Published online Feb 24, 2024. doi: 10.5306/wjco.v15.i2.169
Peer-review started: December 3, 2023
First decision: December 18, 2023
Revised: December 19, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: February 24, 2024
Processing time: 78 Days and 9 Hours
Prostate cancer poses a significant health challenge globally, demanding proactive prevention strategies. This editorial explores the emerging role of vitamin D in prostate cancer prevention. While traditionally associated with bone health, vitamin D is increasingly recognized for its broader impact on immune function, cellular signaling, and cancer prevention. Epidemiological studies suggest an intriguing link between vitamin D deficiency and elevated prostate cancer risk, particularly in regions with limited sunlight exposure. Mechanistically, vitamin D regulates cellular processes, inhibiting unchecked cancer cell growth and bols
Core Tip: This editorial highlights the evolving role of vitamin D in prostate cancer prevention, going beyond its traditional association with bone health. Emerging evidence suggests a link between vitamin D deficiency and increased prostate cancer risk, especially in regions with limited sunlight exposure. Mechanistically, vitamin D regulates cellular processes, inhibiting cancer cell growth and enhancing immune surveillance. To unlock its full potential, personalized prevention strategies are crucial. Robust research, public awareness, dietary improvements, and vigilant medical guidance are essential components. Collaborative efforts aim to position vitamin D as a sentinel in prostate cancer prevention, offering hope and improved health for men globally.
- Citation: Cassell A, Konneh S. Unlocking the potential-vitamin D in prostate cancer prevention. World J Clin Oncol 2024; 15(2): 169-174
- URL: https://www.wjgnet.com/2218-4333/full/v15/i2/169.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i2.169
Prostate cancer, a prevalent urological malignancy, poses a significant health burden globally, particularly in developed nations. In 2020, it accounted for a substantial share of cancer cases and deaths among males, contributing to 14.1% and 6.8%, respectively[1]. The sheer magnitude of the impact is evident in the staggering number of 1414259 new cases and 375304 deaths attributed to prostate cancer in the year 2020 alone.
The Age-Standardized Rate of incidence globally was at 30.7 per 100000 persons, unveiling a notable 13-fold variation across different regions[1]. This variability underscores the complex interplay of factors influencing the incidence of prostate cancer, from genetic predispositions to environmental influences.
Prostate cancer, a formidable adversary in the realm of men's health, demands our unwavering attention. In the quest for effective prevention, an unlikely hero has emerged-vitamin D. This unassuming nutrient, long associated with bone health, is now revealing its hidden potential as a guardian against prostate cancer.
Vitamin is performing the magic trick and the scientific community is hoping that this nutrient can assume a more robust role in the prevention of prostate cancer.
In this editorial, we embark on a journey to explore the evolving landscape of vitamin D in prostate cancer prevention, unveiling its promise and the work yet to be done (Figure 1).
Vitamin D, commonly dubbed the "sunshine vitamin," has long held its place in the annals of nutritional science. It regulates calcium and phosphorus metabolism, crucial for skeletal health. However, its influence extends far beyond bones, penetrating the intricate realms of the immune system, cell signaling, and cancer prevention.
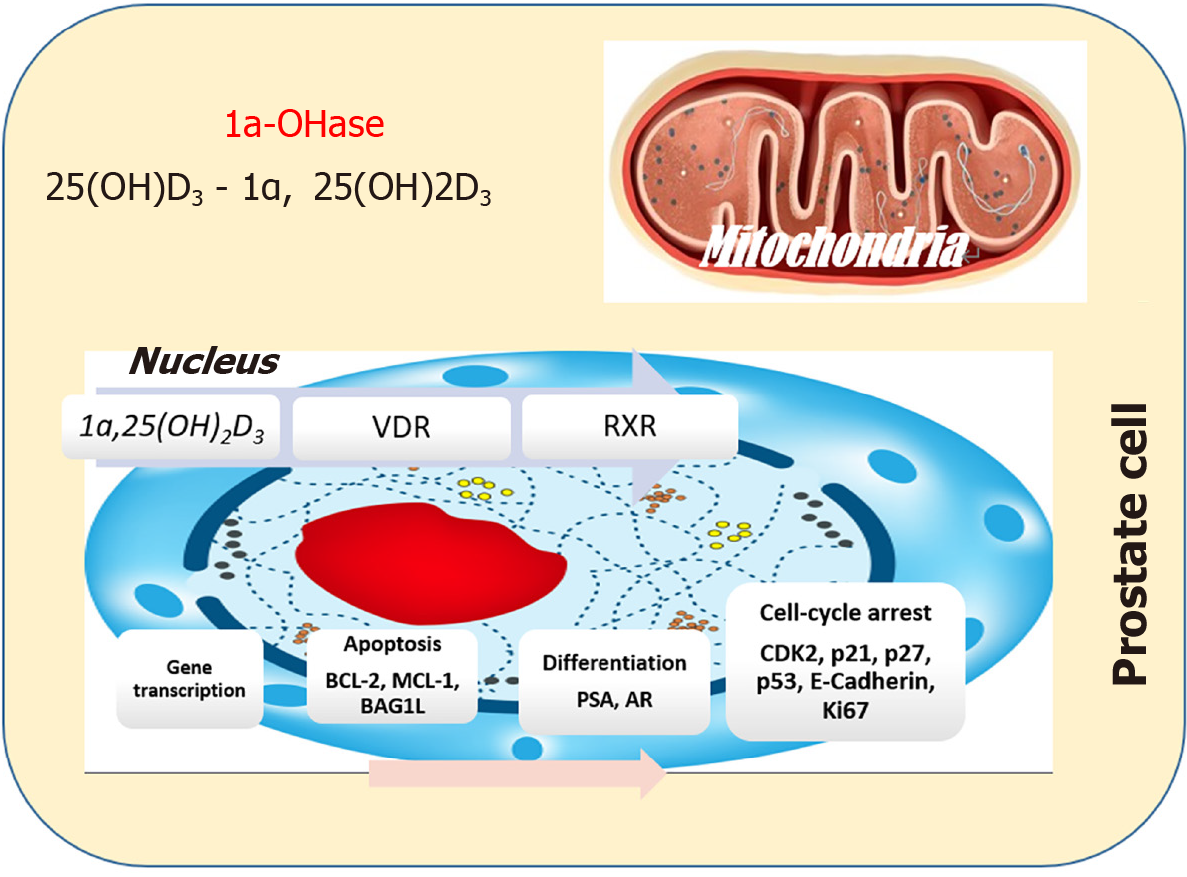
Vitamin D compounds constitute integral elements of the vitamin D hormone system, and their synthesis in the body is a complex process initiated in the skin under ultraviolet light influence. Unlike traditional vitamins, vitamin D is intricately linked to a series of steps, commencing with the conversion of a cholesterol precursor molecule (7-dehydrocholesterol) into cholecalciferol (vitamin D3). This synthesis continues with successive hydroxylations in the liver and kidney, ultimately producing the most active hormone form, 1,25-dihydroxycholecalciferol or calcitriol.
The metabolic journey of vitamin D involves crucial enzymatic processes, primarily facilitated by cytochrome P450 (CYP) enzymes CYPR1, CYP27B1, and CYP24A1[2]. Liver and kidneys serve as major organs for metabolism and excretion, but notably, various tissues, including tumor cells and microenvironment cells, also partake in the metabolism of these hormones. This intricate interplay highlights the multifaceted nature of vitamin D metabolism, extending its impact beyond traditional metabolic organs.
Vitamin D's potential in prostate cancer prevention is grounded in its ability to regulate fundamental cellular processes. It influences cell proliferation, apoptosis (programmed cell death), and angiogenesis (the formation of new blood vessels). These actions collectively thwart the unchecked growth and spread of malignant cells.
Furthermore, vitamin D plays a pivotal role in immune modulation, potentially enhancing the body's ability to identify and combat cancerous cells. These mechanisms are complex, reinforcing the need for rigorous scientific exploration.
The inhibitory influence of vitamin D on prostate cell proliferation operates through the vitamin D Receptor (VDR), a member of the steroid/nuclear receptor superfamily. Within target cells, VDR forms a high-affinity bond with 1a,25(OH)2D and subsequently engages with the retinoid X receptor[3]. This collaborative partnership forms a heterodimeric complex equipped with distinctive zinc-finger motifs. These motifs strategically attach to a specific DNA-sequence motif known as a vitamin D-response element residing in the promoter region of vitamin D-regulated genes[3]. The orchestrated interplay within this complex regulates the pace of RNA polymerase II-mediated transcription for these genes, finely tuning the cellular processes impacted by vitamin D in prostate cancer cells.
The intricacies of how 1a,25(OH)2D transmits signals to impede proliferation in prostate cells remain a puzzle with evolving facets. Recent investigations hint at the likelihood that 1a,25(OH)2D might engage diverse pathways, exerting its inhibitory effects on cell proliferation in a cell-type-dependent manner[4]. For instance, androgen-sensitive prostate-cancer cells like LNCaP exhibit a notable accumulation in the G0–G1 phase of the cell cycle upon 1a,25(OH)2D3 treatment. In contrast, the same treatment induces growth inhibition in ALVA-31 and PC-3 cells without eliciting a comparable cell cycle phase accumulation. This nuanced understanding underscores the multifaceted nature of 1a,25(OH)2D's impact on cell proliferation across distinct prostate cell types[4].
In certain experimental scenarios, 1a,25(OH)2D emerges as a catalyst for apoptosis in LNCaP cells, shedding light on potential therapeutic avenues. Employing the TUNEL assay in tandem with flow cytometric analysis, Blutt et al[5] demonstrated DNA fragmentation indicative of apoptosis upon exposing LNCaP cells to 1a,25(OH)2D3[5]. This apoptotic cascade is intricately linked to the downregulation of antiapoptotic proteins, including Bcl2 and BclXL. Interestingly, overexpression of the Bcl2-encoding gene proved effective in preventing this process. Moreover, 1a,25(OH)2D3 orchestrates the downregulation of additional antiapoptotic players (Mcl-1, BAG1L, XIAP, cIAP1, and cIAP2) in LNCaP cells, while leaving proapoptotic Bax and Bak levels unaltered[6]. This nuanced orchestration suggests a promising avenue for targeted interventions in modulating apoptotic responses in prostate cells.
Epidemiological studies have long tantalized us with hints of a connection between vitamin D and prostate cancer. Regions with limited sunlight exposure, leading to decreased vitamin D synthesis, often correlate with higher prostate cancer incidence. Similarly, individuals with lower vitamin D levels in their blood have been found to be at an elevated risk. These observations, while intriguing, begged the question: Could vitamin D truly be a sentinel against prostate cancer?
Compelling evidence linking vitamin D to prostate cancer unfolds in studies focused on African-American men. These individuals often exhibit low levels of 25(OH)D3, attributed primarily to the impact of skin pigmentation limiting vitamin D synthesis. Intriguingly, this demographic faces a notably higher risk of prostate cancer and mortality compared to their Caucasian counterparts. While this correlation has been repeatedly observed, the underlying mechanisms remain elusive[2].
Access to medical care disparities among African-American men emerges as a significant contributing factor to their unfavorable cancer outcomes. If diminished serum levels of 25(OH)D3 indeed contribute to heightened cancer risks, exploring potential associations between different cancer risks or outcomes and vitamin D-metabolizing genes, and even vitamin D-binding protein, becomes a logical avenue for investigation. Unraveling these intricacies could provide valuable insights into the complex interplay between vitamin D and prostate cancer in specific demographic contexts.
A nationwide, randomized, placebo-controlled trial involving men aged 50 or older in the United States[7]. The primary endpoints were invasive cancer and major cardiovascular events, with secondary endpoints including site-specific cancers and additional cardiovascular events. The results showed that vitamin D supplementation did not lower the risk of invasive cancer or cardiovascular events compared to the placebo. However, the rate of death from cancer was significantly lower with vitamin D than with placebo after 2 years of follow-up).
Novel data have revealed an intriguing relationship between 25(OH)D concentration and cancer[8]. The findings indicated a moderate inverse association with total cancer incidence and a more pronounced inverse association with cancer mortality. Interestingly, it suggests that vitamin D might play a more robust role in supporting survival and improving outcomes rather than solely decreasing cancer incidence.
One noteworthy United States study revealed a negative correlation between serum 1,25 vitamin D3 and prostate cancer, particularly in men above the median age of 57 years[9]. A Finnish study, spanning 13 years and involving approximately 19000 men, discovered that lower serum 25(OH)D3 concentrations were associated with a heightened risk of earlier and more aggressive onset of prostate cancer[10].
Furthermore, more recent reports with follow-up periods of 18 years and a median time of 44 months[11] suggested that individuals with circulating 25(OH)D3 and 1,25(OH)2D3, or 25(OH)D3 alone, at median or higher-than-medium levels exhibited a reduced risk of prostate cancer progression.
Data has shown that engaging in active surveillance coupled with a daily vitamin D3 supplementation of 4000 IU demonstrated a notable reduction in the number of positive cores during repeat biopsy for over half of individuals diagnosed with low-risk prostate cancer[12]. This promising regimen not only addresses the specific needs of patients with low-risk prostate cancer but also holds the potential to minimize the likelihood of overtreatment for those who, based on repeat biopsy results, exhibit positive responses and maintain stability or improvement in their condition.
Despite these findings, it is crucial to acknowledge the inconsistent nature of the existing research, emphasizing the need for further exploration and comprehensive studies to establish a clearer understanding of the relationship between vitamin D levels and prostate cancer risk reduction.
The road to harnessing the full potential of vitamin D in prostate cancer prevention is paved with complexity. Individual variations in genetics, diet, sunlight exposure, and geographical location contribute to one's vitamin D status. A one-size-fits-all approach falls short in this nuanced arena. Personalized strategies that consider these factors are paramount.
To unlock the potential of vitamin D in prostate cancer prevention, we must set a course guided by science, public health, and individual empowerment:
Continued research into the intricate interplay between vitamin D and prostate cancer is essential. Mechanistic insights and clinical trials will pave the way for evidence-based recommendations.
Raising awareness about the importance of maintaining optimal vitamin D levels and prostate health is paramount. Public health campaigns can be instrumental in educating individuals and dispelling myths.
Encouraging a balanced diet rich in vitamin D and safe sun exposure practices can empower individuals to take control of their health.
Healthcare providers must be vigilant in assessing and addressing vitamin D deficiency, particularly in individuals at risk. Routine prostate cancer screenings can catch the disease in its early stages.
The evolving narrative of vitamin D in prostate cancer prevention beckons us to explore the untapped potential of a humble nutrient. As we embark on this journey, we must heed the call for rigorous research, individual empowerment, and public health enlightenment. Though, vitamin may not uprightly reduce the risk of prostate cancer, it has been shown to be effective in preventing disease progression.
Together, with collaborative efforts from the scientific community, healthcare providers, policymakers, and the public, we can hope for a future where prostate cancer's grip on men's lives is loosened, and where the promise of vitamin D is fully realized. In this vision, we find renewed determination to embrace the dawn of vitamin D as a sentinel in prostate cancer prevention, ushering in a new era of hope and health.
Special thanks to the department of surgery of the John F Kennedy Medical Center.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Liberia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu XL, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Huang J, Chan EO, Liu X, Lok V, Ngai CH, Zhang L, Xu W, Zheng ZJ, Chiu PK, Vasdev N, Enikeev D, Shariat SF, Ng CF, Teoh JY, Wong MCS. Global Trends of Prostate Cancer by Age, and Their Associations With Gross Domestic Product (GDP), Human Development Index (HDI), Smoking, and Alcohol Drinking. Clin Genitourin Cancer. 2023;21:e261-e270.e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Trump DL, Aragon-Ching JB. Vitamin D in prostate cancer. Asian J Androl. 2018;20:244-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Moukayed M, Grant WB. Molecular link between vitamin D and cancer prevention. Nutrients. 2013;5:3993-4021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667-677. [PubMed] |
| 7. | Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE; VITAL Research Group. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med. 2019;380:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1136] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 8. | Bandera Merchan B, Morcillo S, Martin-Nuñez G, Tinahones FJ, Macías-González M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J Steroid Biochem Mol Biol. 2017;167:203-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Corder EH, Guess HA, Hulka BS, Friedman GD, Sadler M, Vollmer RT, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2:467-472. [PubMed] |
| 10. | Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Marshall DT, Savage SJ, Garrett-Mayer E, Keane TE, Hollis BW, Horst RL, Ambrose LH, Kindy MS, Gattoni-Celli S. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab. 2012;97:2315-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |