Cetuximab in Metastatic Colorectal Cancer: A Narrative Review. Adv Ther. 2018;35:1497-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 98. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3126] [Article Influence: 195.4] [Reference Citation Analysis (1)] |
| 99. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 100. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 101. | Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, Schilsky RL. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol. 2016;34:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 102. | Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:678-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 314] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 103. | Petrelli F, Ardito R, Ghidini A, Zaniboni A, Ghidini M, Barni S, Tomasello G. Different Toxicity of Cetuximab and Panitumumab in Metastatic Colorectal Cancer Treatment: A Systematic Review and Meta-Analysis. Oncology. 2018;94:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 104. | Lo L, Patel D, Townsend AR, Price TJ. Pharmacokinetic and pharmacodynamic evaluation of panitumumab in the treatment of colorectal cancer. Expert Opin Drug Metab Toxicol. 2015;11:1907-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 106. | Kim TW, Elme A, Park JO, Udrea AA, Kim SY, Ahn JB, Valencia RV, Krishnan S, Manojlovic N, Guan X, Lofton-Day C, Jung AS, Vrdoljak E. Final Analysis of Outcomes and RAS/BRAF Status in a Randomized Phase 3 Study of Panitumumab and Best Supportive Care in Chemorefractory Wild Type KRAS Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Rossini D, Boccaccino A, Carullo M, Antoniotti C, Dima G, Ciracì P, Marmorino F, Moretto R, Masi G, Cremolini C. Primary tumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: a systematic review and pooled analysis of randomised trials. Eur J Cancer. 2023;184:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 108. | Liu T, Jiang S, Teng X, Zhong L, Liu M, Jin Y, Dong M. A comparison of panitumumab and cetuximab in the treatment of KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Immunopharmacol Immunotoxicol. 2023;45:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 109. | Zhang Y, Zou JY, Wang Z, Wang Y. Fruquintinib: a novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag Res. 2019;11:7787-7803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 110. | Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J, Cubillo Gracian A, Sartore-Bianchi A, Satoh T, Randrian V, Tomasek J, Chong G, Paulson AS, Masuishi T, Jones J, Csőszi T, Cremolini C, Ghiringhelli F, Shergill A, Hochster HS, Krauss J, Bassam A, Ducreux M, Elme A, Faugeras L, Kasper S, Van Cutsem E, Arnold D, Nanda S, Yang Z, Schelman WR, Kania M, Tabernero J, Eng C; FRESCO-2 Study Investigators. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 111. | Dakowicz D, Zajkowska M, Mroczko B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 112. | Bhattacharya R, Ye XC, Wang R, Ling X, McManus M, Fan F, Boulbes D, Ellis LM. Intracrine VEGF Signaling Mediates the Activity of Prosurvival Pathways in Human Colorectal Cancer Cells. Cancer Res. 2016;76:3014-3024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Bhattacharya R, Fan F, Wang R, Ye X, Xia L, Boulbes D, Ellis LM. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br J Cancer. 2017;117:848-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 114. | Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 727] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 115. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7733] [Article Influence: 368.2] [Reference Citation Analysis (1)] |
| 116. | Zhang C, Liu L, Lv Y, Li J, Cao C, Lu J, Wang S, Du B, Yang X. Chemotherapy plus panitumumab/cetuximab versus chemotherapy plus bevacizumab in wild-type KRAS/RAS metastatic colorectal cancer: a meta-analysis. Expert Rev Anticancer Ther. 2022;22:1333-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 117. | Cui Y, Guo Y. Publisher Correction to: The effectiveness and safety of bevacizumab versus cetuximab in the treatment of colorectal cancer: a systematic review and meta-analysis. Int J Clin Pharm. 2022;44:1572. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 118. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1024] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 119. | Thakur A, Chorawala MR, Patel RS. A systemic review and meta-analysis of Aflibercept plus FOLFIRI regimen as a second-line treatment for metastatic colorectal cancer: A PRISMA compliant pooled analysis of randomized controlled trials and single arm studies to assess efficacy and safety. Crit Rev Oncol Hematol. 2023;188:104034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 664] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 121. | Ge P, Wan N, Han X, Wang X, Zhang J, Long X, Wang X, Bian Y. Efficacy, safety, and cost-effectiveness analysis of aflibercept in metastatic colorectal cancer: A rapid health technology assessment. Front Pharmacol. 2022;13:914683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 122. | Gao Z, Cao C, Bao Y, Fan Y, Chen G, Fu P. Systematic Review and Meta-Analysis of Multitargeted Tyrosine Kinase Inhibitors in Patients With Intractable Metastatic Colorectal Cancer. Technol Cancer Res Treat. 2020;19:1533033820943241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 123. | Ros J, Rodríguez-Castells M, Saoudi N, Baraibar I, Salva F, Tabernero J, Élez E. Treatment of BRAF-V600E mutant metastatic colorectal cancer: new insights and biomarkers. Expert Rev Anticancer Ther. 2023;23:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, Desai J, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Elez E, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol. 2021;39:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 326] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 125. | Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 688] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 126. | Casak SJ, Horiba MN, Yuan M, Cheng J, Lemery SJ, Shen YL, Fu W, Moore JN, Li Y, Bi Y, Auth D, Fesenko N, Kluetz PG, Pazdur R, Fashoyin-Aje LA. FDA Approval Summary: Tucatinib with Trastuzumab for Advanced Unresectable or Metastatic, Chemotherapy Refractory, HER2-Positive RAS Wild-Type Colorectal Cancer. Clin Cancer Res. 2023;29:4326-4330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 127. | Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 128. | Strickler JH, Cercek A, Siena S, André T, Ng K, Van Cutsem E, Wu C, Paulson AS, Hubbard JM, Coveler AL, Fountzilas C, Kardosh A, Kasi PM, Lenz HJ, Ciombor KK, Elez E, Bajor DL, Cremolini C, Sanchez F, Stecher M, Feng W, Bekaii-Saab TS; MOUNTAINEER investigators. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 2023;24:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 129. | Koury J, Lucero M, Cato C, Chang L, Geiger J, Henry D, Hernandez J, Hung F, Kaur P, Teskey G, Tran A. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J Immunol Res. 2018;2018:9585614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 130. | Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1361] [Cited by in RCA: 1789] [Article Influence: 357.8] [Reference Citation Analysis (0)] |
| 131. | Shimu AS, Wei HX, Li Q, Zheng X, Li B. The new progress in cancer immunotherapy. Clin Exp Med. 2023;23:553-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 132. | Zhang Y, Zheng J. Functions of Immune Checkpoint Molecules Beyond Immune Evasion. Adv Exp Med Biol. 2020;1248:201-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 133. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10352] [Article Influence: 796.3] [Reference Citation Analysis (34)] |
| 134. | Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K, Kulasinghe A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29:3044-3060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 667] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 135. | Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 974] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 136. | Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015;75:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 137. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4139] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 138. | Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, Xia Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 271] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 139. | Immunotherapy for Colorectal Cancer. Cancer Research Institute. Available from: https://www.cancerresearch.org/cancer-types/colorectal-cancer. |
| 140. | Research C for DE. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2019 Preprint. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. |
| 141. | Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T; KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 511] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 142. | Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089-1096. [PubMed] |
| 143. | Research C for DE. FDA grants nivolumab accelerated approval for MSI-H or dMMR colorectal cancer. 2019 Preprint. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-msi-h-or-dmmr-colorectal-cancer. |
| 144. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2091] [Article Influence: 261.4] [Reference Citation Analysis (0)] |
| 145. | Kooshkaki O, Derakhshani A, Hosseinkhani N, Torabi M, Safaei S, Brunetti O, Racanelli V, Silvestris N, Baradaran B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 146. | Singh V, Sheikh A, Abourehab MAS, Kesharwani P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 147. | GSK. US FDA Advisory Committee votes in support of trials designed to evaluate Jemperli (dostarlimab-gxly) as a potential treatment for mismatch repair-deficient/microsatellite instability-high locally advanced rectal cancer 2023. Available from: https://www.gsk.com/en-gb/media/press-releases/us-fda-advisory-committee-votes-in-support-of-trials-designed-to-evaluate-jemperli-dostarlimab-gxly-as-a-potential-treatment-for-dmmrmsi-h-locally-advanced-rectal-cancer/. |
| 148. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 900] [Article Influence: 300.0] [Reference Citation Analysis (0)] |
| 149. | Watanabe T, Ishino T, Ueda Y, Nagasaki J, Sadahira T, Dansako H, Araki M, Togashi Y. Activated CTLA‐4‐independent immunosuppression of Treg cells disturbs CTLA‐4 blockade‐mediated antitumor immunity. Cancer Sci. 2023;114:1859-1870.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 150. | Derakhshani A, Hashemzadeh S, Asadzadeh Z, Shadbad MA, Rasibonab F, Safarpour H, Jafarlou V, Solimando AG, Racanelli V, Singh PK, Najafi S, Javadrashid D, Brunetti O, Silvestris N, Baradaran B. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers (Basel). 2021;13:2414.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 151. | Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo). 2013;2013:857519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 152. | André T, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Abdullaev S, Memaj A, Lei M, Dixon M, Kopetz S, Overman MJ. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 153. | Boland PM, Ma WW. Immunotherapy for Colorectal Cancer. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 154. | June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7:280ps7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 155. | Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023;14:1188049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 194] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 156. | Du H, Yang J, Zhang Y. Cytokine-induced killer cell/dendritic cell combined with cytokine-induced killer cell immunotherapy for treating advanced gastrointestinal cancer. BMC Cancer. 2020;20:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 157. | Zhang C, Wang L, Zhang Q, Shen J, Huang X, Wang M, Huang Y, Chen J, Xu Y, Zhao W, Qi Y, Li Y, Ou Y, Yang Z, Qian C. Screening and characterization of the scFv for chimeric antigen receptor T cells targeting CEA-positive carcinoma. Front Immunol. 2023;14:1182409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 158. | Juat DJ, Hachey SJ, Billimek J, Del Rosario MP, Nelson EL, Hughes CCW, Zell JA. Adoptive T-Cell Therapy in Advanced Colorectal Cancer: A Systematic Review. Oncologist. 2022;27:210-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 159. | Martinis E, Ricci C, Trevisan C, Tomadini G, Tonon S. Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 160. | Wu Z, Yang M, Cao Y. Tumor antigens and vaccines in colorectal cancer. Med Drug Discov. 2022;16:100144. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 161. | Jia W, Zhang T, Huang H, Feng H, Wang S, Guo Z, Luo Z, Ji X, Cheng X, Zhao R. Colorectal cancer vaccines: The current scenario and future prospects. Front Immunol. 2022;13:942235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 162. | Janssen E, Subtil B, de la Jara Ortiz F, Verheul HMW, Tauriello DVF. Combinatorial Immunotherapies for Metastatic Colorectal Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 163. | Tizona Therapeutics, Inc. TTX-080 HLA-G Antagonist in Subjects With Advanced Cancers. [accessed 2024 Jan 9]. In: ClinicalTrials.gov [Internet]. A Phase 1a/1b Dose Escalation/Expansion Study of TTX-080, an HLA-G Antagonist, as Monotherapy and in Combination With Pembrolizumab or Cetuximab in Patients With Advanced Solid Refractory/Resistant Malignancies. Available from: https://clinicaltrials.gov/study/NCT04485013 Clinicaltrials.gov Identifier: NCT04485013. |
| 164. | MedImmune LLC. Durvalumab Alone or in Combination With Novel Agents in Subjects With NSCLC (COAST). [accessed 2023 Dec 12]. In: ClinicalTrials.gov [Internet]. A Phase 2 Open-label, Multicenter, Randomized, Multidrug Platform Study of Durvalumab (MEDI4736) Alone or in Combination With Novel Agents in Subjects With Locally Advanced, Unresectable (Stage III) Non-small Cell Lung Cancer (COAST). Available from: https://clinicaltrials.gov/study/NCT03822351 Clinicaltrials.gov Identifier: NCT03822351. |
| 165. | Daiichi Sankyo Co. , Ltd. Study of Ifinatamab Deruxtecan (DS-7300a, I-DXd) in Participants With Advanced Solid Malignant Tumors. [accessed 2024 Jul 10]. In: ClinicalTrials.gov [Internet]. Phase I/II, Two-Part, Multicenter First-in-Human Study of DS-7300a in Subjects With Advanced Solid Malignant Tumors. Available from: https://clinicaltrials.gov/study/NCT04145622 Clinicaltrials.gov Identifier: NCT04145622. |
| 166. | BioNTech SE. Evaluation of the Safety and Tolerability of i.v. Administration of a Cancer Vaccine in Patients With Advanced Melanoma (Lipo-MERIT). [accessed 2023 Jul 19]. In: ClinicalTrials.gov [Internet]. Clinical First-in-human Dose Escalation Study Evaluating the Safety and Tolerability of Intravenous Administration of a Tetravalent RNA-lipoplex Cancer Vaccine Targeting the Tumor-associated Antigens NY-ESO-1, Tyrosinase, MAGE-A3, and TPTE in Patients With Advanced Melanoma. Available from: https://clinicaltrials.gov/study/NCT02410733 Clinicaltrials.gov Identifier: NCT02410733. |
| 167. | Neel DS, Bivona TG. Resistance is futile: overcoming resistance to targeted therapies in lung adenocarcinoma. NPJ Precis Oncol. 2017;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 168. | Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A, Zecchin D, Apicella M, Migliardi G, Galimi F, Lauricella C, Zanon C, Perera T, Veronese S, Corti G, Amatu A, Gambacorta M, Diaz LA Jr, Sausen M, Velculescu VE, Comoglio P, Trusolino L, Di Nicolantonio F, Giordano S, Siena S. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 169. | Jahangiri A, De Lay M, Miller LM, Carbonell WS, Hu YL, Lu K, Tom MW, Paquette J, Tokuyasu TA, Tsao S, Marshall R, Perry A, Bjorgan KM, Chaumeil MM, Ronen SM, Bergers G, Aghi MK. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res. 2013;19:1773-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 170. | LaFargue CJ, Amero P, Noh K, Mangala LS, Wen Y, Bayraktar E, Umamaheswaran S, Stur E, Dasari SK, Ivan C, Pradeep S, Yoo W, Lu C, Jennings NB, Vathipadiekal V, Hu W, Chelariu-Raicu A, Ku Z, Deng H, Xiong W, Choi HJ, Hu M, Kiyama T, Mao CA, Ali-Fehmi R, Birrer MJ, Liu J, Zhang N, Lopez-Berestein G, de Franciscis V, An Z, Sood AK. Overcoming adaptive resistance to anti-VEGF therapy by targeting CD5L. Nat Commun. 2023;14:2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 171. | Hao M, Hou S, Li W, Li K, Xue L, Hu Q, Zhu L, Chen Y, Sun H, Ju C, Zhang C. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 172. | Tang YL, Li DD, Duan JY, Sheng LM, Wang X. Resistance to targeted therapy in metastatic colorectal cancer: Current status and new developments. World J Gastroenterol. 2023;29:926-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 173. | Manzi J, Hoff CO, Ferreira R, Pimentel A, Datta J, Livingstone AS, Vianna R, Abreu P. Targeted Therapies in Colorectal Cancer: Recent Advances in Biomarkers, Landmark Trials, and Future Perspectives. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 174. | Ramzy GM, Norkin M, Koessler T, Voirol L, Tihy M, Hany D, McKee T, Ris F, Buchs N, Docquier M, Toso C, Rubbia-Brandt L, Bakalli G, Guerrier S, Huelsken J, Nowak-Sliwinska P. Platform combining statistical modeling and patient-derived organoids to facilitate personalized treatment of colorectal carcinoma. J Exp Clin Cancer Res. 2023;42:79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 175. | Ghiringhelli F, Fumet JD. Is There a Place for Immunotherapy for Metastatic Microsatellite Stable Colorectal Cancer? Front Immunol. 2019;10:1816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 176. | Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 177. | Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat Rev. 2016;48:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 178. | Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current Challenges in Cancer Treatment. Clin Ther. 2016;38:1551-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 513] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 179. | van Velzen MJM, Derks S, van Grieken NCT, Haj Mohammad N, van Laarhoven HWM. MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev. 2020;86:102024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 180. | Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf). 2020;8:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 181. | Trimaglio G, Tilkin-Mariamé AF, Feliu V, Lauzéral-Vizcaino F, Tosolini M, Valle C, Ayyoub M, Neyrolles O, Vergnolle N, Rombouts Y, Devaud C. Colon-specific immune microenvironment regulates cancer progression versus rejection. Oncoimmunology. 2020;9:1790125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 182. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1917] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 183. | Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: A broad overview. Crit Rev Oncol Hematol. 2020;155:103109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 184. | Vaseghi Maghvan P, Jeibouei S, Akbari ME, Niazi V, Karami F, Rezvani A, Ansarinejad N, Abbasinia M, Sarvari G, Zali H, Talaie R. Personalized medicine in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2020;13:S18-S28. [PubMed] |
| 185. | Dey A, Mitra A, Pathak S, Prasad S, Zhang AS, Zhang H, Sun XF, Banerjee A. Recent Advancements, Limitations, and Future Perspectives of the use of Personalized Medicine in Treatment of Colon Cancer. Technol Cancer Res Treat. 2023;22:15330338231178403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |