Published online Jun 24, 2024. doi: 10.5306/wjco.v15.i6.695
Revised: April 25, 2024
Accepted: May 15, 2024
Published online: June 24, 2024
Processing time: 116 Days and 21.2 Hours
Gallbladder cancer (GBC) is one of the commonest biliary malignancies seen in India, Argentina, and Japan. The disease has dismal outcome as it is detected quite late due to nonspecific symptoms and signs. Early detection is the only way to improve the outcome. There have been several advances in basic as well as clinical research in the hepatobiliary and pancreatic diseases in the West and other developed countries but not enough has been done in GBC. Therefore, it is imp
Core Tip: This review is important to know the work done on gallbladder cancer from the country with high incidence of the disease and struggling to do its best. Research outcome may help to compare with the work being done in rest of the world and for the future collaborative research to find way for early diagnosis and to improve the treatment outcome of this aggressive disease.
- Citation: Kumar A, Sarangi Y, Gupta A, Sharma A. Gallbladder cancer: Progress in the Indian subcontinent. World J Clin Oncol 2024; 15(6): 695-716
- URL: https://www.wjgnet.com/2218-4333/full/v15/i6/695.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i6.695
Gallbladder cancer (GBC) is the most common and most aggressive malignancy of the biliary tree. Early diagnosis is crucial for better prognosis of this dreaded disease. However nonspecific clinical presentations often hinder the accurate diagnosis of GBC at an early stage. GBC remains a highly lethal disease, with only 10% of all patients are upfront resectable. People diagnosed with early GBC typically have a 5-year survival rate of 30%-40%, whereas individuals with locally advanced lesions have a one-year survival rate of around 10%[1].
India carries one of the most substantial burdens of this condition, with its peak incidence among women in northern India reaching 21.5 cases per 100000 individuals. The substantial morbidity and mortality from GBC significantly hinder global cancer control efforts, particularly in high-risk populations. Currently, effective strategies to reduce GBC mortality are lacking.
Chile has the highest incidence of GBC globally, with 9.7 new cases per 100000 inhabitants each year. This is followed by Bolivia (8.1 per 100000 inhabitants), South Korea (6.5 per 100000 inhabitants), Laos (4.7 per 100000 inhabitants), and Japan (4.7 per 100000 inhabitants)[2]. In the United Kingdom, GBC accounts for less than 1% of all new cancer cases. In contrast, United States has significantly lower incidence rate compared to global trends, with rates of 1.4 per 100000 among women and 0.8 per 100000 among men.
Despite of large case load of GBC in Indian subcontinent, the extent of advancement in clinical and basic research related to the etiology, pathogenesis, diagnosis, prevention, and treatment of GBC remains unclear. This review seeks to evaluate India's progress in various aspects of GBC research and provide a comprehensive summary of GBC studies conducted across the Indian subcontinent. In this review, we have endeavored to encompass all the significant research conducted in India on GBC.
We systematically reviewed published literature from India on gall bladder (GB) cancer between year 2000 to 2023 from online search engine PubMed and Medline using the search terms gallbladder, etiopathogenesis, prevention, Indian studies, basic research, epidemiological studies, clinical studies, and bullion operators like AND, OR, NOT. The secondary sources retrieved from these publications were identified through a manual search and assessed for relevance. Publications have been categorized in detail covering the areas of epidemiology, basic and clinical work. The result has been discussed in detail.
We have categorized the research published from 2000 to 2023 into five distinct areas: Demography and Etiopathogenesis, Clinicopathogenesis, Genetics and Polymorphism, Diagnostics and Imaging, Surgical Approaches and Resection, and Multimodality Therapy.
India has a high incidence of GBC and accounts for approximately 10% of the global burden of this disease. There is a pronounced regional disparity in GBC incidence across India, with considerably higher rates observed in the northern and northeastern regions compared to southern states. For instance, GBC incidence in North India (8.9/100000) is approximately 10 times greater than that in Chennai (0.8/100000). According to data from the National Cancer Registry, Delhi exhibits a similar Age-Standardized Rate (ASR) for GBC comparable to that of Chile. According to a study by Dutta et al[3], the ASRs for GBC in northern India and northeastern India are 11.8 per 100000 population and 17.1 per 100000 population, respectively. These rates are comparable to the high incidence areas of Bolivia (14/100000) and Chile (9.3/100000). Phadke et al[4] conducted a study to calculate the Annual Percentage Change (APC) in age-adjusted incidence rates of a specific health condition. They found significant APC, in the high-risk regions of Cachar [7.0 (P = 0.02)], Delhi [4.0 (P = 0.04)], and Kamrup [4.3 (P = 0.02)]. Meanwhile, in the low-risk regions of Bengaluru and Pune, the APC was 5.7 (P = 0.04) and 3.4 (P = 0.04), respectively. S et al[5] discovered that GB cancer incidence among women in Kamrup urban (ASR 16.2) was second highest after Chile[5]. Some epidemiological studies have also shown that, the population living in high-risk regions is associated with an elevated risk of developing GBC. Mhatre et al[6] from Tata Memorial Hospital (TMH) in Mumbai conducted a study revealing demographic disparities in GBC. The study revealed 4.82-fold higher odds (95%CI: 3.87-5.99) of developing GBC among individuals born in high-risk regions compared to those born in low-risk regions. Additionally, there was a dose-response relationship observed between GBC risk and length of stay in high-risk regions, showing a lifetime exposure odds ratio (OR) of 5.58 (95%CI: 4.42-7.05) with a significant P value (≤ 0.001). Notably, the risk of GBC persisted even among individuals who migrated from high-risk to low-risk regions. All these studies indicate a higher disease burden of GBC in India.
Numerous risk factors contribute to the development of GBC in Indian patients, particularly in high-risk regions, notably in the northern and northeastern states where many studies have been conducted. The Sutlej, Ganges, Yamuna, and Brahmaputra rivers, originating from glaciers in the northern Himalayas, flow east and became contaminated dow
Many studies were conducted to find the other demographics risk factors for this dreaded disease. Mhatre et al[11] discovered that there is an increased risk of GBC associated with higher parity and a shorter reproductive lifespan. Same authors in another study reported that consumption of mustard oil was associated with increased risk of GBC[12]. In a gallstone-matched study, Mishra et al[13] identified several factors associated with GBC, notably age 50 years or older, low literacy, below-poverty-line socioeconomic status, infrequent bowel habits, history of hypertension, use of antihypertensive medications, non-vegetarian diet, cooking with firewood, consumption of hand pump water, and high coffee intake[13]. Gallstones are the most significant risk factor for GBC, present in 60%–90% of GBC patients compared to 20%–25% of an age-matched population[14]. Several studies conducted in the early 2000s explored the relationship between gallstones and GBC. Mhatre et al[15] identified that the presence of gallstones is associated with an elevated risk of developing GBC. Dutta et al[16] found that the presence of gallstones was an independent determinant associated with a younger age of patients with GBC (OR 4, 95%CI: 1.5-11; P = 0.006). Sharma et al[17] identified that the duration of symptom for gall stone disease is a significant risk factor for GBC. Conversely, Narang et al[18] found that ,a higher number and larger size of gallstones, along with the presence of cholesterol in gallstones, may also elevate the risk of developing GBC. Singh et al[19] reported that as the size of gallstones increases, there is a progression in the gallbladder mucosa response, transitioning from cholecystitis and hyperplasia to metaplasia, ultimately into carcinoma. Studies conducted in India highlighted the composition of bile and its role in gallbladder carcinoma. Sharma et al[19] discovered that patients with GBC had a substantial decrease in lipid species and an increase in bacterial taxa in their bile. The summary of other relevant publications on demography and etiopathogenesis is presented in Table 1[20-37].
| Serial No. | Sample size | Findings | Ref. |
| 1 | GBC (214) controls (214) | Biomass burning was recognized as a significant risk factor for GBC | Shridhar et al[20] |
| 2 | GBC (200); Gall stone disease (200) controls (200) | Residence in the Gangetic belt, consumption of tea, tobacco, joint family structure, chemical exposure, fried food, and high levels of secondary bile salts are risk factors of GBC | Jain et al[21] |
| 3 | GBC (54) | Cholelithiasis is a predisposing factor for GBC | Bhattacharjee and Nanda[22] |
| 4 | GBC (1291) | Exposure to high soil arsenic levels and proximity to river ganga are risk factors for GBC | Madhawi et al[23] |
| 5 | GBC (333) | Smoking, cholelithiasis, alcohol consumption, typhoid in the past, post-menopausal women are risk factors for GBC | Tyagi et al[24] |
| 6 | GBC (63) | Poor hygiene and water supply, malnutrition, cholelithiasis, tobacco and alcohol consumption are modifiable risk factors for GBC | Khan et al[25] |
| 7 | GBC (122); controls (122) | Education, intake of vitamin C, parity, and type of fuel used were significant factors for GBC | Panda et al[26] |
| 8 | GBC (49) | About 75% of patients diagnosed with GSD showed detectable H. pylori DNA in their gallbladder tissue | Bansal et al[27] |
| 9 | GSD (330) | As the stone size increases, gallbladder mucosa changes progress from cholecystitis to carcinoma | Mathur et al[28] |
| 10 | GBC (n = 11), Chronic cholecystitis (n = 23), Xantho-granulomatous cholecystitis (n = 11) | The cholesterol content in gallstones of GBC was significantly lower compared to that in benign gallbladder diseases | Srivastava et al[29] |
| 11 | GBC (390) | Chronic bacterial infection of bile is considered an etiological factor in the development of gallbladder carcinoma | Sharma et al[30] |
| 12 | GSD (101) | H. pylori colonizes regions of gastric metaplasia within the gallbladder | Misra et al[31] |
| 13 | GBC (328); controls (328) | Females, consumption of mustard oil, Family history, low socioeconomic status and drinking water from hand pump were the risk factors for GBC | Kumar et al[32] |
| 14 | GBC (27), GSD (196) | High prevalence of salmonella typhi in gall bladder carcinoma | Vaishnavi et al[33] |
| 15 | GBC (38) | Higher levels of biliary nitrate associated with the gallbladder carcinogenesis | Shukla et al[34] |
| 16 | GBC (n = 30); controls (n = 30) | Decreased levels of selenium (Se), zinc (Zn), and vitamin E are associated with an increased risk of gallbladder carcinoma | Shukla et al[35] |
| 17 | GBC (n = 30); controls (n = 30) | Significantly high biliary benzene hexachloride and dichloro diphenyl trichloroethane associated with gallbladder carcinogenesis | Shukla et al[36] |
| 18 | 150 GBC | Gall stones associated with development of metaplastic, dysplastic and neoplastic mucosal changes of gall bladder mucosa | Gupta et al[37] |
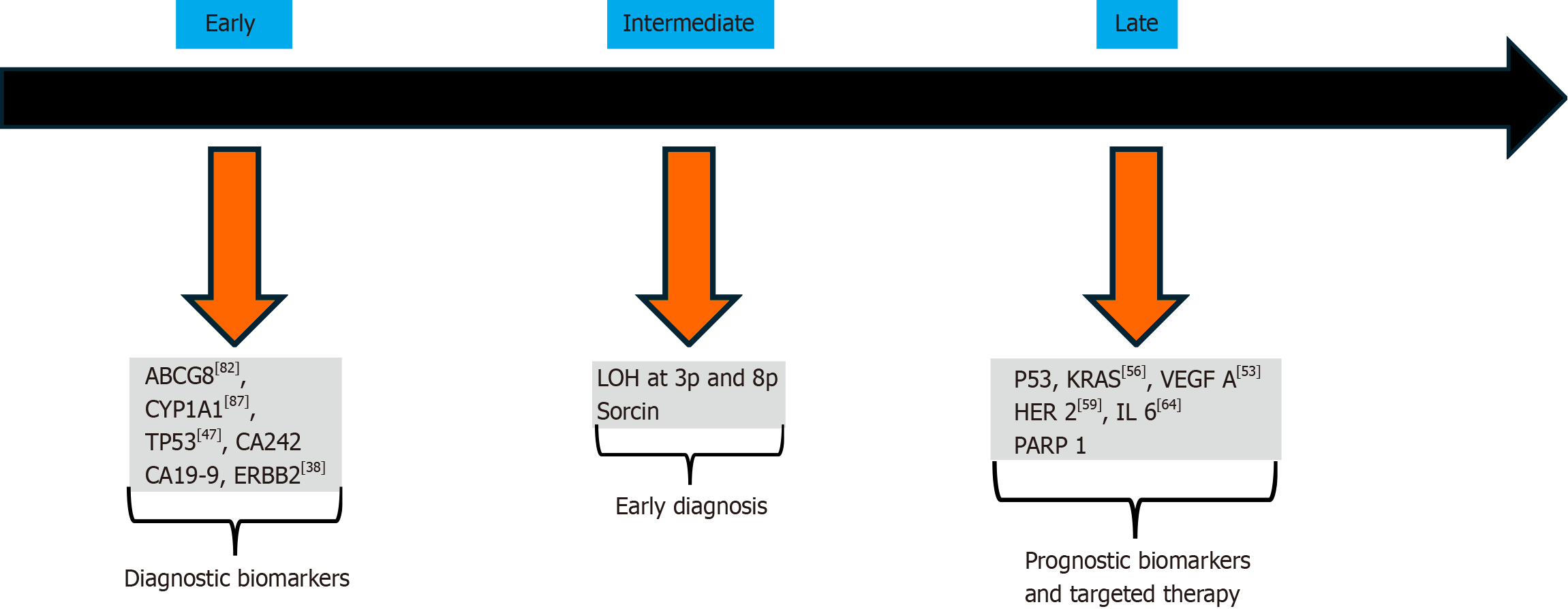
Our current understanding of the genetic and molecular changes associated with GBC remains limited. GBC, like other tumors, is driven by multiple genetic alterations. Several studies in India have aimed to elucidate the genetic mutations and molecular pathogenesis underlying gallbladder carcinoma. Studies from Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, have identified KRAS, PIK3CA, and EGFR are the some of commonly mutated genes in GBC. A summary of genes related to different stages of pathogenesis is illustrated in Figure 1. Iyer et al[38] identified ERBB2 alterations in early-stage GBC and proposed that anti-EGFR therapy (Afatinib) could be a promising therapeutic option. Kazmi et al[39] studied the role of KRAS and found that K-ras codon 12 mutations were highly prevalent in GBC and were associated with bad prognoses in resected GBC. Gupta et al[40] identified a new protein called Survivin, which was significantly associated with GBC, suggesting its role in the pathogenesis of GBC. Many studies were conducted regarding single gene expression and Global gene expression in GBC.
Many studies were conducted in India exploring single gene expression in GBC. Kumari et al[41] found that that C-erbB2 positive tumors exhibited a longer median survival compared to C-erbB2 negative tumors. Misra et al[42] studies p53 protein overexpression in gallbladder carcinoma. Singh and colleagues reported that MASPIN and THBS1 have an important epigenetic role in GBC[43]. Tekcham et al[44] identified downregulation of PTEN in GBC. Rai et al[45] found that the expression of CCKAR was significantly higher in GBC compared to GSD.
Several studies have also investigated global gene expression in GBC. Yadav et al[46] conducted a study on targeted gene sequencing of GBC and identified 184 somatic mutations and 60 germline mutations in cancer driver genes such as SMAD4, lysine methyltransferase 2C (KMT2C), and tumor protein p53. Kumari et al[47] used high-throughput methods and detected mutations in P53, STK11, RICTOR, TSC2, as well as FGF3-TACC fusion and FGF10 amplification using next-generation sequencing platform.
Loss of heterozygosity (LOH) is frequently observed in GBC. In GBCs this change is commonly seen as the loss of one copy of a gene (heterozygous allelic loss) in multiple chromosomal regions. This phenomenon has been extensively studied and is known as LOH. Priya et al[48] observed LOH in FHIT gene. Jain et al[49] detected LOH at 8 Loci, that is 3p12, 3p14.2, 5q21, 9p21, 9q, 13q, 17p13, and 18q for tumor suppressor genes (DUTT1, FHIT, APC, p16, FCMD, RB1, p53, and DCC genes). There are few studies that examined the aberrant promoter methylation gene. Pandey et al[50] from Lucknow studied candidate genes and identified GSTT1, NAT2, APOB, and MTHFR mutations. Numerous other studies on genetics and polymorphisms have been published, and some of them are summarized in Table 2[51-93].
| Serial No. | Sample size | Findings | Ref. |
| 1 | - | IGF-MAPK cascade, p38 MAPK pathway, p53 pathway, and FAS signaling pathway as highly enriched among dysregulated miRNAs in GBC | Saxena et al[51] |
| 2 | - | PARP1 rs1136410 (A/G) associated with early onset of GBC | Anjali et al[52] |
| 3 | GBC (29), controls (29) | VEGF-A expression can be used as potential prognostic biomarker in GBC | Singh et al[53] |
| 4 | - | Studied the prognostic significance of the oxidative stress marker 8-OH-dG and genes associated with the BER pathway | Singh et al[54] |
| 5 | GBC (25) | In gallbladder cancer patients, mutations were identified in both P53 and codon 12 of KRAS | Shukla et al[55] |
| 6 | - | Mutations in the sorcin gene associated with poor overall survival in GBC | Shabnam et al[56] |
| 7 | GBC (523), controls (274) | Studied the TERT-CLPTM1L and 8q24 Genetic Variants in GBC | Yadav et al[57] |
| 8 | GBC (50) | Individual and repetitive mutations of shh gene in GBC can be used as diagnostic marker | Dixit et al[58] |
| 9 | GBC (50) | Overexpression of Her2/neu and Ki67 in gallbladder cancer associated with lymph node metastasis | Pujani et al[59] |
| 10 | GBC (541), controls (307) | KRAS rs61764370 polymorphism is significantly associated with GBC | Kazmi et al[60] |
| 11 | - | Studied Epigenetic silencing of APC in advanced GBC. | Tekcham et al[61] |
| 12 | GBC (24) | Found 7 hypermethylated or down-regulated (e.g., FBN1, LPP, and SOD3) and 61 hypomethylated or up-regulated markers (e.g., HBE1, SNRPF, TPD52) for GBC | Sharma et al[62] |
| 13 | Cases (50) | The level of EGFR expression correlates with the aggressiveness of the disease | Kumar et al[63] |
| 14 | GBC (52) | Tγδ17 could serve as a potential predictive biomarker in GBC | Patil et al[64] |
| 15 | GBC (30) | The MTHFR A1298C polymorphism associated with development of GBC | Dixit et al[65] |
| 16 | GBC (37) | Telomere dysfunction and alterations are the earlier events in progression of GBC | Poojary et al[66] |
| 17 | GBC (148), controls (256) | CYP-17 gene polymorphism is associated with risk of gallbladder cancer | Dwivedi et al[67] |
| 18 | - | LXR-β polymorphisms associated with GBC | Sharma et al[68] |
| 19 | GBC (195), controls (300) | Vascular endothelial growth factor single nucleotide polymorphism associated with GBC | Mishra et al[69] |
| 20 | GBC (35) | mitochondrial D-loop mutation associated with GBC | Maurya et al[70] |
| 21 | GBC (410), controls (210) | Estrogen and progesterone receptor sequence associated with increased risk of GBC | Srivastava et al[71] |
| 22 | - | KRAS p.Q25H polymorphism associated with development of GBC | Parmanik et al[72] |
| 23 | GBC (230), controls (230) | Caspase-8 polymorphisms associated with GBC | Srivastava et al[73] |
| 24 | GBC (51) | p53 mutation are early events in the evolution of GBC | Agrawal et al[74] |
| 25 | GBC (185), controls (195) | CYP7A1 haplotype associated with GBC | Srivastava et al[75] |
| 26 | GBC (230), controls (230) | The role of pre-microRNA variants in GBC uncertain | Srivastava et al[76] |
| 27 | GBC (62) | Most common alteration in the p53 was frameshift mutation at codon 271 | Nigam et al[77] |
| 28 | GBC (40) | High LOH in CDH1 associated with pathogenesis of GBC | Priya et al[78] |
| 29 | GBC (212), controls (219) | Studied the DNMT3B -579 G > T promoter polymorphism | Srivastava et al[79] |
| 30 | GBC (233), controls (260) | Angiotensin I-converting enzyme insertion/deletion polymorphism associated with GBC | Srivastava et al[80] |
| 31 | GBC (126), controls (190) | Role of DNA repair pathways GB carcinogenesis | Srivastava et al[81] |
| 32 | GBC (171), controls (221) | Patients with ABCG8 variant allele are at a higher risk of GBC | Srivastava et al[82] |
| 33 | GBC (185), controls (200) | Complement receptor polymorphism associated with pathogenesis of GBC | Srivastava and Mittal[83] |
| 34 | GBC (173), controls (204) | Single nucleotide polymorphisms of DNA repair genes OGG1 and XRCC1; associated with low risk for GBC | Srivastava et al[84] |
| 35 | GBC (144), controls (210) | Role of CCR5+/Delta32 polymorphism associated with risk of GBC | Srivastava et al[85] |
| 36 | GBC (124), controls (166) | IL-1 gene polymorphisms associated with GBC | Vishnoi et al[86] |
| 37 | GBC (142), controls (217) | CYP1A1 C allele frequency associated with GBC | Pandey et al[87] |
| 38 | - | CYP7A1 polymorphism associated with GBC | Srivastava et al[88] |
| 39 | - | The X (+), D haplotype of APOB is associated risk for development of GBC | Pandey et al[89] |
| 40 | - | NAT2 slow acetylator phenotype associated with risk of GBC | Pandey et al[90] |
| 41 | GBC (129), controls (208) | LRPAP1 polymorphism associated with GBC | Pandey et al[91] |
| 42 | GBC (39) | Mutation in codon 12 of the K-ras oncogene associated with GBC, which indicate role of chronic inflammation in gallbladder carcinogenesis. | Singh et al[92] |
| 43 | GBC (117), controls (137) | The apoB-XbaI gene polymorphism associated with GBC | Singh et al[93] |
In this section, we have described clinicopathological parameters including presenting symptoms, screening methods, histopathological findings, prognostic factors, and tumor characteristics.
Patients with GBC typically present with pain in the right upper quadrant and epigastrium region of the abdomen, with or without changes in the character of the pain. They may also exhibit symptoms such as jaundice, features of GOO (gastric outlet obstruction), and an incidentally detected mass on imaging. In a study conducted by Pandey et al[94] the most common symptoms observed were weight loss (reported in 201 patients, 99%) followed by loss of appetite (reported in 197 patients, 97%). Other observed symptoms included pain in the right hypochondrium (reported in 143 patients, 70%), a mass in the right hypochondrium (reported in 107 patients, 53%), jaundice (reported in 79 patients, 39%), and nausea and vomiting (reported in 21 patients, 10%).
Many patients in India who undergo cholecystectomy do not routinely have their gallbladder specimens sent for histopathology due to financial burden. However, Agarwal et al[95] found that patients whose cholecystectomy specimens were sent for histopathological examination (HPE) experienced earlier management if GBC was found in the specimen, achieved a better R0 resection rate, and had longer overall survival (OS) compared to those in whom specimens were not sent. Histologically most of the GBCS are adenocarcinoma whereas most common architectural pattern observed was sheets and acini, with a predominance of columnar cells[96]. Yadav et al[97] classified GBC based on fine needle aspiration cytology (FNAC) into various subtypes, including Adenosquamous, mucinous, signet ring, squamous, small cell, mixed adenoneuroendocrine, undifferentiated carcinomas, as well as spindle and giant cell subtypes.
Chandra et al[98] have introduced a classification system based on FNAC, which includes the following categories: Category 1 (inadequate), Category 2 (negative for malignancy), Category 3 (atypical cells), Category 4 (highly atypical cells suggestive of malignancy), and Category 5 (positive for malignancy). Yadav et al[99] reported 28 cases of neuroendocrine tumors (NETs) of the gallbladder. They classified these tumors into three categories based on their differentiation: Well-differentiated (grades 1 and 2), small cell carcinoma (grade 3), and mixed adenoneuroendocrine carcinoma. A positive diagnosis of NET was confirmed by the presence of TTF-1 in the nucleus. Kamboj et al[100] published 19 cases of NEC of GB and described the aggressive nature of diseases. Krishnani et al[101] reported that FNAC demonstrated a high sensitivity of 90.63% and specificity of 94.74% in detecting carcinoma. For locally advanced or metastatic GBC, preoperative histopathological confirmation is mandatory before initiating chemotherapy. Goyal et al[102] concluded that FNAC can be a precise and reliable method for diagnosing GBC in these settings. Shukla et al[103] found that the diagnostic accuracy of ultrasonography (USG)-guided FNAC was 95%. This is significantly higher compared to a dia
Xanthogranulomatous cholecystitis (XGC) is malignant masquerade of GBC. Shukla et al[104] defined the pathological features of XGC, proposing that they include the presence of foam cells, histiocytes, bile, multinucleate giant cells, and a mixed population of inflammatory cells in varying proportions. These features are observed in a background of pink and granular debris.
Some immunohistochemistry (IHC) based study also published to diagnosis and prognosticate the disease. Some studies based on IHC have also been published to aid in the diagnosis and prognosis of the disease. Study conducted by Shukla et al[105], revealed that although it is a common occurrence in females, GBC does not express hormone receptor. Yadav et al[106] suggest that the overexpression of MMP9 and the loss of membranous beta-catenin may be indicative of poor clinical outcomes and advanced disease. Jain et al[107] found significant overexpression of KRT7 and SRI in node positive GBC patients compared to node negative GBC patients. This finding may have important implications for the diagnosis and treatment of GBC.
Analysis of cancer-derived extracellular vesicles (EVs) is an emerging method employed to identify potential bio
Numerous studies have been conducted to identify prognostic markers for GBC. Mishra et al[111] found that presence of Higher TNM stage, adjacent organ infiltration, nodal involvement, and jaundice predict poor survival. Negi et al[112] found that lymph node (LN) ratio is a strong predictor of outcome after curative resection (CR) for GBC. Shah et al[113] found that LN micro metastasis did not correlate with survival. Balachandran et al[114] found that Adjuvant therapy, R0 resection and extended cholecystectomy associated with improved survival in patient with GBC. Some other pathological studies are described in Table 3[115-126].
| Serial No. | Sample size | Findings | Ref. |
| 1 | GBC (50) | Her2 neu, p53, p16, survivin, COX-2, and EZH-2 expression associated with GBC | Gupta et al[115] |
| 2 | GBC (200), Dysplasia (32), CC (100) | HER-2/neu overexpression seen in patients with GBC | Jain et al[116] |
| 3 | GBC (128) | HER 2 and Ki-67 can be used as a prognostic biomarker for gallbladder carcinoma | Halder et al[117] |
| 4 | - | Combination of ALU247 and cfDNA provides good sensitivity, specificity in diagnosis of GBC | Kumari et al[118] |
| 5 | - | Proposed a scoring system for XGC | Rajaguru et al[119] |
| 6 | GBC (34) | Quantitative analysis of cfDNA may aid in early diagnosis | Kumari et al[120] |
| 7 | GBC (39), cholelithiasis (30), and control (25) | Overexpression of survivin is associated with poor prognosis | Nigam et al[121] |
| 8 | (GSD, n =30; GBC, n = 39) healthy control (n = 25) | Expression of survivin in peripheral blood could be useful both in the diagnosis and prognosis of GBC | Nigam et al[122] |
| 9 | GBC (80) | P53 expression is positively correlated with increasing tumor grade, whereas beta-catenin nuclear expression is associated with tumor grade and depth of invasion | Ghosh et al[123] |
| 10 | 9 case of Squamous cell carcinoma | Ultrasound-guided fine-needle aspiration is a useful minimally invasive investigation in the preoperative diagnosis of squamous cell carcinoma of the gallbladder | Gupta and Gupta[124] |
| 11 | GBC (55), vontrols (8) | Assay of CA242, CA19-9, CA15-3, and CA125 can be used as marker of carcinoma of the gallbladder | Shukla et al[125] |
| 12 | GBC (40) | Intraoperative bile cytology can be used for diagnosis of in situ and early invasive GBC | Arora et al[126] |
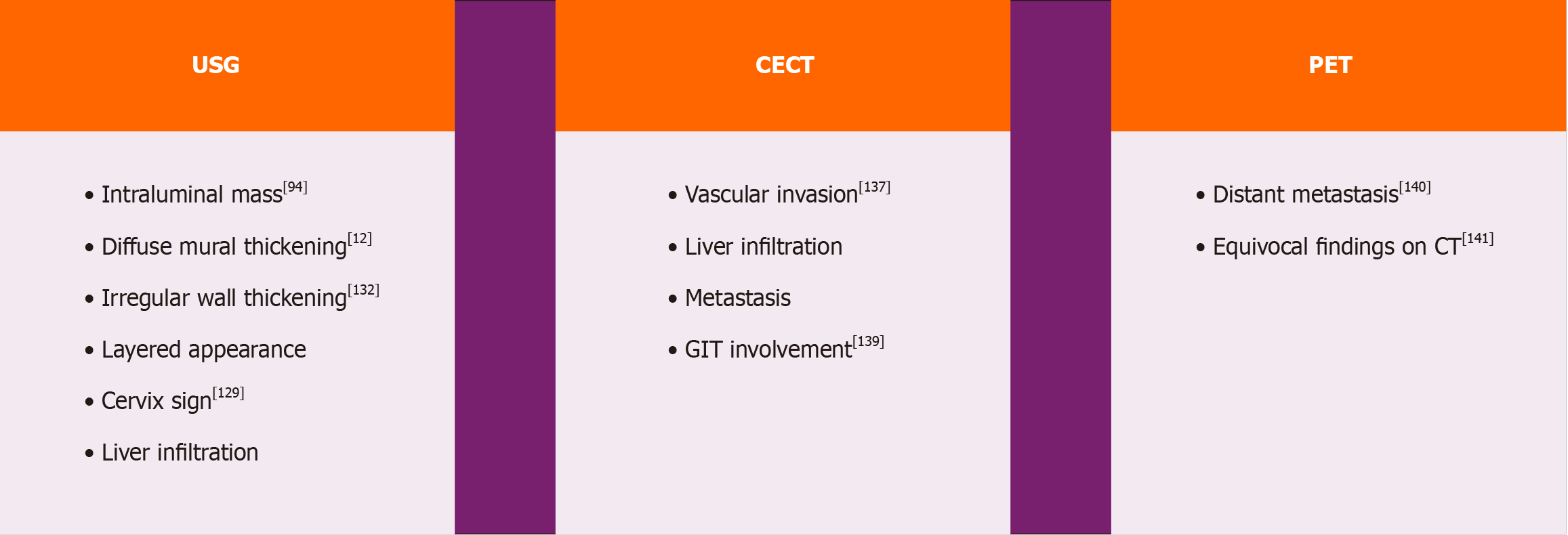
GBC presents significant diagnostic challenges due to its often-asymptomatic nature in the early stages and nonspecific symptoms as it progresses. Early diagnosis of GBC is crucial for improving patient outcomes. Various imaging modalities are critical for detecting and characterizing gallbladder lesions. USG is frequently chosen as the initial imaging modality for its accessibility and capability to visualize gallbladder abnormalities. Computed tomography (CT) and magnetic resonance imaging (MRI) offer detailed information on tumor extent, invasion of adjacent structures, and the presence of distant metastases. Endoscopic ultrasound (EUS) is a valuable tool for staging GBC due to its high sensitivity in detecting small lesions, assessing local tumor invasion, and facilitating biopsy when necessary. Additionally, positron emission tomography (PET) imaging can help evaluate distant metastases and guide treatment decisions.
Ultrasound is an essential diagnostic tool used for investigating medical conditions in patients. It can detect various abnormalities such as masses, ascites, metastasis, liver infiltration, and involvement of surrounding structures. It is considered as the primary investigation method in most cases. Gallbladder adenocarcinomas can appear as one of three morphologies in USG: Intraluminal mass, diffuse mural thickening, or a mass that replaces the gallbladder. Gallstones are commonly present along with mass in 60%–90% cases. Some retrospective studies were conducted to determine the accuracy of USG in diagnosing GBC. In a study conducted by Pandey et al[94], a mass lesion in the gallbladder was found in 87% of the 177 patients examined via abdominal sonography. The masses were classified as either intraluminal (59%) or infiltrative (41%), with irregular margins and higher echogenicity compared to the liver. Infiltrative lesions were more common at the gallbladder neck and fundus. In study by Batra et al[127] mass replacing GB was most common findings (73%) on USG followed by presence of gall stone along with mass (54%). Chhabra et al[128] demonstrated a new com
It’s quite challenging to differentiate between malignant and benign Thick-walled GB (TWGB). Many studies were conducted in this regard. Gupta et al[132] a recommended scoring system called Gallbladder Reporting and Data System can be used to evaluate gallbladder wall features in ultrasound scans. This system considers various factors such as layered appearance, interface with the liver, symmetry and extent of involvement, intramural features like cysts and echogenic foci. By using this system to risk stratify GB lesions; Surgeon can make more informed decisions about trea
Recently some authors studied the utility of EUS fine-needle aspiration (FNA) for evaluation of gallbladder mass. Singla et al[136] demonstrated that EUS-FNA is a highly sensitive tool for evaluating gallbladder mass lesions associated with obstructive jaundice. However, due to the low negative predictive value of the test, further evaluation is necessary for cases where FNA results are negative.
CT is the diagnosis modality of choice for staging the disease. Kalra et al[137] discovered that MDCT exhibited a sensitivity of 72.7%, a specificity of 100%, and an accuracy of 85% in assessing the resectability of GBC. To diagnose hepatic and vascular invasion by the tumor, CT showed a 100% correlation with surgical findings. According to the study conducted by Kumaran et al[138] CT scan has an overall accuracy of 93.3% in staging of GBC. Therefore Dual-phase helical CT is a comprehensive method that can be used for preoperative staging of GBC to determine if it is resectable. Soundararajan et al[139] proposed CT can be routinely used to determine gastro-intestinal tract involvement on GBC.
GBC are not pet avid tumors. Standard guidelines do not advocate routine pet scan in GBC. In India Many studies were published exploring the utility of PET-CT in GBC. According to a study conducted by Patkar et al[140] found that PET-CT can detect metastatic disease in 46.6% of patients that could not be confidently detected using standard cross-sectional imaging. Goel et al[141] found that PET-CT altered the management plan in approximately 25% of resectable GBC cases and 30%-35% of locally advanced cases. Shukla et al[142] studied role of PET-CT in incidental GBC and found that MDCT had a sensitivity and positive predictive value (PPV) of 42.8% each for determining residual disease, while PET-CT showed a sensitivity of 28.5% and PPV of 20%. In patients diagnosed incidentally with GBC and no metastatic disease, PET-CT and MDCT appear to offer complementary roles in clinical assessment. Goel et al[143] found that, the patients with PET-negative pT1b tumors showed no residual disease, in contrast to PET-positive patients (0% vs 33%, P = 0.028). They proposed that PET-negative pT1b patients could be monitored closely due to the low risk of relapse. Kumar et al[144] investigated the efficacy of PET-CT in detecting tumor recurrence in GBC. Their study demonstrated that PET/CT exhibited a high sensitivity (97.6%) and specificity (90%) for detecting tumor recurrence. These findings suggest that PET-CT can be a valuable tool in the detection of tumor recurrence, Residual tumor in incidental GBC and in cases with suspicious of distant metastasis.
In patients with GBC Sinha et al[145]discovered a significant association between serum levels of tumor markers (such as CA19-9, CEA, CA125, and CA242) and GBC. Agrawal et al[146] suggested that CA19-9, CA125 and CEA may serve as predictors of resectability in GBC. They found that elevated levels of CA19-9 and CA125 associated with poorer prognosis in affected patients. The role of different imaging modalities in the diagnosis of GBC is illustrated in Figure 2. Some other studies on diagnostics and imaging modality are described in Table 4[147-157].
| Serial No. | Sample size | Findings | Ref. |
| 1 | - | PET-CT can be used to rule out metastatic disease and for post-therapy surveillance for recurrence in patients of GBC | Bisht et al[147] |
| 2 | GBC (38) | Potential utility of CT texture-based radiomics analysis in patients with GBC. | Gupta et al[148] |
| 3 | GBC (141) | Raised levels of serum CA19-9 beyond 20 units/mL should be used for prognostication purposes after EC | Agrawal et al[149] |
| 4 | GBC (141) | NMR-based methods can be used as a diagnostic modality for GBC | Sharma et al[150] |
| 5 | GBC (41) | CEA expression may help in diagnosis of GBC | Mondal et al[151] |
| 6 | GBC (203) | Discontinuous mucosal lining, diffuse wall thickening, intramural nodules, and cholelithiasis may indicate XGC rather than gallbladder carcinoma | Sureka et al[152] |
| 7 | GBC (74) | Duodenal involvement significantly decreases resectability but does not preclude resection | Kalayarasan et al[153] |
| 8 | XGC (31) | Mass-forming XGC mimics GBC | Agarwal et al[154] |
| 9 | GBC (117) | CA 242 is a promising tumor marker for GBC and performs better than CEA and CA19-9. | Rana et al[155] |
| 10 | GBC (15) | Dynamic MRI with MRCP is a reliable method of showing gall bladder carcinoma. | Kaza et al[156] |
| 11 | GBC (60) | Color Doppler USG together can improve pickup rate of GBC | Pradhan et al[157] |
In this section, we have described various landmark studies published in the literature from India regarding the surgical approaches for GBC. Staging laparoscopy is highly accurate in identifying peritoneal deposits and sub centimetric metastases over liver surface and should be considered for all patients of GBC undergoing surgery. Agarwal et al[158] found, staging laparoscopy prevented laparotomy in 23% of patients with GBC. They suggested performing routine staging laparoscopy for GBC patients undergoing surgery. Another study by Agrawal et al[159] reported that staging laparoscopy prevented a nontherapeutic laparotomy in 43% of patients initially deemed resectable based on pre-operative imaging. Both of these studies underscored the importance of staging laparoscopy and advocated for its mandatory use in GBC.
Surgeons around the world do not widely accept laparoscopic cholecystectomy for TWGB due to the risk of malignancy. Srikanth et al[160] in their study proposed that laparoscopic cholecystectomy can be performed in patients with diffuse TWGB. However, there is an increased risk of to open cholecystectomy. Kapoor et al[161] proposed an approach of anticipatory extended cholecystectomy for TWGB (Luckow Approach).
After staging laparoscopy, the usual first step is the sampling of interaortocaval (IAC) LNs. GBC involving the 16b1 (IAC) LN is considered to be metastatic. Agarwal et al[162] studied the role of routine frozen-section HPE of the 16b1 LN in the management of GBC. They recommended the routine sampling of the 16b1 node during surgical procedures to prevent non-therapeutic radical resection. Their study found that routine 16b1 LN biopsy prevented such resections in 18.6% of patients. When 16b1 nodes tested positive, Agarwal et al[162] treated them along with systemic disease, and abandoned surgery when these nodes were found to be positive. Ghosh et al[163] from SGPGIMS, Lucknow echoed the same finding, they found that the median survival for IAC node positive group and patients with distant metastasis were same. Ho
According to previous studies, only 10% of patients with GBC are deemed resectable. For Tis and T1a lesions, a simple cholecystectomy with negative margins at the cystic duct is typically effective and can achieve cure rates ranging from 85% to 100%. However, gallbladder lesions classified as T1b and higher often come with nodal metastases, necessitating radical resection. This typically involves en bloc hepatic bed resection (which may include removing 2-3 cm of liver wedge or performing a formal segment IVb and V resection) along with regional lymphadenectomy. The surgical goal is R0 resection, aiming to achieve negative cystic duct margins (CDMs). In cases of positive margins, revision of the duct margin or extrahepatic bile duct (EHBD) excision may be necessary. Studies published in India exploring the ideal surgical approach for GBC have not yet provided concrete recommendations.
Singh et al[165] found that CR is the only chance of cure, aggressive surgical approach and improve OS. Wagholikar et al[166] advocated that simple cholecystectomy is effective only for GBC limited to the mucosa. However, patients diagnosed with pT1b tumors should undergo extended cholecystectomy. The study proposes that for cases where incidental GBC extends to the muscularis layer, early reoperation to complete an extended cholecystectomy is reco
There has been considerable debate regarding the optimal approach to liver resection in GBC, specifically whether anatomical segment IVb and V resection is superior to non-anatomical wedge excision of the liver. There are some studies regarding the same with conflicting results. Pottakkat et al[167] demonstrated that the extended criteria for radical resection (Segment 4b and 5) are highly effective, achieving R0 status in over 80% of GBC patients and yielding acceptable long-term outcomes. Nag et al[168] from GB Pant Hospital, New Delhi, discovered that there was no significant difference in mean recurrence-free survival (RFS) between segment 4b and 5 resections compared to wedge resection, with 58.2 months vs 42.3 months, respectively (P = 0.264). The OS was also similar between both of these groups. Therefore, they concluded that segment 4b and 5 resections were not superior to wedge resection. Tewari et al[169] from Banaras Hindu University (BHU) found that wedge resection of the gallbladder achieves adequate oncological clearance in early GBC.
Studies have shown that the surgical margin status is the most important factor in determining the overall outcome of liver resection surgeries, rather than the type of resection performed. Behari et al[170] found similar results, with patients who underwent R0 resections having significantly better survival rates than those who underwent R1 resections (median 25.8 months vs 17.0 months; P = 0.03). Hence, the primary objective should be to achieve complete removal of the disease with clear histologic margins.
Routine use of CDM frozen section analysis is controversial. Some study from India refuted this practice. Jajal et al[171] suggest that routine CDM frozen analysis may not be necessary in patients with resectable GBC who do not have jaundice. They found that a positive CDM has comparable survival outcomes to a negative CDM, with similar R0 resection rates and tumor stages. Some authors suggest that routine resection of the common bile duct during surgery may aid in lymphadenectomy and increase LN yield. However, a study by Behari et al[170] from India found that EHBD resection does not offer significant survival benefits.
The standard lymphadenectomy for GBC involves removing LNs located along the hepatoduodenal ligament, common hepatic artery, and peri-pancreatic region (stations 8, 12, and 13). Pandey et al[172] from BHU proposed this systemic regional lymphadenectomy in GBC gives a good lymph nodal yield and better outcome. Negi et al[112] suggested that the retrieval and examination of at least 6 nodes can have a positive impact on the quality of staging and disease-free survival (DFS) in node-negative patients. Goel et al[173] from TMH found that for individuals diagnosed with T1b GB cancer, the likelihood of nodal positivity is approximately 21%. Therefore, they recommended that radical surgery, including comprehensive periportal lymphadenectomy, should be considered the standard of care.
Some authors believe that the presence of obstructive jaundice is a sign of inoperability. Agarwal et al[174] conducted a study on 51 patients with GB cancer and obstructive jaundice. Of these 51 patients, 14 underwent resection with curative intent, resulting in a resectability rate of 27.45%. Despite being jaundiced, these patients were able to undergo successful resection. They found a mortality rate of 7.14% and a morbidity rate of 50%. The mean DFS was 23.46 months, with a median of 26 months and a range spanning from 2 to 62 months. Among patients who underwent resection, 50% survived beyond two years. They concluded that Biliary obstruction in GB cancer is not signs of inoperability and resection results in prolongation of survival[174]. Pandey et al[172] found that EHBD resection to achieve R0 resection is safe, associated with acceptable postoperative morbidity, and may increase survival. A positive CDM, direct infiltration of the hepatoduodenal ligament, and densely adherent peri-choledochal LNs were the indicators of EHBD resection. K et al[175] studied 59 GBC patients who had jaundice. Total of 61.7% and 84%, respectively, of patients underwent CR and non-CR had common bile duct involvement (P = 0.062). They found that Patients with GBC and jaundice have better significant survival after CR.
There are some reserves among surgeons for extended resection in carcinoma GB. As operative techniques improve and new technology develops, there is an increasing trend in extended resection. Commonly performed extended procedures are segmentectomy 4B + 5, extended right hepatectomy, median sectorectomy and hepatopancreaticoduodenectomy. Pottakkat et al[167] studied the outcomes of extended resection in GBC and identified postoperative complications in 60% of patients. They achieved acceptable R0 resection (83%) and survival rates. But Behari et al[170] could find that only 43% of GBC undergoing extended resection could achieved R0 resection. Singh et al[176] from Rajiv Gandhi Cancer institute first to explore PVE followed by extended hepatectomy in GBC. In their study on 14 patients following Neo adjuvant chemo therapy (NACT) and portal vein embolization. Seven (50%) underwent CR (extended hepatectomy). The median OS was 27 months for resectable patients and 15 months for unresectable patients.
Agarwal et al's[177] study, which examined the duodenum's involvement in GBC, revealed that, of the 43 patients with duodenal involvement, 26 had R0 resection (61.9%). Of these, 16 had duodenal sleeve resection (61.54%), 9 had distal gastrectomy with resection of the first part of the duodenum (34.62%), and 1 patient had hepato-pancreatoduodenectomy (HPD; 3.85%). They concluded that duodenal infiltration does not serve as a sign of unresectability or a reason to undergo HPD. For the majority of these patients, a distal gastrectomy combined with resection of the first segment of the duodenum, or a duodenal sleeve resection can accomplish an oncologically appropriate R0 resection[178].
With surgeons now willing for extended resection, further some studies published for resection in presence of metastasis. Patkar et al[179] from TMH propose a potential role for aggressive treatment of advanced GBC with limited metastatic burden, such as microscopic disease in the station 16b1 node, low-burden peritoneal disease with deposits smaller than 1 cm in adjacent omentum or diaphragm, isolated N2 disease, or a solitary discontinuous liver metastasis in adjacent liver parenchyma.
With advance laparoscopic and robotic surgery now getting prominence, Agarwal et al[180] found that laparoscopic radical cholecystectomy is safe and feasible for selected patients with GBC, and the outcomes are comparable to those of open radical cholecystectomy. Nag et al[181] found laparoscopic hepatic bisegmentectomy is safe and feasible. Palanisamy et al[182] from GEM hospital published their study and suggested that laparoscopic radical cholecystectomy may serve as a viable and feasible option for managing GBC, providing effective oncological clearance. Additionally, it offers the typical benefits associated with minimal access procedures. Goel et al[183] from TMH suggested robotic radical cholecystectomy is safe and feasible and the short-term results are compared with Open radical cholecystectomy.
Incidentally detected carcinoma GB is another debatable topic. Some studies have been published on incidentally detected gallbladder carcinoma from India. Shukla et al[184] studied the factors influencing operability in incidentally detected GBC and found that stage is the most important factor in determining resectability. They found that as the T-stage of the disease progressed, the probability of finding residual disease increased, while operability decreased. They also found that incidence of lymph nodal disease is higher for pT1b cancers. Wagholikar et al[166] suggested that, incidental GBC extending up to the muscularis requires completion extended cholecystectomy. Although there are no prospective clinical trials, it is widely believed that complete resection of residual disease leads to improved survival. Multiple studies have shown a survival advantage with CR, thereby supporting re-resection in incidental GBC beyond pT1b. Patkar et al[185] from TMH, found contrasting results in their study on 425 patients of PT2 of GBC out of which 118 (27.7%) and 307 (72.23%) patients were in the upfront operated GBC and incidental GBC groups, respectively. They found that incidentally detected pT2 GBCs have significantly worse outcomes compared to similarly staged patients undergoing an upfront radical cholecystectomy. Some other studies on surgical management are described in Table 5[186-189].
| Serial No. | Sample size | Findings | Ref. |
| 1 | GBC (521) | Surgical resection improves survival in GBC with jaundice | Goel et al[186] |
| 2 | GBC (97) | Radical cholecystectomy with wedge resection of the liver oncological equivalence compared to formal segment IVb/V excision | Patkar et al[187] |
| 3 | GBC (20) | The technique of LHBRL is safe and feasible for patients with GBC | Nag et al[188] |
| 4 | 07 incidental GBC | Re-exploration and aggressive resection followed by adjuvant chemotherapy for incidental gallbladder carcinoma are safe and provide hope for long-term survival | Kaman et al[189] |
Despite improvement in surgical techniques, survival and resection rate of GBC remained the same. Gradually multi-modality therapy evolved with the aim of improving survival. Patkar et al[190] published the experience of TMH, Mumbai with largest cohort of 1307 patients with GBC. They advocated peri-operative chemotherapy both in adjuvant and neo adjuvant settings in properly selected patients. We will first discuss adjuvant therapy followed by neoadjuvant therapy and advances made in these fields.
The role of adjuvant therapy on increasing survival in GBC is controversial. To date, there have been only a few randomized studies conducted in India exploring the role of adjuvant radiotherapy (RT) or chemo RT (CRT) in resected GBC.
Ostwal et al[191] from TMH, Mumbai in their study of 242 resectable stage II and III GBC patients found that patients undergoing R0 resections receiving adjuvant Gemcitabine and cisplatin bases chemotherapy have high completion rates and good tolerance. Kattepur et al[192] found that adjuvant chemotherapy reduces distant failure rates but did not improve OS in completely resected GBC patients. They found that lymphovascular invasion important predictor of RFS in T2N0 patients. Some centers tried RT in adjuvant setting. Mahantshetty et al[193] from TMH suggested adjuvant RT along with CT improve survival in GBC after resection. Authors used 5 FU bases regimen in their cohort of patients. Choudhary and Asthana[194] studied the effect of adjuvant treatment, including RT, CT, or CRT, following CR and found improved survival compared to surgery alone but the difference was not statistically significant. Agrawal et al[195] found that Adjuvant CRT followed by adjuvant chemotherapy improves outcomes in patients with R1 and node-positive disease. There is only one Randomized control trial available that examines the function of CT and RT in adjuvant situations.
The GB-GECCOR trial, a phase II multicenter study, investigated two treatment arms: CAPE-RT (consisting of 2-4 cycles of capecitabine followed by RT: 45 Gy over 25 fractions concurrent with capecitabine: 825 mg/m2 twice daily, followed by 2-4 additional cycles of capecitabine) vs GC (gemcitabine at 1000 mg/m2 and cisplatin at 25 mg/m2 administered on days 1 and 8, every 3 wk) following resection. The DFS rate for the GC group was 88.9% vs 77.8% for CAPE-RT group. These outcomes support the application of both regimens as appropriate care[196].
The appropriate regimen for unresectable and metastatic disease has not yet been defined. Many studies have been published studying different therapies in these settings. Many studies shown that Gemcitabine/cisplatin combination well tolerated and in advanced unresectable GBC[197-199]. Chatni et al[200] studied infusion chemotherapy with cisplatin and fluorouracil. Sharma et al[201] studied the gemcitabine and oxaliplatin regimen in unresectable GB cancer and found a survival benefit. Same authors studied the efficacy of chemotherapy compared to best supportive care and reported that chemotherapy improved OS and progression free survival (PFS) in unresectable GBC. Ali et al[202] in their comparative study between Cisplatin-5-Fluorouracil and Gemcitabine-Cisplatin and found overall more benefit in Gemcitabine-Cisplatin arm. Singh et al[203] advocated use of Gemcitabine based regimen in metastatic and advanced GBC which improved PFS.
Currently there is no “standard” second-line therapy after failure of first-line gemcitabine and cisplatin in patients with GBC. Ramaswamy et al[204] found that CAP-IRI is a well-tolerated second-line chemotherapeutic regimen in advanced GBC. In GB-SELECT trial they found that single agent irinotecan can be used as second line treatment in palliative setting. Some other important study on Adjuvant therapy in metastatic disease are described in Table 6[205-214].
| Serial No. | Sample size | Findings | Ref. |
| 1 | GBC (176) | CT followed by CTRT improves outcomes in patients with limited volume disease | Alam et al[205] |
| 2 | GBC (550) | CT followed by cCTRT appears to improve survival in responders with good PS | Alam et al[206] |
| 3 | GBC (38) | Her2neu directed therapy significantly improved survival | Das et al[207] |
| 4 | GBC (66) | FOLFOX-4 is an effective and well-tolerated regimen as a second-line treatment | Dodagoudar et al[208] |
| 5 | GBC (87) | CAP-IRI is a well-tolerated second-line chemotherapeutic regimen in advanced GBC | Ramaswamy et al[209] |
| 6 | GBC (121) | Reduced dose intensity of chemotherapy in GBC | Gangopadhyay et al[210] |
| 7 | GBC (210) | Use of gem-platinum in Indian patients associated with slightly worse outcomes | Sirohi et al[211] |
| 8 | - | Autologous immune enhancement therapy associated with, an improvement of the quality of life | Bhamare et al[212] |
| 9 | GBC (104) | Adjuvant chemoradiation improve survival | Mallick et al[213] |
| 10 | - | Chemoradiation improve survival in locally advanced GBC | Engineer et al[214] |
Surgical resection is the only potential cure for GBC. However, many patients are present with locally advanced state, making it difficult to perform curative surgery. Neoadjuvant therapy can effectively downstage tumors in these patients and help assess disease biology to identify the most suitable candidates for surgery. TMH has taken a step ahead and started using NACT as an option in locally advanced GBC. Chaudhari et al[215] introduced the TMH criteria for neoadjuvant chemotherapy in locally advanced or borderline resectable GBC, leading to curative surgical resection in a significant percentage of patients. Agrawal et al[216] demonstrated the benefit of neoadjuvant chemo-RT in locally advanced GBC. In their study they have reported Radiologic downstaging of liver involvement is 40.5% and lymphadenopathy is 67.5%[216].
A group of investigators is presently carrying out a prospective clinical experiment called POLCA-GB at TMH in Mumbai. This experiment aims to assess the efficacy of chemotherapy administered alone (four cycles of gemcitabine-cisplatin or gemcitabine-oxaliplatin) vs chemotherapy administered in combination with radiation treatment (RT) contemporaneous with gemcitabine, followed by two rounds of gemcitabine-cisplatin or gemcitabine-oxaliplatin chemotherapy. This trial aims to enable potentially CR by downstaging locally advanced GBC. The trial is now in the recruiting stage[217]. Few other series from India have demonstrated a benefit of gemcitabine-platinum based neoadjuvant therapy in GBC described in Table 7[218-221].
| Serial No. | Sample size | Findings | Ref. |
| 1 | GBC (510) | Surgery and peri-operative systemic therapy associated with improved survival | Patkar et al[218] |
| 2 | GBC (28) | Locally advanced GBC may benefit from neoadjuvant chemoradiation | Engineer et al[219] |
| 3 | GBC (37) | Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer | Sirohi et al[220] |
| 4 | GBC (21) | Resection after neoadjuvant chemotherapy improve survival | Selvakumar et al[221] |
This review has found significant work and efforts made by India in addressing GBC over the past two decades. India has produced a substantial number of quality research publications on GBC-epidemiology, etiopathogenesis, pathology, surgery, and multimodal treatment approaches. Country has progressed well on surgical management and multimodal treatment strategies. However, there remains a lack of specific research on etiopathogenesis, early diagnosis, preventive measures, and emerging treatments such as immunotherapy. The coming decades should prioritize efforts towards screening strategy in high-risk zone, early detection, and the development of well-defined treatment protocol/ policy. Collaborative international research would be useful to achieve and overcome the deficient areas.
| 1. | Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 7890] [Article Influence: 7890.0] [Reference Citation Analysis (2)] |
| 3. | Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Chin Clin Oncol. 2019;8:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Phadke PR, Mhatre SS, Budukh AM, Dikshit RP. Trends in gallbladder cancer incidence in the high- and low-risk regions of India. Indian J Med Paediatr Oncol. 2019;40:90-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | S ST, Krishnan SK, Das P, Sudarshan KL, Kotian CM, Santhappan S, Vishwakarma MB, N S, Mathur P. Descriptive Epidemiology of Gastrointestinal Cancers: Results from National Cancer Registry Programme, India. Asian Pac J Cancer Prev. 2022;23:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Mhatre SS, Nagrani RT, Budukh A, Chiplunkar S, Badwe R, Patil P, Laversanne M, Rajaraman P, Bray F, Dikshit R. Place of birth and risk of gallbladder cancer in India. Indian J Cancer. 2016;53:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Gupta SK, Ansari MA, Shukla VK. What makes the Gangetic belt a fertile ground for gallbladder cancers? J Surg Oncol. 2005;91:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mishra RR, Tewari M, Shukla HS. Helicobacter pylori and pathogenesis of gallbladder cancer. J Gastroenterol Hepatol. 2011;26:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Nath G, Singh YK, Kumar K, Gulati AK, Shukla VK, Khanna AK, Tripathi SK, Jain AK, Kumar M, Singh TB. Association of carcinoma of the gallbladder with typhoid carriage in a typhoid endemic area using nested PCR. J Infect Dev Ctries. 2008;2:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tewari M, Mishra RR, Shukla HS. Salmonella typhi and gallbladder cancer: report from an endemic region. Hepatobiliary Pancreat Dis Int. 2010;9:524-530. [PubMed] |
| 11. | Mhatre S, Lacey B, Sherliker P, Chatterjee N, Rajaraman P, Goel M, Patkar S, Ostwal V, Patil P, Shrikhande SV, Chitkara G, Badwe R, Lewington S, Dikshit R. Reproductive factors and gall-bladder cancer, and the effect of common genetic variants on these associations: a case-control study in India. Int J Epidemiol. 2022;51:789-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mhatre S, Rajaraman P, Chatterjee N, Bray F, Goel M, Patkar S, Ostwal V, Patil P, Manjrekar A, Shrikhande SV, Badwe R, Dikshit R. Mustard oil consumption, cooking method, diet and gallbladder cancer risk in high- and low-risk regions of India. Int J Cancer. 2020;147:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Mishra K, Behari A, Shukla P, Tsuchiya Y, Endoh K, Asai T, Ikoma T, Nakamura K, Kapoor VK. Risk factors for gallbladder cancer development in northern India: A gallstones-matched, case-control study. Indian J Med Res. 2021;154:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 14. | Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Mhatre S, Richmond RC, Chatterjee N, Rajaraman P, Wang Z, Zhang H, Badwe R, Goel M, Patkar S, Shrikhande SV, Patil PS, Davey Smith G, Relton CL, Dikshit RP. The Role of Gallstones in Gallbladder Cancer in India: A Mendelian Randomization Study. Cancer Epidemiol Biomarkers Prev. 2021;30:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Dutta U, Nagi B, Garg PK, Sinha SK, Singh K, Tandon RK. Patients with gallstones develop gallbladder cancer at an earlier age. Eur J Cancer Prev. 2005;14:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Narang S, Goyal P, Bal M, Bandlish U, Goyal S. Gall stones size, number, biochemical analysis and lipidogram- an association with gall bladder cancer: a study of 200 cases. Int J Cancer Ther Oncol. 2014;2:020310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Singh A, Singh G, Kaur K, Goyal G, Saini G, Sharma D. Histopathological Changes in Gallbladder Mucosa Associated with Cholelithiasis: A Prospective Study. Niger J Surg. 2019;25: 21-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sharma N, Yadav M, Tripathi G, Mathew B, Bindal V, Falari S, Pamecha V, Maras JS. Bile multi-omics analysis classifies lipid species and microbial peptides predictive of carcinoma of gallbladder. Hepatology. 2022;76:920-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Shridhar K, Krishnatreya M, Kumar R, Kondal D, Bhattacharyya M, Kalita B, Snehil P, Singh AK, Kataki AC, Ghosh A, D Prabhakaran, Prabhakaran P, Dhillon PK. Household cooking fuel and gallbladder cancer risk: a multi-centre case-control study in India. Cancer Causes Control. 2024;35:281-292. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Jain K, Sreenivas V, Velpandian T, Kapil U, Garg PK. Risk factors for gallbladder cancer: a case-control study. Int J Cancer. 2013;132:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Bhattacharjee PK, Nanda D. Prospective observational study on cholelithiasis in patients with carcinoma gall bladder in a tertiary referral hospital of Eastern India. J Cancer Res Ther. 2019;15:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Madhawi R, Pandey A, Raj S, Mandal M, Devi S, Sinha PK, Singh RK. Geographical pattern of carcinoma gallbladder in Bihar and its association with river Ganges and arsenic levels: Retrospective individual consecutive patient data from Regional Cancer Centre. South Asian J Cancer. 2018;7:167-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tyagi BB, Manoharan N, Raina V. Risk factors for gallbladder cancer : A population based case-control study in Delhi. Indian J Med Paediatr Oncol. 2008;29:16-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Khan I, Panda N, Banerjee M, Das R. Epidemiological factors in gall bladder cancer in eastern India-a single centre study. Indian J Surg Oncol. 2013;4:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Panda D, Sharma A, Shukla NK, Jaiswal R, Dwivedi S, Raina V, Mohanti BK, Deo SV, Patra S. Gall bladder cancer and the role of dietary and lifestyle factors: a case-control study in a North Indian population. Eur J Cancer Prev. 2013;22:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Bansal VK, Misra MC, Chaubal G, Datta Gupta S, Das B, Ahuja V, Sagar S. Helicobacter pylori in gallbladder mucosa in patients with gallbladder disease. Indian J Gastroenterol. 2012;31:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Mathur SK, Duhan A, Singh S, Aggarwal M, Aggarwal G, Sen R, Garg S. Correlation of gallstone characteristics with mucosal changes in gall bladder. Trop Gastroenterol. 2012;33:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Srivastava M, Sharma A, Kapoor VK, Nagana Gowda GA. Stones from cancerous and benign gallbladders are different: A proton nuclear magnetic resonance spectroscopy study. Hepatol Res. 2008;38:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Sharma V, Chauhan VS, Nath G, Kumar A, Shukla VK. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology. 2007;54:1622-1625. [PubMed] |
| 31. | Misra V, Misra SP, Dwivedi M, Shouche Y, Dharne M, Singh PA. Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology. 2007;39:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Kumar JR, Tewari M, Rai A, Sinha R, Mohapatra SC, Shukla HS. An objective assessment of demography of gallbladder cancer. J Surg Oncol. 2006;93:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Vaishnavi C, Kochhar R, Singh G, Kumar S, Singh S, Singh K. Epidemiology of typhoid carriers among blood donors and patients with biliary, gastrointestinal and other related diseases. Microbiol Immunol. 2005;49:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Shukla VK, Prakash A, Chauhan VS, Singh S, Puneet. Biliary nitrate and risk of carcinoma of the gallbladder. Eur J Cancer Prev. 2004;13:355-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Shukla VK, Adukia TK, Singh SP, Mishra CP, Mishra RN. Micronutrients, antioxidants, and carcinoma of the gallbladder. J Surg Oncol. 2003;84:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Shukla VK, Rastogi AN, Adukia TK, Raizada RB, Reddy DC, Singh S. Organochlorine pesticides in carcinoma of the gallbladder: a case-control study. Eur J Cancer Prev. 2001;10:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Gupta SC, Misra V, Singh PA, Roy A, Misra SP, Gupta AK. Gall stones and carcinoma gall bladder. Indian J Pathol Microbiol. 2000;43:147-154. [PubMed] |
| 38. | Iyer P, Shrikhande SV, Ranjan M, Joshi A, Gardi N, Prasad R, Dharavath B, Thorat R, Salunkhe S, Sahoo B, Chandrani P, Kore H, Mohanty B, Chaudhari V, Choughule A, Kawle D, Chaudhari P, Ingle A, Banavali S, Gera P, Ramadwar MR, Prabhash K, Barreto SG, Dutt S, Dutt A. ERBB2 and KRAS alterations mediate response to EGFR inhibitors in early stage gallbladder cancer. Int J Cancer. 2019;144:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Kazmi HR, Chandra A, Nigam J, Noushif M, Parmar D, Gupta V. Prognostic significance of K-ras codon 12 mutation in patients with resected gallbladder cancer. Dig Surg. 2013;30:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Gupta V, Goel MM, Chandra A, Gupta P, Kumar S, Nigam J. Expression and clinicopathological significance of antiapoptotis protein survivin in gallbladder cancer. Indian J Pathol Microbiol. 2016;59:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Kumari N, Kapoor VK, Krishnani N, Kumar K, Baitha DK. Role of C-erbB2 expression in gallbladder cancer. Indian J Pathol Microbiol. 2012;55:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 42. | Misra S, Chaturvedi A, Goel MM, Mehrotra R, Sharma ID, Srivastava AN, Misra NC. Overexpression of p53 protein in gallbladder carcinoma in North India. Eur J Surg Oncol. 2000;26:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Singh TD, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic profiling of gallbladder cancer and gall stone diseases: Evaluation of role of tumour associated genes. Gene. 2016;576:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Tekcham DS, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic Downregulation of PTEN in Gallbladder Cancer. J Gastrointest Cancer. 2017;48:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Rai R, Tewari M, Kumar M, Singh TB, Shukla HS. Expression profile of cholecystokinin type-A receptor in gallbladder cancer and gallstone disease. Hepatobiliary Pancreat Dis Int. 2011;10:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Yadav S, DE Sarkar N, Kumari N, Krishnani N, Kumar A, Mittal B. Targeted Gene Sequencing of Gallbladder Carcinoma Identifies High-impact Somatic and Rare Germline Mutations. Cancer Genomics Proteomics. 2017;14:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Kumari N, Corless CL, Warrick A, Beadling C, Nelson D, Neff T, Krishnani N, Kapoor VK. Mutation profiling in gallbladder cancer in Indian population. Indian J Pathol Microbiol. 2014;57:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Priya TP, Kapoor VK, Krishnani N, Agrawal V, Agarwal S. Fragile histidine triad (FHIT) gene and its association with p53 protein expression in the progression of gall bladder cancer. Cancer Invest. 2009;27:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Jain K, Mohapatra T, Das P, Misra MC, Gupta SD, Ghosh M, Kabra M, Bansal VK, Kumar S, Sreenivas V, Garg PK. Sequential occurrence of preneoplastic lesions and accumulation of loss of heterozygosity in patients with gallbladder stones suggest causal association with gallbladder cancer. Ann Surg. 2014;260:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Pandey SN, Jain M, Nigam P, Choudhuri G, Mittal B. Genetic polymorphisms in GSTM1, GSTT1, GSTP1, GSTM3 and the susceptibility to gallbladder cancer in North India. Biomarkers. 2006;11:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Saxena R, Chakrapani B, Sarath Krishnan MP, Gupta A, Gupta S, Das J, Gupta SC, Mirza AA, Rao S, Goyal B. Next generation sequencing uncovers multiple miRNAs associated molecular targets in gallbladder cancer patients. Sci Rep. 2023;13:19101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 52. | Anjali K, Singh D, Kumar P, Kumar T, Narayan G, Singh S. PARP1 rs1136410 (A/G) polymorphism is associated with early age of onset of gallbladder cancer. Eur J Cancer Prev. 2022;31:311-317. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Singh P, Jain SL, Sakhuja P, Agarwal A. Expression of VEGF-A, HER2/neu, and KRAS in gall bladder carcinoma and their correlation with clinico-pathological parameters. Indian J Pathol Microbiol. 2021;64:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Singh N, Kazim SN, Sultana R, Tiwari D, Borkotoky R, Kakati S, Nath Das N, Kumar Saikia A, Bose S. Oxidative stress and deregulations in base excision repair pathway as contributors to gallbladder anomalies and carcinoma - a study involving North-East Indian population. Free Radic Res. 2019;53:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Shukla SK, Singh G, Shahi KS, Bhuvan, Pant P. Genetic Changes of P(53) and Kras in Gallbladder Carcinoma in Kumaon Region of Uttarakhand. J Gastrointest Cancer. 2020;51:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Shabnam B, Padmavathi G, Banik K, Girisa S, Monisha J, Sethi G, Fan L, Wang L, Mao X, Kunnumakkara AB. Sorcin a Potential Molecular Target for Cancer Therapy. Transl Oncol. 2018;11:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Yadav S, Chandra A, Kumar A, Mittal B. Association of TERT-CLPTM1L and 8q24 Common Genetic Variants with Gallbladder Cancer Susceptibility and Prognosis in North Indian Population. Biochem Genet. 2018;56:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Dixit R, Pandey M, Tripathi SK, Dwivedi AN, Shukla VK. Comparative Analysis of Mutational Profile of Sonic hedgehog Gene in Gallbladder Cancer. Dig Dis Sci. 2017;62:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Pujani M, Makker I, Makker A, Jetley S, Goel MM. Expression of Human Epidermal Growth Factor Receptor (Her 2/neu) and Proliferative Marker Ki-67: Association with Clinicopathological Parameters in Gallbladder Carcinoma. Asian Pac J Cancer Prev. 2016;17:3903-3909. [PubMed] |
| 60. | Kazmi HR, Chandra A, Kumar S, Satyam LK, Gupta A, Nigam J, Srivastava M, Mittal B. A let-7 microRNA binding site polymorphism in the KRAS 3'UTR is associated with increased risk and reduced survival for gallbladder cancer in North Indian population. J Cancer Res Clin Oncol. 2016;142:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Tekcham DS, Poojary SS, Bhunia S, Barbhuiya MA, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic regulation of APC in the molecular pathogenesis of gallbladder cancer. Indian J Med Res. 2016;143:S82-S90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Sharma P, Bhunia S, Poojary SS, Tekcham DS, Barbhuiya MA, Gupta S, Shrivastav BR, Tiwari PK. Global methylation profiling to identify epigenetic signature of gallbladder cancer and gallstone disease. Tumour Biol. 2016;37:14687-14699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Kumar N, Khan MA, Kumar N; Rigvardhan, Ranjan R, Hazra N. Epidermal growth factor receptor expression in carcinoma gallbladder: A prospective study in Indian scenario. J Cancer Res Ther. 2016;12:959-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Dixit R, Singh G, Pandey M, Basu S, Bhartiya SK, Singh KK, Shukla VK. Association of Methylenetetrahydrafolate Reductase Gene Polymorphism (MTHFR) in Patients with Gallbladder Cancer. J Gastrointest Cancer. 2016;47:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Poojary SS, Mishra G, Gupta S, Shrivastav BR, Tiwari PK. Dysfunction of subtelomeric methylation and telomere length in gallstone disease and gallbladder cancer patients of North Central India. J Hepatobiliary Pancreat Sci. 2016;23:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Dwivedi S, Agrawal S, Singh S, Madeshiya AK, Singh D, Mahdi AA, Chandra A. Association of Cytochrome-17 (MspA1) Gene Polymorphism with Risk of Gall Bladder Stones and Cancer in North India. Asian Pac J Cancer Prev. 2015;16:5557-5563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Sharma KL, Misra S, Kumar A, Mittal B. Association of liver X receptors (LXRs) genetic variants to gallbladder cancer susceptibility. Tumour Biol. 2013;34:3959-3966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Mishra K, Behari A, Kapoor VK, Khan MS, Prakash S, Agrawal S. Vascular endothelial growth factor single-nucleotide polymorphism in gallbladder cancer. J Gastroenterol Hepatol. 2013;28:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Maurya SK, Tewari M, Shukla HS. Gallbladder carcinoma: high rate of mitochondrial D-loop mutations. Diagn Mol Pathol. 2013;22:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Srivastava A, Sharma KL, Srivastava N, Misra S, Mittal B. Significant role of estrogen and progesterone receptor sequence variants in gallbladder cancer predisposition: a multi-analytical strategy. PLoS One. 2012;7:e40162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Pramanik V, Sarkar BN, Kar M, Das G, Malay BK, Sufia KK, Lakkakula BV, Vadlamudi RR. A novel polymorphism in codon 25 of the KRAS gene associated with gallbladder carcinoma patients of the eastern part of India. Genet Test Mol Biomarkers. 2011;15:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Srivastava K, Srivastava A, Mittal B. Caspase-8 polymorphisms and risk of gallbladder cancer in a northern Indian population. Mol Carcinog. 2010;49:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Agrawal V, Goel A, Krishnani N, Pandey R, Agrawal S, Kapoor VK. p53, carcinoembryonic antigen and carbohydrate antigen 19.9 expression in gall bladder cancer, precursor epithelial lesions and xanthogranulomatous cholecystitis. J Postgrad Med. 2010;56:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Srivastava A, Choudhuri G, Mittal B. CYP7A1 (-204 A>C; rs3808607 and -469 T>C; rs3824260) promoter polymorphisms and risk of gallbladder cancer in North Indian population. Metabolism. 2010;59:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Srivastava K, Srivastava A, Mittal B. Common genetic variants in pre-microRNAs and risk of gallbladder cancer in North Indian population. J Hum Genet. 2010;55:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Nigam P, Misra U, Negi TS, Mittal B, Choudhuri G. Alterations of p53 gene in gallbladder cancer patients of North India. Trop Gastroenterol. 2010;31:96-100. [PubMed] |
| 78. | Priya TP, Kapoor VK, Krishnani N, Agrawal V, Agrawal S. Role of E-cadherin gene in gall bladder cancer and its precursor lesions. Virchows Arch. 2010;456:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Srivastava K, Srivastava A, Mittal B. DNMT3B -579 G>T promoter polymorphism and risk of gallbladder carcinoma in North Indian population. J Gastrointest Cancer. 2010;41:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Srivastava K, Srivastava A, Mittal B. Angiotensin I-converting enzyme insertion/deletion polymorphism and increased risk of gall bladder cancer in women. DNA Cell Biol. 2010;29:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Srivastava K, Srivastava A, Mittal B. Polymorphisms in ERCC2, MSH2, and OGG1 DNA repair genes and gallbladder cancer risk in a population of Northern India. Cancer. 2010;116:3160-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Srivastava A, Tulsyan S, Pandey SN, Choudhuri G, Mittal B. Single nucleotide polymorphism in the ABCG8 transporter gene is associated with gallbladder cancer susceptibility. Liver Int. 2009;29:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Srivastava A, Mittal B. Complement receptor 1 (A3650G RsaI and intron 27 HindIII) polymorphisms and risk of gallbladder cancer in north Indian population. Scand J Immunol. 2009;70:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Srivastava A, Srivastava K, Pandey SN, Choudhuri G, Mittal B. Single-nucleotide polymorphisms of DNA repair genes OGG1 and XRCC1: association with gallbladder cancer in North Indian population. Ann Surg Oncol. 2009;16:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Srivastava A, Pandey SN, Choudhuri G, Mittal B. CCR5 Delta32 polymorphism: associated with gallbladder cancer susceptibility. Scand J Immunol. 2008;67:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Vishnoi M, Pandey SN, Choudhuri G, Mittal B. IL-1 gene polymorphisms and genetic susceptibility of gallbladder cancer in a north Indian population. Cancer Genet Cytogenet. 2008;186:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Pandey SN, Choudhuri G, Mittal B. Association of CYP1A1 Msp1 polymorphism with tobacco-related risk of gallbladder cancer in a north Indian population. Eur J Cancer Prev. 2008;17:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Srivastava A, Pandey SN, Choudhuri G, Mittal B. Role of genetic variant A-204C of cholesterol 7alpha-hydroxylase (CYP7A1) in susceptibility to gallbladder cancer. Mol Genet Metab. 2008;94:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Pandey SN, Srivastava A, Dixit M, Choudhuri G, Mittal B. Haplotype analysis of signal peptide (insertion/deletion) and XbaI polymorphisms of the APOB gene in gallbladder cancer. Liver Int. 2007;27:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Pandey SN, Modi DR, Choudhuri G, Mittall B. Slow acetylator genotype of N-acetyl transferase2 (NAT2) is associated with increased susceptibility to gallbladder cancer: the cancer risk not modulated by gallstone disease. Cancer Biol Ther. 2007;6:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Pandey SN, Dixit M, Choudhuri G, Mittal B. Lipoprotein receptor associated protein (LRPAP1) insertion/deletion polymorphism: association with gallbladder cancer susceptibility. Int J Gastrointest Cancer. 2006;37:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Singh MK, Chetri K, Pandey UB, Kapoor VK, Mittal B, Choudhuri G. Mutational spectrum of K-ras oncogene among Indian patients with gallbladder cancer. J Gastroenterol Hepatol. 2004;19:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Singh MK, Pandey UB, Ghoshal UC, Srivenu I, Kapoor VK, Choudhuri G, Mittal B. Apolipoprotein B-100 XbaI gene polymorphism in gallbladder cancer. Hum Genet. 2004;114:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Pandey M, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28:227-232. [PubMed] [DOI] [Full Text] |
| 95. | Agarwal AK, Kalayarasan R, Singh S, Javed A, Sakhuja P. All cholecystectomy specimens must be sent for histopathology to detect inapparent gallbladder cancer. HPB (Oxford). 2012;14:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Rana C, Krishnani N, Kumari N. Ultrasound-guided fine needle aspiration cytology of gallbladder lesions: a study of 596 cases. Cytopathology. 2016;27:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Yadav R, Jain D, Mathur SR, Sharma A, Iyer VK. Gallbladder carcinoma: An attempt of WHO histological classification on fine needle aspiration material. Cytojournal. 2013;10:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Chandra S, Chandra H, Shukla SK, Sahu S. Fine-needle aspiration cytology of gallbladder with an attempt of cytomorphological classification. Cytojournal. 2019;16:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Yadav R, Jain D, Mathur SR, Iyer VK. Cytomorphology of neuroendocrine tumours of the gallbladder. Cytopathology. 2016;27:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Kamboj M, Gandhi JS, Gupta G, Sharma A, Pasricha S, Mehta A, Chandragouda D, Sinha R. Neuroendocrine Carcinoma of Gall Bladder: A Series of 19 Cases with Review of Literature. J Gastrointest Cancer. 2015;46:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Krishnani N, Shukla S, Jain M, Pandey R, Gupta RK. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000;44:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Goyal S, Prasad G, Chaudhary D, Sakhuja P, Srivastava S, Aggarwal AK. Role of Guided FNA in Gallbladder Cancer: A Retrospective 3-Year Study. J Cytol. 2023;40:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Shukla VK, Pandey M, Kumar M, Sood BP, Gupta A, Aryya NC, Shukla RC, Verma DN. Ultrasound-guided fine needle aspiration cytology of malignant gallbladder masses. Acta Cytol. 1997;41:1654-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Shukla S, Krishnani N, Jain M, Pandey R, Gupta RK. Xanthogranulomatous cholecystitis. Fine needle aspiration cytology in 17 cases. Acta Cytol. 1997;41:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Shukla PJ, Barreto SG, Gupta P, Neve R, Ramadwar M, Deodhar K, Mehta S, Shrikhande SV, Mohandas KM. Is there a role for estrogen and progesterone receptors in gall bladder cancer? HPB (Oxford). 2007;9:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Yadav K, Agarwal P, Kumar M, Gupta S, Mishra M, Maurya MK, Qayoom S, Goel MM. Immunohistochemical appraisal of epithelial mesenchymal transition type III in gall bladder cancer. Indian J Pathol Microbiol. 2023;66:44-53. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Jain V, Akhtar J, Priya R, Sakhuja P, Goyal S, Agarwal AK, Ghose V, Polisetty RV, Sirdeshmukh R, Siraj F, Gautam P. Tissue proteome analysis for profiling proteins associated with lymph node metastasis in gallbladder cancer. BMC Cancer. 2023;23:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 108. | Priya R, Jain V, Akhtar J, Saklani N, Sakhuja P, Agarwal AK, Polisetty RV, Sirdeshmukh R, Kar S, Gautam P. Proteomic profiling of cell line-derived extracellular vesicles to identify candidate circulatory markers for detection of gallbladder cancer. Front Oncol. 2022;12:1027914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 109. | Akhtar J, Jain V, Kansal R, Priya R, Sakhuja P, Goyal S, Agarwal AK, Ghose V, Polisetty RV, Sirdeshmukh R, Kar S, Gautam P. Quantitative tissue proteome profile reveals neutrophil degranulation and remodeling of extracellular matrix proteins in early stage gallbladder cancer. Front Oncol. 2022;12:1046974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Priya R, Jain V, Akhtar J, Chauhan G, Sakhuja P, Goyal S, Agarwal AK, Javed A, Jain AP, Polisetty RV, Sirdeshmukh R, Kar S, Gautam P. Plasma-derived candidate biomarkers for detection of gallbladder carcinoma. Sci Rep. 2021;11:23554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Mishra PK, Saluja SS, Prithiviraj N, Varshney V, Goel N, Patil N. Predictors of curative resection and long term survival of gallbladder cancer - A retrospective analysis. Am J Surg. 2017;214:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg. 2011;15:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Shah SH, Gupta N, Gupta G, Mehta A, Singh S. Lymph node micrometastasis in gallbladder cancer. Indian J Gastroenterol. 2017;36:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Balachandran P, Agarwal S, Krishnani N, Pandey CM, Kumar A, Sikora SS, Saxena R, Kapoor VK. Predictors of long-term survival in patients with gallbladder cancer. J Gastrointest Surg. 2006;10:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Gupta A, Gupta S, Mani R, Durgapal P, Goyal B, Rajput D, Rao S, Dhar P, Gupta M, Kishore S, Kant R. Expression of Human epidermal growth factor receptor 2, Survivin, Enhancer of zeste homolog -2, Cyclooxygenase-2, p53 and p16 molecular markers in Gall bladder carcinoma. J Carcinog. 2021;20:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Jain P, Goyal S, Chauhan G, Majumdar K, Ali S, Sakhuja P, Agarwal AK. HER-2/neu over expression in gall bladder adenocarcinoma: A quest for potential therapeutic target. Indian J Pathol Microbiol. 2020;63:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | Halder S, Kundu S, Chakraborty J, Chakrabarti S. Significance of HER2 and Ki-67 in Preneoplastic Lesions and Carcinoma of Gallbladder. J Gastrointest Cancer. 2019;50:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Kumari S, Husain N, Agarwal A, Neyaz A, Gupta S, Chaturvedi A, Lohani M, Sonkar AA. Diagnostic Value of Circulating Free DNA Integrity and Global Methylation Status in Gall Bladder Carcinoma. Pathol Oncol Res. 2019;25:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Rajaguru K, Mehrotra S, Lalwani S, Mangla V, Mehta N, Nundy S. New scoring system for differentiating xanthogranulomatous cholecystitis from gall bladder carcinoma: a tertiary care centre experience. ANZ J Surg. 2018;88:E34-E39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Kumari S, Tewari S, Husain N, Agarwal A, Pandey A, Singhal A, Lohani M. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol Oncol Res. 2017;23:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 121. | Nigam J, Chandra A, Kazmi HR, Parmar D, Singh D, Gupta V. Prognostic significance of survivin in resected gallbladder cancer. J Surg Res. 2015;194:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Nigam J, Chandra A, Kazmi HR, Parmar D, Singh D, Gupta V, M N. Expression of survivin mRNA in gallbladder cancer: a diagnostic and prognostic marker? Tumour Biol. 2014;35:9241-9246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Ghosh M, Sakhuja P, Singh S, Agarwal AK. p53 and beta-catenin expression in gallbladder tissues and correlation with tumor progression in gallbladder cancer. Saudi J Gastroenterol. 2013;19:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Gupta P, Gupta RK. Preoperative diagnosis of squamous cell carcinoma of the gallbladder by ultrasound-guided aspiration cytology: clinical and cytological findings of nine cases. J Gastrointest Cancer. 2012;43:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Shukla VK, Gurubachan, Sharma D, Dixit VK, Usha. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol. 2006;27:160-165. [PubMed] |
| 126. | Arora VK, Kumar S, Singh N, Bhatia A. Intraoperative bile cytology of the dysplasia-carcinoma in situ sequence of gallbladder carcinoma. Cancer. 2005;105:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Batra Y, Pal S, Dutta U, Desai P, Garg PK, Makharia G, Ahuja V, Pande GK, Sahni P, Chattopadhyay TK, Tandon RK. Gallbladder cancer in India: a dismal picture. J Gastroenterol Hepatol. 2005;20:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 128. | Chhabra M, Kalage D, Gupta P, Siddiqui R, Singh S, Yadav TD, Gupta V, Kaman L, Singh H, Irrinki S, Das C, Prakash G, Saikia UN, Nada R, Dutta U, Sandhu MS. Proposal for a new morphological "combined type" of gallbladder cancer: description of radiopathological characteristics and comparison with other morphological types. Abdom Radiol (NY). 2024;49:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Rana P, Pruthi H, Gupta P, Chhabra M, Soundararajan R, Singh S, Gulati A, Das CK, Yadav TD, Gupta V, Saikia UN, Dutta U, Sandhu M. Sonographic "Cervix Sign": A New Ancillary Sign of Gallbladder Neck Malignancy. J Clin Exp Hepatol. 2023;13:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Patel A, Singh H, Nair R, Buva D. 50P Ultrasound guided screening of gallbladder cancer in healthy volunteers: A prospective study from Northern India. Annals of Oncology. 2020;31:S260. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 131. | Anadure R, Sreen A, Singh H, Sharma A, Sharma R, Mohimen A, Gupta S. The utility of screening ultrasound in early diagnosis of gall bladder cancer among high-risk population. Oncol J India. 2021;5:8. [DOI] [Full Text] |
| 132. | Gupta P, Dutta U, Rana P, Singhal M, Gulati A, Kalra N, Soundararajan R, Kalage D, Chhabra M, Sharma V, Gupta V, Yadav TD, Kaman L, Irrinki S, Singh H, Sakaray Y, Das CK, Saikia U, Nada R, Srinivasan R, Sandhu MS, Sharma R, Shetty N, Eapen A, Kaur H, Kambadakone A, de Haas R, Kapoor VK, Barreto SG, Sharma AK, Patel A, Garg P, Pal SK, Goel M, Patkar S, Behari A, Agarwal AK, Sirohi B, Javle M, Garcea G, Nervi F, Adsay V, Roa JC, Han HS. Gallbladder reporting and data system (GB-RADS) for risk stratification of gallbladder wall thickening on ultrasonography: an international expert consensus. Abdom Radiol (NY). 2022;47:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 133. | Gupta P, Marodia Y, Bansal A, Kalra N, Kumar-M P, Sharma V, Dutta U, Sandhu MS. Imaging-based algorithmic approach to gallbladder wall thickening. World J Gastroenterol. 2020;26:6163-6181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (3)] |
| 134. | Kapoor A, Kapoor A, Mahajan G. Differentiating malignant from benign thickening of the gallbladder wall by the use of acoustic radiation force impulse elastography. J Ultrasound Med. 2011;30:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Kalage D, Gupta P, Gulati A, Yadav TD, Gupta V, Kaman L, Nada R, Singh H, Irrinki S, Das C, Dutta U, Sandhu M. Multiparametric MR imaging with diffusion-weighted, intravoxel incoherent motion, diffusion tensor, and dynamic contrast-enhanced perfusion sequences to assess gallbladder wall thickening: a prospective study based on surgical histopathology. Eur Radiol. 2023;33:4981-4993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 136. | Singla V, Agarwal R, Anikhindi SA, Puri P, Kumar M, Ranjan P, Kumar A, Sharma P, Bansal N, Bakshi P, Verma K, Arora A. Role of EUS-FNA for gallbladder mass lesions with biliary obstruction: a large single-center experience. Endosc Int Open. 2019;7:E1403-E1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Kalra N, Suri S, Gupta R, Natarajan SK, Khandelwal N, Wig JD, Joshi K. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol. 2006;186:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 138. | Kumaran V, Gulati S, Paul B, Pande K, Sahni P, Chattopadhyay K. The role of dual-phase helical CT in assessing resectability of carcinoma of the gallbladder. Eur Radiol. 2002;12:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 139. | Soundararajan R, Vanka S, Gupta P, Chhabra M, Rana P, Gulati A, Das CK, Saikia UN, Yadav TD, Gupta V, Kaman L, Singh H, Irrinki S, Dutta U, Sandhu MS. Gastrointestinal involvement in gallbladder cancer: Computed tomography findings and proposal of a classification system. Indian J Gastroenterol. 2023;42:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 140. | Patkar S, Chaturvedi A, Goel M, Rangarajan V, Sharma A, Engineer R. Role of positron emission tomography-contrast enhanced computed tomography in locally advanced gallbladder cancer. J Hepatobiliary Pancreat Sci. 2020;27:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 141. | Goel S, Aggarwal A, Iqbal A, Gupta M, Rao A, Singh S. 18-FDG PET-CT should be included in preoperative staging of gall bladder cancer. Eur J Surg Oncol. 2020;46:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Shukla PJ, Barreto SG, Arya S, Shrikhande SV, Hawaldar R, Purandare N, Rangarajan V. Does PET-CT scan have a role prior to radical re-resection for incidental gallbladder cancer? HPB (Oxford). 2008;10:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 143. | Goel M, Tamhankar A, Rangarajan V, Patkar S, Ramadwar M, Shrikhande SV. Role of PET CT scan in redefining treatment of incidental gall bladder carcinoma. J Surg Oncol. 2016;113:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Kumar R, Sharma P, Kumari A, Halanaik D, Malhotra A. Role of 18F-FDG PET/CT in detecting recurrent gallbladder carcinoma. Clin Nucl Med. 2012;37:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Sinha SR, Prakash P, Singh RK, Sinha DK. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World J Gastrointest Surg. 2022;14:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 146. | Agrawal S, Gupta A, Gupta S, Goyal B, Siddeek RAT, Rajput D, Chauhan U, Kishore S, Gupta M, Kant R. Role of carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125 as the predictors of resectability and survival in the patients of Carcinoma Gall Bladder. J Carcinog. 2020;19:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 147. | Bisht N, Lohia N, Singh S, Sarin A, Mahato A, Paliwal D, Sinha I, Bhatnagar S. Utility of 18-Flurodeoxyglucose Positron Emission Tomography-Computed Tomography ( (18) FDG PET-CT) in Gallbladder Cancer: Experience from a Tertiary Care Hospital. World J Nucl Med. 2023;22:276-283. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 148. | Gupta P, Rana P, Ganeshan B, Kalage D, Irrinki S, Gupta V, Yadav TD, Kumar R, Das CK, Gupta P, Endozo R, Nada R, Srinivasan R, Kalra N, Dutta U, Sandhu M. Computed tomography texture-based radiomics analysis in gallbladder cancer: initial experience. Clin Exp Hepatol. 2021;7:406-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Agrawal S, Lawrence A, Saxena R. Does CA 19-9 Have Prognostic Relevance in Gallbladder Carcinoma (GBC)? J Gastrointest Cancer. 2018;49:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 150. | Sharma RK, Mishra K, Farooqui A, Behari A, Kapoor VK, Sinha N. (1)H nuclear magnetic resonance (NMR)-based serum metabolomics of human gallbladder inflammation. Inflamm Res. 2017;66:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 151. | Mondal SK, Bhattacharjee D, Mandal PK, Biswas S. Histopathological study of gallbladder carcinoma and its mimics with role of carcinoembryonic antigen immunomarker in resolving diagnostic difficulties. Indian J Med Paediatr Oncol. 2017;38:411-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 152. | Sureka B, Singh VP, Rajesh SR, Laroia S, Bansal K, Rastogi A, Bihari C, Bhadoria AS, Agrawal N, Arora A. Computed Tomography (CT) and Magnetic Resonance (MR) Findings in Xanthogranulomatous Cholecystitis: Retrospective Analysis of Pathologically Proven 30 Cases - Tertiary Care Experience. Pol J Radiol. 2017;82:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 153. | Kalayarasan R, Javed A, Puri AS, Puri SK, Sakhuja P, Agarwal AK. A prospective analysis of the preoperative assessment of duodenal involvement in gallbladder cancer. HPB (Oxford). 2013;15:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 154. | Agarwal AK, Kalayarasan R, Javed A, Sakhuja P. Mass-forming xanthogranulomatous cholecystitis masquerading as gallbladder cancer. J Gastrointest Surg. 2013;17:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Rana S, Dutta U, Kochhar R, Rana SV, Gupta R, Pal R, Jain K, Srinivasan R, Nagi B, Nain CK, Singh K. Evaluation of CA 242 as a tumor marker in gallbladder cancer. J Gastrointest Cancer. 2012;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 156. | Kaza RK, Gulati M, Wig JD, Chawla YK. Evaluation of gall bladder carcinoma with dynamic magnetic resonance imaging and magnetic resonance cholangiopancreatography. Australas Radiol. 2006;50:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 157. | Pradhan S, Shukla VK, Agrawal S, Dixit VK, Sharma OP. Sonographic and colour doppler morphology in carcinoma gallbladder. Indian J Cancer. 2002;39:143-148. [PubMed] |
| 158. | Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 159. | Agrawal S, Sonawane RN, Behari A, Kumar A, Sikora SS, Saxena R, Kapoor VK. Laparoscopic staging in gallbladder cancer. Dig Surg. 2005;22:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 160. | Srikanth G, Kumar A, Khare R, Siddappa L, Gupta A, Sikora SS, Saxena R, Kapoor VK. Should laparoscopic cholecystectomy be performed in patients with thick-walled gallbladder? J Hepatobiliary Pancreat Surg. 2004;11:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Kapoor VK, Singh R, Behari A, Sharma S, Kumar A, Prakash A, Singh RK, Saxena R. Anticipatory extended cholecystectomy: the 'Lucknow' approach for thick walled gall bladder with low suspicion of cancer. Chin Clin Oncol. 2016;5:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 162. | Agarwal AK, Kalayarasan R, Javed A, Sakhuja P. Role of routine 16b1 lymph node biopsy in the management of gallbladder cancer: an analysis. HPB (Oxford). 2014;16:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Ghosh NK, Rahul R, Singh A, Sharma S, Kumar A, Singh RK, Behari A, Kapoor VK, Saxena R. Retroperitoneal Lymph Node Metastasis in Gallbladder Cancer: As Bad as Distant Metastasis. South Asian J Cancer. 2022;11:195-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 164. | Aggarwal A, Goel S, Sayed AI, Goel V, Talwar V, Singh S. Interaortocaval Lymph Node Metastasis in Gall Bladder Cancer: Is It Regional Node or Metastatic Disease? J Gastrointest Cancer. 2023;54:1252-1260. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 165. | Singh SK, Talwar R, Kannan N, Tyagi AK, Jaiswal P, Kumar A. Aggressive Surgical Approach for Gallbladder Cancer: a Single-Center Experience from Northern India. J Gastrointest Cancer. 2015;46:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 166. | Wagholikar GD, Behari A, Krishnani N, Kumar A, Sikora SS, Saxena R, Kapoor VK. Early gallbladder cancer. J Am Coll Surg. 2002;194:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 167. | Pottakkat B, Kapoor A, Prakash A, Singh RK, Behari A, Kumar A, Kapoor VK, Saxena R. Evaluation of a prospective surgical strategy of extended resection to achieve R0 status in gall bladder cancer. J Gastrointest Cancer. 2013;44:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Nag HH, Nekarakanti PK, Sachan A, Nabi P, Tyagi S. Bi-segmentectomy versus wedge hepatic resection in extended cholecystectomy for T2 and T3 gallbladder cancer: A matched case-control study. Ann Hepatobiliary Pancreat Surg. 2021;25:485-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 169. | Tewari M, Kumar S, Shukla S, Shukla HS. Analysis of wedge resection of gallbladder bed and lymphadenectomy on adequate oncologic clearance for gallbladder cancer. Indian J Cancer. 2016;53:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Behari A, Sikora SS, Wagholikar GD, Kumar A, Saxena R, Kapoor VK. Longterm survival after extended resections in patients with gallbladder cancer. J Am Coll Surg. 2003;196:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 171. | Jajal V, Nekarakanti PK, K S, Nag H. Effects of Cystic Duct Margin Involvement on the Survival Rates of Patients With Gallbladder Cancer: A Propensity Score-Matched Case-Control Study. Cureus. 2023;15:e50585. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 172. | Pandey D, Garg PK, Jakhetiya A, Jain N, Rai S. Surgico-pathological Outcomes of 148 Radical Cholecystectomies Using Systematic Regional Lymphadenectomy Protocol: a Retrospective Study. J Gastrointest Cancer. 2018;49:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 173. | Goel M, Pandrowala S, Patel P, Patkar S. Node positivity in T1b gallbladder cancer: A high volume centre experience. Eur J Surg Oncol. 2022;48:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Agarwal AK, Mandal S, Singh S, Bhojwani R, Sakhuja P, Uppal R. Biliary obstruction in gall bladder cancer is not sine qua non of inoperability. Ann Surg Oncol. 2007;14:2831-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 175. | K S, Jajal VM, Nekarakanti PK, Choudhary D, Nag HH. Gallbladder Cancer With Jaundice: Surgery Versus No Surgery. Cureus. 2022;14:e30594. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 176. | Singh S, Goel S, Aggarwal A, Iqbal A, Hazarika D, Talwar V. Combination of portal vein embolization and neoadjuvant chemotherapy for locally advanced gallbladder cancer requiring extended hepatectomy - A novel approach. Indian J Gastroenterol. 2021;40:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 177. | Agarwal AK, Mandal S, Singh S, Sakhuja P, Puri S. Gallbladder cancer with duodenal infiltration: is it still resectable? J Gastrointest Surg. 2007;11:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 178. | Sharma A, Nagar A, Varshney P, Tomar M, Sarin S, Choubey RP, Kapoor VK. Pancreas-preserving limited duodenal resection: Minimizing morbidity without compromising oncological adequacy. Ann Hepatobiliary Pancreat Surg. 2022;26:149-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 179. | Patkar S, Patel S, Kazi M, Goel M. Radical surgery for stage IV gallbladder cancers: Treatment strategies in patients with limited metastatic burden. Ann Hepatobiliary Pancreat Surg. 2023;27:180-188. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 180. | Agarwal AK, Javed A, Kalayarasan R, Sakhuja P. Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford). 2015;17:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 181. | Nag HH, Sachan A, Nekarakanti PK. Laparoscopic versus open extended cholecystectomy with bi-segmentectomy (s4b and s5) in patients with gallbladder cancer. J Minim Access Surg. 2021;17:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 182. | Palanisamy S, Patel N, Sabnis S, Palanisamy N, Vijay A, Palanivelu P, Parthasarthi R, Chinnusamy P. Laparoscopic radical cholecystectomy for suspected early gall bladder carcinoma: thinking beyond convention. Surg Endosc. 2016;30:2442-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 183. | Goel M, Khobragade K, Patkar S, Kanetkar A, Kurunkar S. Robotic surgery for gallbladder cancer: Operative technique and early outcomes. J Surg Oncol. 2019;119:958-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 184. | Shukla PJ, Barreto G, Kakade A, Shrikhande SV. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumours. HPB (Oxford). 2008;10:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 185. | Patkar S, Kunte A, Chaudhari V, Goel M. Outcomes of incidental versus non-incidental T2 gallbladder cancer: A single-institute experience of 425 cases. J Surg Oncol. 2024;129:754-764. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 186. | Goel M, Gupta AM, Patkar S, Parray AM, Shetty N, Ramaswamy A, Patil P, Chopra S, Ostwal V, Kulkarni S, Engineer R, Mehta S. Towards standardization of management of gallbladder carcinoma with obstructive jaundice: Analysis of 113 cases over 10 years at a single institution. J Surg Oncol. 2021;124:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 187. | Patkar S, Patil V, Acharya MR, Kurunkar S, Goel M. Achieving margin negative resection-doing less is justified: oncological outcomes of wedge excision of liver in gallbladder cancer (GBC) surgery. Chin Clin Oncol. 2019;8:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 188. | Nag HH, Raj P, Sisodia K. The technique of laparoscopic hepatic bisegmentectomy with regional lymphadenectomy for gallbladder cancer. J Minim Access Surg. 2018;14:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 189. | Kaman L, Behera A, Singh G, Nedounsejiane M. Radical surgery for incidental cancer gallbladder after laparoscopic cholecystectomy. Trop Gastroenterol. 2009;30:233-236. [PubMed] |
| 190. | Patkar S, Patel S, Gupta A, Ostwal V, Ramaswamy A, Shetty N, Goel M. Lessons learnt from 1300 consecutive gallbladder cancer surgeries: Evolving role of peri-operative chemotherapy in the treatment paradigm. Eur J Surg Oncol. 2023;49:107035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 191. | Ostwal V, Swami R, Patkar S, Majumdar S, Goel M, Mehta S, Engineer R, Mandavkar S, Kumar S, Ramaswamy A. Gemcitabine-cisplatin (GC) as adjuvant chemotherapy in resected stage II and stage III gallbladder cancers (GBC): a potential way forward. Med Oncol. 2018;35:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 192. | Kattepur AK, Patkar S, Goel M, Ramaswamy A, Ostwal V. Role of Adjuvant Chemotherapy in Resected T2N0 Gall Bladder Cancer. J Gastrointest Surg. 2019;23:2232-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 193. | Mahantshetty UM, Palled SR, Engineer R, Homkar G, Shrivastava SK, Shukla PJ. Adjuvant radiation therapy in gall bladder cancers: 10 years experience at Tata Memorial Hospital. J Cancer Res Ther. 2006;2:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 194. | Choudhary S, Asthana AK. Impact of Adjuvant Therapy on Survival in Curatively Resected Gallbladder Carcinoma. J Clin Diagn Res. 2015;9:XC01-XC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 195. | Agrawal S, Gupta PK, Rastogi N, Lawrence A, Kumari N, Das KJ, Saxena R. Outcomes of adjuvant chemoradiation and predictors of survival after extended cholecystectomy in gall bladder carcinoma: a single institution experience from an endemic region. J Gastrointest Cancer. 2015;46:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 196. | Ramaswamy A, Ostwal VS, Engineer R, Parulekar M, Mandavkar S, Aier N, Bhargava PG, Srinivas S, Patkar S, Krishnatry R, Gudi S, Kapoor A, Pandey D, Patel S, Shinghal A, Goel A, Dora TK, Chaudhary D, Ankathi SK, Goel M. A multicenter, open-label, randomized phase II study evaluating adjuvant gemcitabine plus cisplatin (GC) and capecitabine with concurrent capecitabine radiotherapy (CAPE-RT) in patients with operated gallbladder adenocarcinoma (GBC): The GECCOR-GB trial. JCO. 2023;41:4017-4017. [DOI] [Full Text] |
| 197. | Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, Awasthy BS. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 198. | Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:110-114. [PubMed] |
| 199. | Ramaswamy A, Ostwal V, Pinninti R, Kannan S, Bhargava P, Nashikkar C, Mirani J, Banavali S. Gemcitabine-cisplatin versus gemcitabine-oxaliplatin doublet chemotherapy in advanced gallbladder cancers: a match pair analysis. J Hepatobiliary Pancreat Sci. 2017;24:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 200. | Chatni SS, Sainani RS, Mehta SA, Mohandas KM. Infusion chemotherapy with cisplatinum and fluorouracil in the treatment of locally-advanced and metastatic gallbladder cancer. J Cancer Res Ther. 2008;4:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 201. | Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581-4586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 202. | Ali D, Alam N, Yasmin F, Ali S, Anwer T, Alam S, Ahmad S. Comparative Study Between Two Treatment Regimens of Cisplatin-5-Fluorouracil and Gemcitabine-Cisplatin in Gallbladder Cancer Patients. Iran J Pharm Res. 2017;16:798-804. [PubMed] |
| 203. | Singh SK, Talwar R, Kannan N, Tyagi AK, Jaiswal P, Kumar A. Chemotherapy Compared with Best Supportive Care for Metastatic/Unresectable Gallbladder Cancer: a Non-randomized Prospective Cohort Study. Indian J Surg Oncol. 2016;7:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 204. | Ramaswamy A, Ostwal V, Sharma A, Bhargava P, Srinivas S, Goel M, Patkar S, Mandavkar S, Jadhav P, Parulekar M, Choudhari A, Gupta S. Efficacy of Capecitabine Plus Irinotecan vs Irinotecan Monotherapy as Second-line Treatment in Patients With Advanced Gallbladder Cancer: A Multicenter Phase 2 Randomized Clinical Trial (GB-SELECT). JAMA Oncol. 2021;7:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 205. | Alam MN, Agrawal S, Rastogi N, Saxena R. Chemotherapy or chemotherapy followed by consolidation chemoradiation in postoperative (simple cholecystectomy) gall bladder cancer with residual disease, unsuitable for revision surgery? Risk stratification and outcomes. J Cancer Res Ther. 2023;19:259-264. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 206. | Alam MN, Agrawal S, Rastogi N, Maria Das KJ. Consolidation chemoradiation (cCTRT) improves survival in responders to first-line chemotherapy (CT) in locally advanced gallbladder cancer (LA-GBC): A new standard of care? Indian J Cancer. 2022;59:577-583. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 207. | Das CK, Patel A, Raj A, Gupta VG, Mehta P, Bhethanabhotla S, Reddy R, Yadav A. Anti-Her2neu directed therapy in advanced gall bladder cancer: A prospective, multicenter experience from India. JCO. 2020;38:e16682-e16682. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 208. | Dodagoudar C, Doval DC, Mahanta A, Goel V, Upadhyay A, Goyal P, Talwar V, Singh S, John MC, Tiwari S, Patnaik N. FOLFOX-4 as second-line therapy after failure of gemcitabine and platinum combination in advanced gall bladder cancer patients. Jpn J Clin Oncol. 2016;46:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 209. | Ramaswamy A, Ostwal V, Pande N, Sahu A, Jandyal S, Ramadwar M, Shetty N, Patkar S, Goel M, Gupta S. Second-Line Palliative Chemotherapy in Advanced Gall Bladder Cancer, CAP-IRI: Safe and Effective Option. J Gastrointest Cancer. 2016;47:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 210. | Gangopadhyay A, Nath P, Biswas J. Reduced Dose Intensity of Chemotherapy may not Lead to Inferior Palliation in Locally Advanced Carcinoma of the Gall Bladder: An Experience from a Regional Cancer Centre in Eastern India. J Gastrointest Cancer. 2015;46:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 211. | Sirohi B, Rastogi S, Singh A, Sheth V, Dawood S, Talole S, Ramadwar M, Kulkarni S, Shrikhande SV. Use of gemcitabine-platinum in Indian patients with advanced gall bladder cancer. Future Oncol. 2015;11:1191-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 212. | Bhamare S, Prabhakar P, Dharmadhikari A, Dedeepiya VD, Terunuma H, Senthilkumar R, Srinivasan T, Reena HC, Preethy S, Abraham SJ. Autologous immune enhancement therapy in a case of gall bladder cancer stage IV after surgical resection and chemotherapy yielding a stable non-progressive disease. J Cancer Res Ther. 2014;10:752-754. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 213. | Mallick S, Benson R, Julka PK, Rath GK. Adjuvant chemoradiotherapy for squamous cell carcinoma of gallbladder. J Gastrointest Cancer. 2014;45 Suppl 1:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 214. | Engineer R, Wadasadawala T, Mehta S, Mahantshetty U, Purandare N, Rangarajan V, Kishore Shrivastava S. Chemoradiation for unresectable gall bladder cancer: time to review historic nihilism? J Gastrointest Cancer. 2011;42:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 215. | Chaudhari VA, Ostwal V, Patkar S, Sahu A, Toshniwal A, Ramaswamy A, Shetty NS, Shrikhande SV, Goel M. Outcome of neoadjuvant chemotherapy in "locally advanced/borderline resectable" gallbladder cancer: the need to define indications. HPB (Oxford). 2018;20:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 216. | Agrawal S, Mohan L, Mourya C, Neyaz Z, Saxena R. Radiological Downstaging with Neoadjuvant Therapy in Unresectable Gall Bladder Cancer Cases. Asian Pac J Cancer Prev. 2016;17:2137-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 217. | Engineer R, Patkar S, Lewis SC, Sharma AD, Shetty N, Ostwal V, Ramaswamy A, Chopra S, Agrawal A, Patil P, Mehta S, Goel M. A phase III randomised clinical trial of perioperative therapy (neoadjuvant chemotherapy versus chemoradiotherapy) in locally advanced gallbladder cancers (POLCAGB): study protocol. BMJ Open. 2019;9:e028147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 218. | Patkar S, Ostwal V, Ramaswamy A, Engineer R, Chopra S, Shetty N, Dusane R, Shrikhande SV, Goel M. Emerging role of multimodality treatment in gall bladder cancer: Outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol. 2018;117:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 219. | Engineer R, Goel M, Chopra S, Patil P, Purandare N, Rangarajan V, Ph R, Bal M, Shrikhande S, Shrivastava SK, Mehta S. Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Gallbladder Cancers: A New Paradigm. Ann Surg Oncol. 2016;23:3009-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 220. | Sirohi B, Mitra A, Jagannath P, Singh A, Ramadvar M, Kulkarni S, Goel M, Shrikhande SV. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol. 2015;11:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 221. | Selvakumar VP, Zaidi S, Pande P, Goel A, Kumar K. Resection after neoadjuvant chemotherapy in advanced carcinoma of the gallbladder: a retrospective study. Indian J Surg Oncol. 2015;6:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |