Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.570
Peer-review started: October 5, 2023
First decision: November 9, 2023
Revised: November 9, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 24, 2023
Processing time: 77 Days and 19.1 Hours
Well-differentiated thyroid carcinoma has a favorable prognosis with a 5-year survival rate of over 95%. However, the undifferentiated or anaplastic type accounting for < 0.2%, usually in elderly individuals, exhibits a dismal prognosis with rapid growth and disappointing outcomes. It is the most aggressive form of thyroid carcinoma, with a median survival of 5 mo and poor quality of life (airway obstruction, dysphagia, hoarseness, persistent pain). Early diagnosis and staging are crucial. Diagnostic tools include biopsy (fine needle aspiration, core needle, open surgery), high-resolution ultrasound, computed tomography, magnetic resonance imaging, [(18)F]fluoro-D-glucose positron emission tomo-graphy/computed tomography, liquid biopsy and microRNAs. The BRAF gene (BRAF-V600E and BRAF wild type) is the most often found molecular factor. Others include the genes RET, KRAS, HRAS, and NRAS. Recent management policy is based on surgery, even debulking, chemotherapy (cisplatin or doxorubicin), radiotherapy (adjuvant or definitive), targeted biological agents and immunotherapy. The last two options constitute novel hopeful management modalities improving the overall survival in these otherwise condemned patients. Anti-programmed death-ligand 1 antibody immunotherapy, stem cell targeted therapies, nanotechnology achievements and artificial intelligence imple-mentation provide novel promising alternatives. Genetic mutations determine molecular pathways, thus indicating novel treatment strategies such as anti-BRAF, anti-vascular endothelial growth factor-A, and anti-epidermal growth factor receptor. Treatment with the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib has been approved by the Food and Drug Administration in cases with BRAF-V600E gene mutations and is currently the standard care. This neoadjuvant treatment followed by surgery ensures a two-year overall survival of 80%. Prognostic factors for improved outcomes have been found to be younger age, earlier tumor stage and radiation therapy. A multi
Core Tip: Anaplastic thyroid carcinoma is uncommon but one of the most lethal neoplasms. The optimal management remains unclear. The addition of novel targeted therapy and immunotherapy to the traditional management of surgery, radiation and chemotherapy has improved the outcomes. Multimodality management and the emerging use of individualized treatment based on novel therapeutic agents offers promising results. However, further research efforts involving the molecular microenvironment and biological drivers should be made.
- Citation: Pavlidis ET, Galanis IN, Pavlidis TE. Update on current diagnosis and management of anaplastic thyroid carcinoma. World J Clin Oncol 2023; 14(12): 570-583
- URL: https://www.wjgnet.com/2218-4333/full/v14/i12/570.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i12.570
Τhyroid carcinoma incidence is increasing, but that of the anaplastic and medullary types remains rather stable. The overall increase is due mainly to the rise of the most commonly occurring papillary carcinoma, which is associated with the best prognosis[1-3]. The incidence of anaplastic carcinoma in Europe has been assessed to be far less than 6 cases per 100000 population, more precisely, 0.1-0.3 cases per 100000 population in Denmark, the Netherlands and Wales[2,4,5] and 0.12-0.2 cases per 100000 population in the United States[3]; thus, it has been characterized as a rare disease[6].
The 5-year survival of a well-differentiated thyroid carcinoma exceeds 95%[7]. In contrast, the undifferentiated form, also called by a revised and better term, anaplastic carcinoma determined from World Health Organization classification[8], accounts for less than 0.2%[9] or as much as 1%-2% of all thyroid malignancies[10,11]. It comes from the follicular epithelium and constitutes one of the most lethal neoplasms related to disappointing outcomes[4,9,11]. Its median survival is restricted to only 4 to 6 mo, accompanied by poor quality of life[4,10].
Rapidly growing neck tumors are often accompanied by devastating and occasionally life-threatening events. They may invade the trachea, causing airway obstruction and asphyxia; the esophagus, causing dysphagia; the recurrent laryngeal nerve, causing paralysis and hoarseness; major vessels, causing manifestations of superior vena cava syndrome or brain intermittent ischemia; and neural plexuses, causing persistent pain. Additionally, at the time of diagnosis, metastases are found in half of cases, mainly pulmonary metastases (40%), followed by brain metastases (10%)[4,9,12-14].
A long existing untreated nodular goiter (30% of cases) or known history of papillary carcinoma is usually found mainly in the elderly with female predominance[3,4]. Preexisting papillary carcinoma may indicate a potent divergent transformation[15,16]. Early diagnosis based mainly on ultrasound (US) and core needle biopsy is crucial[17-21]. The following staging after the initial diagnosis of anaplastic carcinoma is of great importance and can be achieved by computed tomography (CT), magnetic resonance imaging (MRI)[18,19], and preferably positron emission tomography/CT (PET-CT)[17,22,23]. Modern molecular testing by revealing implicated genes, basically the BRAF gene (BRAF-V600E and BRAF wild type), and other molecules[24-27] can contribute to more accurate diagnosis but most importantly determine molecular pathways indicating novel treatment strategies by targeted biological factors, i.e., anti-BRAF, anti-vascular endothelial growth factor (VEGF)-A or anti-epidermal growth factor receptor (EGFR) agents[24].
Nanotechnology achievements may offer either a vehicle for advanced drug delivery systems promoting targeted therapy[28,29] or a core for chemo-photothermal (lenvatinib-laser irradiation) therapy[30]. Additionally, these advances may provide tools for diagnosing disease progression in the form of magnetic or radiolabeled probes[29]. Immunotherapy with an anti-programmed cell death-ligand 1 (PD-L1) monoclonal antibody (atezolizumab) may increase the action of radiotherapy on cancer cells and is a novel innovation[31]. Stem cell-targeted therapies are other novel emerging alternatives with promising perspectives[24,32,33].
Machine learning with deep learning along with artificial intelligence implementation has provided preliminary encouraging results for diagnosis, imaging assessment, treatment and outcome prediction. It now remains to be used in clinical practice[10,34]. A multidisciplinary approach must be followed with an individualized therapeutic plan based on surveillance and epidemiology end results (SEER)[3,9,35-37].
The management policy constitutes the standard treatment, including surgery first of all, even debulking surgery, adjuvant chemotherapy that mainly uses cisplatin or doxorubicin and docetaxel-paclitaxel, and accelerated hyperfunctional external beam radiotherapy, preferably neo-adjuvant and definitive. It can increase the median survival up to 10 mo[24]. The novel hopeful management by targeted biological agents and immunotherapy has further improved the overall survival in these otherwise condemned patients[4,9,24,38,39].
Treatment with the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib was approved by the Food and Drug Administration (FDA) of the United States in 2014 for mutated melanoma and in 2018 for mutated anaplastic thyroid carcinoma; thus, it has already been used successfully in cases of metastatic or locally advanced inoperable anaplastic thyroid carcinoma with BRAF-V600E gene mutation. This targeted therapy has been recommended as neoadjuvant treatment followed by surgery. It constitutes the standard care and ensures a two-year overall survival of 80%[4,24,40,41].
The main prognostic factors for improved outcomes have been younger age, earlier tumor stage, tumor size, multifocality, radiation therapy and novel targeted therapy[10,42]. This narrative review evaluates the current knowledge on anaplastic thyroid carcinoma with extreme aggressiveness and a dismal prognosis, emphasizing proper diagnosis and management. This study was based on the data of an extensive literature review from PubMed extending to September 2023, focusing particularly on full-text papers published only in the English language over the last five years.
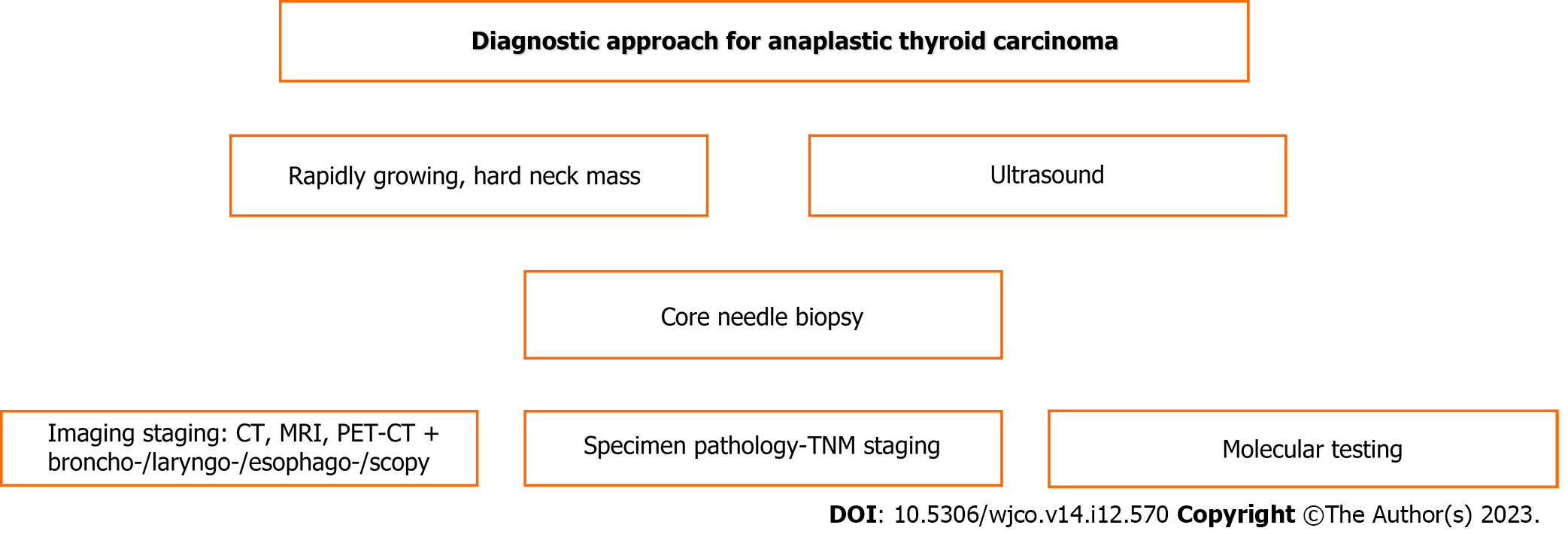
The diagnostic steps are shown schematically in Figure 1.
The anaplastic thyroid carcinoma exhibits a rapid onset with a large, hard, painful neck mass, cough with or without hemoptysis and dyspnea (35%) in cases of trachea invasion, hoarseness (40%) or dysphagia (40%), with local spread in over 50% of cases, lymph node involvement, possible skin invasion, and rapid evolution with dramatic invasion of adjacent structures that may need urgent intervention as mentioned above in the introduction section[4,6,43].
A recent large study from the United States including 5359 patients with anaplastic thyroid carcinoma provides an analysis of several presentation characteristics. The majority of patients were women (58%), non-Hispanic white (80%), with a median age of 70 ± 12 years, a median tumor size of 6.1 cm (range 4.5-8 cm), and distant metastases (29%)[44].
It is important for rapidly growing neck swelling to differentiate anaplastic thyroid carcinoma from thyroid lymphoma by biopsy[45] since they have completely different management strategies. The lymphoma requires no surgical intervention but only R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine or otherwise called oncovin, and prednisolone) accompanied by immediate extreme volume reduction and long-term excellent outcomes[46].
US can be used to detect heterogeneous echogenicity, abnormal shape, calcifications, increased vascularity, diffuse infiltration of adjacent tissues and regional lymph node involvement. US plain or preferably high resolution is the first step of exploration[18,19], followed preferably by core needle biopsy[21]. After establishing the diagnosis of the primary tumor of anaplastic thyroid carcinoma, staging imaging is necessary for local tumor extension assessment and revealing distant metastases.
Contrast-enhanced CT or MRI and magnetic resonance angiography can reveal any involvement of major vessels or lymph nodes and any other local involvement or distant metastases. CT can be used to detect heterogeneous tumor appearance, necrosis, calcifications, hypervascularity, and possible infiltration of the trachea and esophagus. CT is preferred over MRI[18,19]. Additionally, fiberoptic laryngoscopy for vocal cord evaluation, bronchoscopy and esophagoscopy are necessary prerequisites[20,43].
However, more precise assessment has been offered by [(18)F]fluoro-D-glucose PET-CT, which ensures better anatomic location with active metabolic uptake detecting occult deposits[17,23,47]. It can be especially valuable in elderly patients for accurate disease setting assessment that can precisely determine the appropriate management strategy[22].
Prompt diagnosis and on-time management without any delay are imperative tasks, particularly in severe life-threatening complications. Molecular testing is indicated to better determine mutations and proper targeted therapy either as neo-adjuvant in unresectable cases or adjuvant after surgical excision[48], especially in cases of refractory carcinoma[49]. The American Thyroid Association (ATA) recent guidelines include recommendations for molecular testing for anaplastic thyroid carcinoma[50].
The BRAF gene mutation (BRAF-V600E and BRAF wild type) is the most important molecular factor found in 40%-50% of patients with anaplastic thyroid carcinoma[4,6,51]. The MEK gene has a close connection to the BRAF gene. Both are responsible for mitogen-activated protein kinase that promotes cell proliferation, tumor growth and angiogenesis[41,52,53]. Other mutations found involve the following genes: p53 in 63% of cases, RET, RAS (KRAS, HRAS, NRAS) in 22% of cases, TERT promoter in 75% of cases, PIK3CA in 18% of cases, EIF1AX in 14% of cases, PTEN in 14% of cases[6,24,50], and NESTIN, CCND1, POU5F1, MCL1, MYBL2, MCL1, IQGAP1, SOX2, and NANOG[33]. Additionally, epigenetic-related genes, i.e., the chromatin remodeling SWI/SNF complex in 36% of cases, histone methyltransferases in 24% of cases, and DNA mismatch repair pathway genes in 10%-15% of cases, were found[6]. Gong et al[54] recognized 10 hub genes for anaplastic thyroid carcinoma: CXCL8, CDH1, AURKA, CCNA2, FN1, CDK1, ITGAM, CDC20, MMP9, and KIF11. RAS gene mutations have been reported to correlate with increased aggressiveness and increased mortality[55]. Unfortunately, targeted therapy is not yet available[50]. A positive result for mutated neurotrophic tyrosine receptor kinase (NTRK) gene testing is valuable to select patients for therapy by tropomyosin receptor kinase inhibitors (larotrectinib, entrectinib)[56]. miRNA-506 downregulation has been found in anaplastic thyroid carcinoma. This molecule normally regulates the WNT and NOTCH signaling pathways to adjust cell proliferation and migration. Thus, in practical view, as a new therapeutic targeted biological agent, it could suppress tumor progression and dissemination[25].
In an experimental model, intercellular adhesion molecule-1 (ICAM1) was an attractive target for anaplastic and papillary thyroid carcinoma by a monoclonal antibody; its distribution was explored by MRI[57]. Another molecule, collagen triple helix repeat containing-1 (CTHRC1), has been found to decrease the survival of patients with anaplastic thyroid carcinoma by promoting its progression and invasion via the WNT pathway and epithelial-mesenchymal transition. Blocking its action could be particularly useful[58]. Histone lysine lactylation represents a novel epigenetic mark that can boost the proliferation of anaplastic thyroid carcinoma. Blocking this protein may increase the action of BRAF-V600E gene inhibitors, thus preventing the progression of the mutated malignancy[51]. PD-L1 expression was found to be positive in a high proportion in papillary 87% but with weaker and patchy expression, and in anaplastic thyroid carcinoma 73%, indicating that the latter can exhibit a better response to immunotherapy[59].
It is obvious, that in cases of rapidly enlarging neck nodules, the necessary first step is an US imaging performance. Advances in the US technology provide precise diagnostic capability by high resolution US. However, then biopsy is fundamental to make the diagnosis. Fine needle aspiration (FNA) cytology using a 21-25 gauge needle under US guidance has been widely used as an initial step in diagnosis by cytologic examination[20,21,34,45,60]. However, due to its high false-negative results, low sensitivity of 54%-61% vs 77%-80% of core needle biopsy (CNB), and specificity of 87% vs 100% of CNB[20,21] or often inconclusive results, this option tends to be omitted recently in favor of CNB. Because of performance, using it is considered a vain spending of time[21,45].
CNB is performed under US guidance and by local anesthesia using a 16-20 Ga needle to take at least 2-3 tissue samples by separate punctures for histopathologic examination[20,21,45]. In addition, the sample can be immediately used for molecular testing[20]. CNB yields the most accurate diagnostic ability and thus constitutes the method of first choice instead of its application after nondiagnostic FNA, which, in contrast to current guidelines, is advised. There was no patient discomfort or malignant cell seeding or notable complications. A little bleeding requiring simple compression or hematoma formation may occur rarely[20,21,45].
Incision biopsy or open surgery biopsy under local or even general anesthesia and skin incision takes 2-3 cm3 of tissue, avoiding any necrotic area. However, it has been abandoned and substituted by CNB[21]. Liquid biopsy is a new noninvasive genotyping diagnostic method that can detect malignant cells in serum and tumor DNA or other extracellular parts, providing valuable information. It may contribute to diagnosis, prognosis, and follow-up for assessment of the response to treatment or relapse[26,27]. All the abovementioned diagnostic tools are shown in Table 1.
| n | Modality |
| 1 | Plain ultrasound or preferably high resolution ultrasound |
| 2 | Core needle biopsy under ultrasound guidance preferably |
| 3 | FNA cytology under ultrasound guidance |
| 4 | Staging imaging (CT, MRI-MRA, 18F-FDG PET-CT) |
| 5 | Bronchoscopy |
| 6 | Esophagoscopy |
| 8 | Fiberoptic laryngoscopy |
| 9 | Molecular testing (BRAF, MEK, NTRK, RET, RAS, p53 genes) |
| 10 | MicroRNAs |
| 11 | PD-L1 expression |
| 12 | Liquid biopsy |
| 13 | Histopathology |
| 14 | Pathological TNM staging |
By definition, all anaplastic thyroid carcinomas are considered advanced and classified in stage IV by the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) according to the tumor-node-metastasis (TNM) system (tumor size and local extension, regional lymph node status and distant metastases). The 8th edition of TNM classification and staging by the AJCC and UICC are shown in Tables 2 and 3[6,8,61].
| T | Size | Extension |
| T1 | ≤ 2 cm | Limited into thyroid |
| T1a | ≤ 1 cm | Limited into thyroid |
| T1b | > 1 cm and ≤ 2 cm | Limited into thyroid |
| T2 | > 2 cm and ≤ 4 cm | Limited into thyroid |
| T3 | > 4 cm | Limited into thyroid or extrathyroid macroscopic invasion only of thyroid muscles and subcutaneous tissue |
| T3a | > 4 cm | Limited into thyroid |
| T3b | Any size | Extrathyroid macroscopic invasion only of thyroid muscles and subcutaneous tissue |
| T4 | Any size | Macroscopic invasion of major adjacent structures |
| T4a | Any size | Macroscopic invasion of larynx, trachea, esophagus, recurrent laryngeal nerve |
| T4b | Any size | Macroscopic invasion of carotid artery, major vessels in mediastinum, prever-tebral fascia |
| Stage | IVA | IVB | IVC |
| Parameters | T1-T3a, N0, M0 | T1-T3a, N1, M0 | Any T, any N, M1 |
| T3b, any N, M0 | |||
| T4, any N, M0 |
There have been several management options, including novel targeted therapy and immunotherapy[4,6,24,44,62]. They are presented below. In a recent cohort study including 97 patients, these options were presented in combination as follows: (1) Surgical intervention in 45% of cases; (2) Chemotherapy in 41% of cases; (3) Neoadjuvant or definitive radiotherapy in 35% of cases; and (4) Targeted therapy in 29% of cases. The median overall survival was 6.5 mo, and it was inferior in those who did not undergo surgery. Multivariate analysis showed that stage IVC and lack of radiotherapy were associated with worse overall survival[42].
This multimodality management, including additional tyrosine kinase inhibitors, could provide a survival of more than one year[63]. Given that in 433 studied patients with advanced metastatic anaplastic thyroid carcinoma (stage IVC), there was a median overall survival of 2 mo and a one-year overall survival of 6.9%[64], better multimodality mana
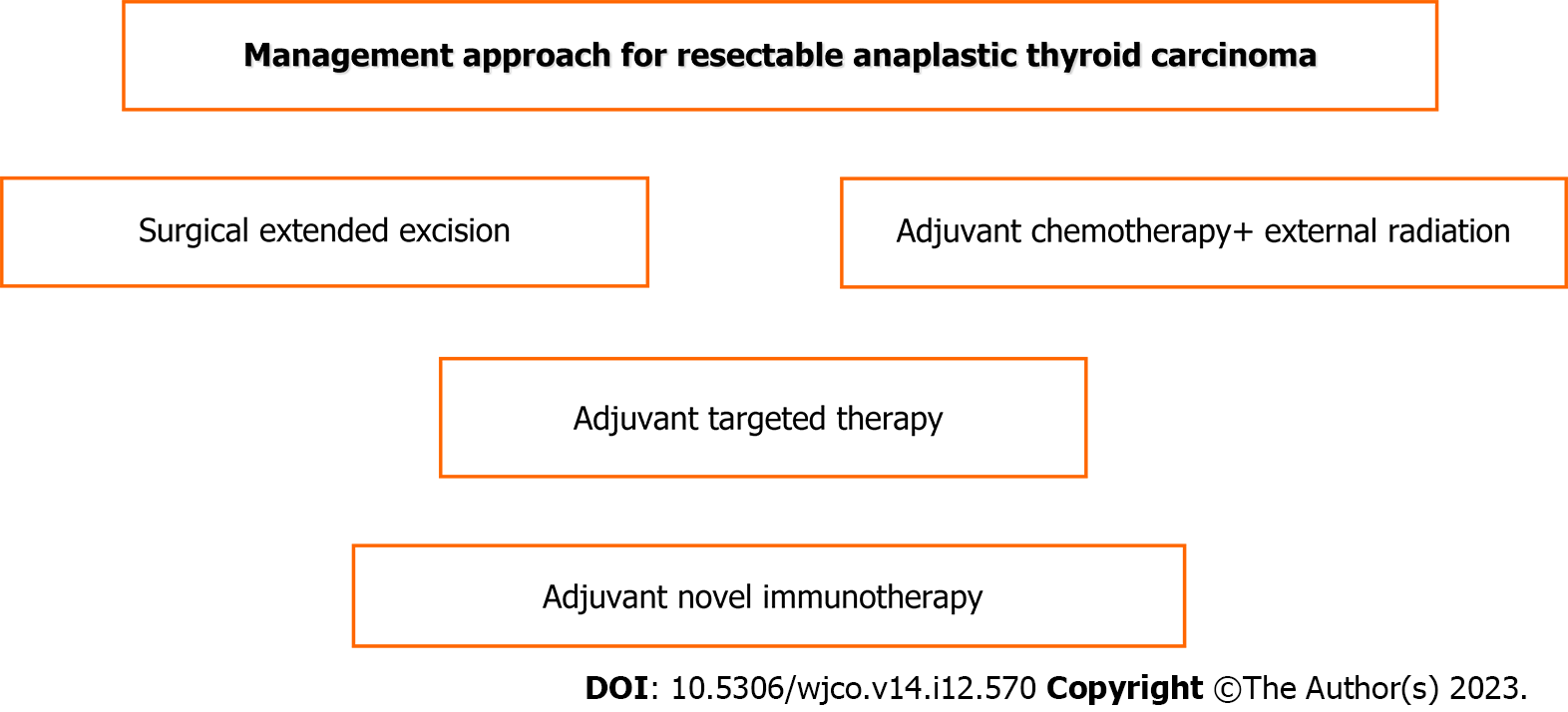
Surgery constitutes the cornerstone of treatment despite the existing debate. It may range from palliative debulking intervention to more radical resections, including total or near total thyroidectomy and extended lymphadenectomy, including the central and lateral lymph node level either unilaterally or bilaterally[6,8,36,53,61]. The above curative surgery may be performed in some patients with earlier disease and may provide, accompanied by adjuvant chemotherapy, occasional long survival over 5 years[18,19]. By multivariate analysis in a systematic review and meta-analysis, surgery and radiotherapy were found to be independent factors predicting increased overall survival[36]. Complete surgical excision followed by adjuvant therapy is the optimal opportunity for cure[4,47]. However, in general, extreme radical resection, such as laryngectomy, tracheal resection, esophagectomy or complete neck dissection without notable oncological contribution, is not indicated[47]. Tumor removal increases the benefits of the treatment. In combination with radiation, new chemotherapy and novel gene targeted therapy can achieve locoregional disease control and improve survival and quality of life[36,41,53,65,67]. The guidelines of the National Comprehensive Cancer Network and ATA recommend surgical resection by lobectomy or near total thyroidectomy with wide lymphadenectomy in stage IVA and IVB, even in stage IVC when an R0 or at least R1 intervention could be achieved in locally resectable tumors[36,43,68-70]. However, many locally unresectable cases may respond to neoadjuvant external beam radiation, chemotherapy or even targeted therapy (dabrafenib and trametinib) for BRAF gene mutation, thus becoming resectable and ensuring surgical excision[43]. Timely detection and proper treatment reduce the number of advanced cases with distant metastases[9].
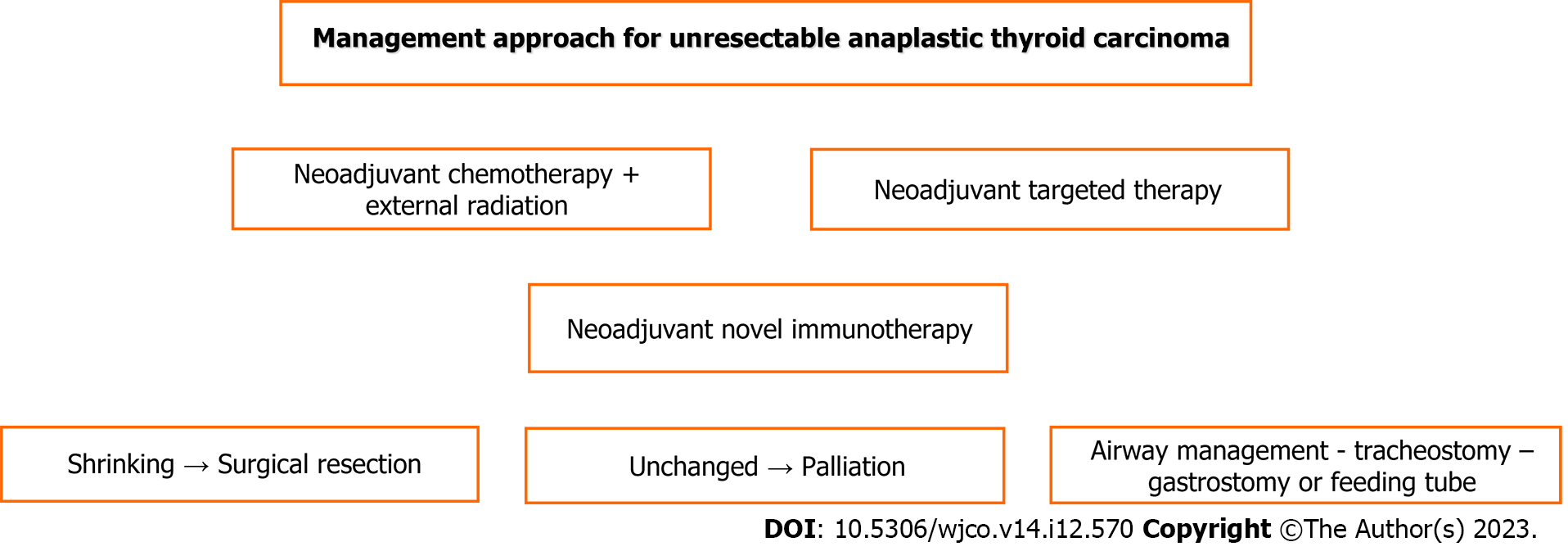
As shown in Figures 2 and 3, the current best practice for respectable tumors is surgery with adjuvant chemotherapy, radiation therapy and targeted therapy-immunotherapy such as dabrafenib and trametinib but immunotherapy if BRAF and MET gene mutations exist; for unresectable tumors, current best practice involves palliative surgery and targeted therapy-immunotherapy in combination with chemoradiation therapy[47].
Patients with inoperable disease may undergo palliative surgery to improve morbidity and avoid complications and life-threatening urgent events[48,71]. Unless there is debulking surgery for decompression, it includes the performance of tracheostomy and gastrostomy, preferably percutaneous by endoscopy assistance for feeding in case prior to radiation, which may cause esophageal stricture[36,48]. In cases of esophageal invasion or stenosis, feeding tube placement by an interventional radiologist can ensure enteral feeding[43].
Palliative airway management for symptom relief must be based on multidisciplinary team collaboration by designing the plan carefully. It is well known that tracheostomy may be related to morbidity and problems affecting quality of life[43]. It should be emphasized overall that patients undergoing extended surgical intervention and receiving adjuvant radiation and chemotherapy gain the chance of the best overall survival[9,48]. Aggressive locoregional surgery and radiotherapy must be performed whenever possible, and adding chemotherapy can lead to further improvement; however, in unresectable cases, radiotherapy and chemotherapy must be preferred[9].
Taxanes (paclitaxel, docetaxel, and cabazitaxel) can be effectively used to treat various forms of cancer by replicating inhibition. Doxorubicin (adriamycin) is an anthracyclin that inhibits cancer cell growth by topoisomerase II activation in the process of DNA repair. Platinum-based chemotherapy (cisplatin, carboplatin, oxaliplatin) is widely used. Attachment to DNA causes destruction of cancer cells by replicating inhibition. These chemotherapeutic drugs have been used in various cancers, including anaplastic thyroid carcinoma[6,53,67].
The ATA guidelines recommend adjuvant or neoadjuvant chemotherapy by combination of: (1) Paclitaxel with carboplatin; (2) Doxorubicin with cisplatin; (3) Doxorubicin with docetaxel; or (4) Paclitaxel alone or doxorubicin alone[6]. Unfortunately, chemoresistance often occurs in anaplastic thyroid carcinoma even in the most effective regimen of paclitaxel[6,72]. Adjuvant chemotherapy increases the median survival and the survival rate[48,67].
Subsequently, other drugs enhancing chemotherapy efficiency have been added. The combination with targeted biological agents such as dabrafenib and trametinib, in cases of mutated BRAF and MEK genes, respectively, may overcome this resistance[6,12,72]. In unmutated cases, novel immunotherapy (anti-PD-1 and anti-PD-L1) has been a recent revolution[6,12,72].
Chemotherapy added to radiation therapy further improves survival compared to radiation alone in resected cases as well as in unresected cases[73]. A randomized controlled phase II trial from 34 centers in the United States including 89 patients showed that the combination of paclitaxel chemotherapy with pazopanib, a multitargeted inhibitor of tyrosine kinase receptors and radiation therapy, was feasible, safe and promising[74].
Anlotinib, another new multitargeted inhibitor of tyrosine kinase receptors [VEGF, FGFR, platelet-derived growth factor receptor (PDGFR), c-kit] approved by the United States FDA, in combination with chemotherapy with paclitaxel, capecitabine or paclitaxel, capecitabine, and carboplatin as first-line therapy is a safe and effective treatment for locally advanced or metastatic thyroid carcinoma. As reported, it had an objective response rate of 60%, a disease control rate of 88%, and provided a progression-free survival of 25.1 wk and a median disease specification survival of 96 wk[75,76].
Local rapid progression of anaplastic thyroid carcinoma and recurrence are related to the extreme malignancy of the disease. Subsequently, local control is of great importance. Radiation therapy is the main stem of every potent successful management, providing cessation of progression and regression of the tumor extent before surgery as well as prevention of recurrence after attempted surgical resection[6].
Radiotherapy can be applied as a neoadjuvant or definitive adjuvant modality generally by external beam radiation therapy (EBRT), which accelerates hyperfunction and improves median overall survival[24,48,77]. It is a necessary part of the current multimodality treatment combined with surgery, chemotherapy, targeted therapy and novel immunotherapy[9,42,63,73,74].
EBRT was assessed by a multivariate analysis in 433 stage IVC patients with anaplastic thyroid carcinoma as an independent prognostic factor of survival along with surgery and chemotherapy[24]. The optimal dose of hyperfunction EBRT varies between 45-70 G, and a subsequent hypofunction dose > 5 G can prevent local recurrence and death[6]. A retrospective study including 491 patients with anaplastic thyroid carcinoma found that the combination of radiation therapy and chemotherapy provided better overall survival than radiotherapy alone regardless of surgery and distant metastases. Prognostic factors for survival were older age, single marital status, local extension, distal metastases and surgery[73]. Among various management modalities, adjuvant chemoradiation after surgical intervention seems to be the better modality for prolonged survival in stage IVA resectable tumors without negative prognostic factors[67]. Radiotherapy may have synergy with immunotherapy by modulating microenvironmental immunity[62]. Brain metastases account for up to 10% of metastatic cases, with an overall survival of 3 mo[13]. Radiation therapy and lenvatinib targeted therapy have been reported in such cases, but with limited efficacy[14].
Targeted therapy by biological agents is based on monoclonal antibodies and is intended to block certain cancer development pathways[56,78-81]. The drugs for mechanisms of some implicated gene mutations include the following: (1) Angiogenesis-lenvatinib, sorafenib, sunitinib, vandetanib, combretastatin; (2) EGFR-docetaxel, gefitinib; (3) BRAF-dabrafenib, vemurafenib, encorafenib; and (4) MEK-trametinib, cobimetinib, binimetinib[24]. The combination of the BRAF inhibitor dabrafenib with the MEK inhibitor trametinib is the most widely used because it is considered more effective than each drug alone[6,24,41,82,83]. Combretastatin targets tumor vascularity. It has been used as adjuvant treatment in combination with paclitaxel - carboplatin but without notable results. Likewise, sorafenib had limited usefulness; instead, other anti-angiogenetic agents, such as vandetanib, sunitinib, and lenvatinib, exhibited significant anti-neoplastic efficacy[24]. Vandetanib, a multitarget tyrosine inhibitor that acts mainly against EGFR and VEGF receptor (VEGFR), promotes apoptosis (programmed cell death) and inhibits tumor growth, migration and invasion[62,84]. Sunitinib, another multitarget tyrosine inhibitor, acts mainly against VEGFR and PDGFR to inhibit tumor growth by deprivation of its blood supply[62]. Lenvatinib is an inhibitor of kinase that inactivates VEGFRs (VEGFR 1, VEGFR 2, and VEGFR 3) and subsequently prevents angiogenesis and tumor growth. It has been used in anaplastic and well-differentiated thyroid carcinoma as an alternative to radioactive iodine, in inoperable hepatocellular carcinoma, and in advanced renal cell carcinoma in combination with everolimus[11,30,85]. Carfilzomib, a proteasome inhibitor approved by the United States FDA for multiple myeloma, is the most effective such drug for treating anaplastic thyroid carcinoma; by acting on cell proliferation and p27 gene overexpression, which promotes apoptosis and cell death[62]. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor approved by the United States FDA for skin T-cell lymphoma, causes p21 gene overexpression that promotes apoptosis and cell death[62].
A recent study from Japan including 36 patients with unresectable anaplastic thyroid carcinoma treated initially with lenvatinib showed an average survival of 5.8 mo, longer than the 2-mo survival from paclitaxel initial treatment, a response rate of 33% and a median overall survival of 5 mo[86].
Glutamin metabolism and subsequent glutaminolysis are biological features that are highly increased in anaplastic thyroid carcinoma and modulate cancer cell survival by sustaining mitochondrial function and oxygen balance. Glutaminolysis inhibition causes cell death. The tyrosine kinase inhibitors lenvatinib and sorafenib affect this signaling pathway of oncogenesis and enhance the efficiency of conventional chemotherapy, such as doxorubicin or taxanes, which otherwise may have minimal influence on patient survival[12]. ICAM1 is an interesting target of monoclonal antibodies with promising results[57]. The polo-like kinase 4 inhibitor has anticancer efficacy and synergy with sorafenib[87]. CTHRC1 promotes the progression of anaplastic thyroid carcinoma and is associated with worse outcomes[58]. The depletion of fibronectin may overcome resistance to BRAF gene inhibitor treatment. It can be targeted by MARK (ERK) pathway inhibition (ipilimumab, vemurafenib)[88]. Additionally, diclofenac added to BRAF gene inhibitors by targeting metabolism overcomes any resistance and maximizes the treatment effect[89]. Targeting the EZH2 complex promotes anticancer activity and can be a promising strategy[90]. Recent research data showed that one-carbon metabolism had a possible role in metabolic stress, and its targeting would be a valuable promising therapy[12].
Immunotherapy has been applied increasingly on a preliminary experimental basis but with promising future perspectives. Thus, it has been included in many research protocols. The existing immunotherapy regimens in combination with targeted therapy (dabrafenib - trametinib) can provide a further potential increase in effectiveness and improved survival benefits[41,91,92]. It is undoubted that the multimodality current treatment plan is accompanied by the best outcomes of this otherwise lethal disease[40]. However, unfortunately, for the mutated BRAF gene wild type, there has not yet been effective treatment[4].
Since the recent innovation of targeted PD-1 and PD-L1 interaction by monoclonal antibodies atezolizumab, spartalizumab and pembrolizumab, the application of immunotherapy has been increasing[6,12,93]. It may be a promising therapeutic choice, especially in those with high PD-1 and PD-L1 expression without BRAF gene mutations[4,40,48,59,94]. Atezolizumab, a monoclonal antibody against PD-L1, gives encouraging results in combination with radiation therapy[31]. Spartalizumab and pembrolizumab are monoclonal antibodies against PD-1[4]. Spartalizumab has been used in a phase II clinical study in unresectable locally advanced or metastatic cases, showing a one-year survival of 40% and a median overall survival of 5.9 mo. Among the side effects, diarrhea, pruritus, fever, and fatigue have been reported[95]. Pembrolizumab has been used in likewise unresectable cases, showing a one-year survival of 38% and a median overall survival of 4.4 mo[96].
Various tumor experimental models of anaplastic thyroid carcinoma microenvironment in mice have been developed for scientific research and monoclonal antibody or other innovative drug production[30,97]. Novel promising therapies, including immunotherapy, multikinase inhibitors, aurora kinase inhibitors, gene therapy by oncolytic viruses, epigenetic modulators, and apoptosis-inducing agents, have been introduced[40,72,76,98,99].
Early diagnosis and treatment yield the best outcome and prognosis[100-102]. A study from the United States including 719 patients found that racial, ethical and socioeconomic status seems to influence survival and prognosis. Nonwhite patients had a lower likelihood of receiving treatment and poorer survival; those living in high poverty had a worse prognosis[103]. A large cohort study from China including 735 patients with multidisciplinary management of anaplastic thyroid carcinoma found an overall survival of 10.7% at 2 years and 8.1% at 5 years. By stage, survival at 2 years was 36.5% (IVA), 15.6% (IVB), and 1.4% (IVC)[9]. A large single institution 20-year study from the United States including 479 patients with multimodality management of anaplastic thyroid carcinoma found a constantly increasing overall survival among three periods of treatment due to progression improvement by the addition of targeted therapy and immunotherapy. The overall survival for 2000-2013 was 35% at 1 year and 18% at 2 years; for 2014-2016, it was 47% at 1 year and 25% at 2 years; for 2017-2019, it was 59% at 1 year and 42% at 2 years[104]. A nationwide cohort study from the Netherlands including 812 patients with management of anaplastic thyroid carcinoma during the period 1989-2016 found a median overall survival of 2.2 mo, overall one-year survival of 12%, and one-year survival of 21.6% in those without distant metastases. Prognostic factors for better survival were age < 65 years, treatment based on more than two to three modalities, without distant metastases and bilateral lymph node involvement[5]. A recent large study including 5359 patients with anaplastic thyroid carcinoma found a total one-year survival of 23%[44]. A nationwide cohort study from Denmark including 320 patients with management of anaplastic thyroid carcinoma during the period 1980-2014 found a 1-year survival of 18% and a 5-year survival of 12%[2].
Another recent large study from China including 1080 patients with stage IVA: 6.3%, IVB: 21.9%, IVC: 71.8% anaplastic thyroid carcinoma management found disease specific survival at 1 mo of 83.1%, at 6 mo, 37.5%, and at 12 mo, 21%. The 1-year disease specific survival was 53.3% for stage IVA, 36.5% for IVB and 13.1% for IVC[35]. A recent study from a tertiary academic hospital in the United States including 45 patients found a median survival of 6.1 mo; smaller tumors and chemotherapy were related to better survival[105]. It was found by regression analysis that age, distant metastases and tumor size were independent factors of worse prognosis[106].
It was found by univariate analysis that distant metastases, lymph node involvement, tumor > 5 cm in size, and local infiltration were predictive factors for worse prognosis[107]. Multivariate analysis showed that the absence of both symptoms and distant metastases was related to longer survival. The asymptomatic patients were younger (≤ 60 years) and had smaller tumors (≤ 5 cm) than symptomatic patients with anaplastic thyroid carcinoma[108]. Another study found by multivariate analysis that age, sex, marital status, multiple primary tumors, distant metastases, and therapy type were independent prognostic factors for cancer-specific survival[109]. A recent large study from China including 1140 patients with anaplastic thyroid carcinoma management found an overall survival of 27.6% at 6 mo, 15.1% at 1 year, and 6.2% at 2 years. The age cutoff was 65 years as a significant predictive factor of improved survival[110]. The predictive factors for favorable prognosis are shown in Table 4.
| n | Factor |
| 1 | Female patients |
| 2 | Age ≤ 60 yr |
| 3 | Married patients |
| 4 | Asymptomatic patients |
| 5 | Tumor ≤ 5 cm in size |
| 6 | Single primary tumor |
| 7 | Without local tissue invasion |
| 8 | Without lymph node involvement (N0) |
| 9 | Without distant metastases (M0) |
| 10 | Kind of therapy |
| 11 | Multimodality treatment |
It has been reported that age ≥ 65 years, palliative surgery and white cell count ≥ 10000/mm3 are predictive factors for worse prognosis[67]. Despite proper management, recurrence with a high incidence may occur. An overall survival of 5-6 mo (specifically 9 mo for stage IVA, 4.8 mo for stage IVB and 3 mo for stage IVC) and a one-year survival of 20% have been reported[43]. Although, the overall prognosis of anaplastic thyroid carcinoma is very poor, in some cases is relatively good. There is evidence that patients with anaplastic carcinoma clearly transformed from papillary thyroid carcinoma, or those with mutated BRAF gene had a significantly better prognosis than other patients. Despite, the conflicting aspects for the above mentioned, it could be possibly explained by the higher expression of PD-L1 in papillary than in anaplastic carcinoma and response to immunotherapy[59] and anti-BRAF targeted therapy[24]. A long-term survival reaching above 5 years has been reported in isolated cases after surgical excision and adjuvant chemotherapy[18,19].
Anaplastic thyroid carcinoma is a rare, rapidly growing and extremely aggressive neoplasm with a dismal prognosis. The correct early diagnosis and treatment are important perquisites for the management of an otherwise lethal condition. US and core needle biopsy are used to make the diagnosis. Preoperative imaging, histopathology and molecular testing establish an accurate natural status and determine the therapeutic strategy plan. Complete surgical resection with wide lymphadenectomy whenever possible is the main step followed by chemoradiation therapy, targeted therapy and immunotherapy. Palliation management may improve the quality of an otherwise unbearable life. Novel multimodal treatment that must be personalized offers the best chance to manage this incurable disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gluvic Z, Serbia; Shi B, China S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association with Changes in Professional Guideline Recommendations. Thyroid. 2020;30:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 2. | Mirian C, Grønhøj C, Jensen DH, Jakobsen KK, Karnov K, Jensen JS, Hahn CH, Klitmøller TA, Bentzen J, von Buchwald C. Trends in thyroid cancer: Retrospective analysis of incidence and survival in Denmark 1980-2014. Cancer Epidemiol. 2018;55:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Janz TA, Neskey DM, Nguyen SA, Lentsch EJ. Is the incidence of anaplastic thyroid cancer increasing: A population based epidemiology study. World J Otorhinolaryngol Head Neck Surg. 2019;5:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Califano I, Smulever A, Jerkovich F, Pitoia F. Advances in the management of anaplastic thyroid carcinoma: transforming a life-threatening condition into a potentially treatable disease. Rev Endocr Metab Disord. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | de Ridder M, Nieveen van Dijkum E, Engelsman A, Kapiteijn E, Klümpen HJ, Rasch CRN. Anaplastic thyroid carcinoma: a nationwide cohort study on incidence, treatment and survival in the Netherlands over 3 decades. Eur J Endocrinol. 2020;183:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, Baudin E, Do Cao C, Hadoux J. Anaplastic Thyroid Carcinoma: An Update. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Pavlidis ET, Pavlidis TE. Role of prophylactic central neck lymph node dissection for papillary thyroid carcinoma in the era of de-escalation. World J Clin Oncol. 2023;14:247-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 8. | Abe I, Lam AK. Anaplastic thyroid carcinoma: Updates on WHO classification, clinicopathological features and staging. Histol Histopathol. 2021;36:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Huang NS, Shi X, Lei BW, Wei WJ, Lu ZW, Yu PC, Wang Y, Ji QH, Wang YL. An Update of the Appropriate Treatment Strategies in Anaplastic Thyroid Cancer: A Population-Based Study of 735 Patients. Int J Endocrinol. 2019;2019:8428547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Xu L, Cai L, Zhu Z, Chen G. Comparison of the cox regression to machine learning in predicting the survival of anaplastic thyroid carcinoma. BMC Endocr Disord. 2023;23:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Sugitani I, Onoda N, Ito KI, Suzuki S. Management of Anaplastic Thyroid Carcinoma: the Fruits from the ATC Research Consortium of Japan. J Nippon Med Sch. 2018;85:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Hwang Y, Yun HJ, Jeong JW, Kim M, Joo S, Lee HK, Chang HS, Kim SM, Fang S. Co-inhibition of glutaminolysis and one-carbon metabolism promotes ROS accumulation leading to enhancement of chemotherapeutic efficacy in anaplastic thyroid cancer. Cell Death Dis. 2023;14:515. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wolff L, Steindl A, Popov P, Dieckmann K, Gatterbauer B, Widhalm G, Berghoff AS, Preusser M, Raderer M, Kiesewetter B. Clinical characteristics, treatment, and long-term outcome of patients with brain metastases from thyroid cancer. Clin Exp Metastasis. 2023;40:217-226. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Obayashi A, Aoki K, Wada T, Furuie H, Kuraoka K, Hamamoto T, Tatsukawa T. Lenvatinib Administration for Anaplastic Thyroid Carcinoma with Brain Metastasis. Acta Med Okayama. 2023;77:227-232. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Lu L, Wang JR, Henderson YC, Bai S, Yang J, Hu M, Shiau CK, Pan T, Yan Y, Tran TM, Li J, Kieser R, Zhao X, Wang J, Nurieva R, Williams MD, Cabanillas ME, Dadu R, Busaidy NL, Zafereo M, Navin N, Lai SY, Gao R. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J Clin Invest. 2023;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 16. | Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratù G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez C, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol. 2018;29:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Roseland ME, Dewaraja YK, Wong KK. Advanced imaging and theranostics in thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2022;29:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Vithana S, Udayakumara E, Gunasena M, Mushraf M. Curative surgery for anaplastic thyroid carcinoma: A case report. SAGE Open Med Case Rep. 2022;10:2050313X221091399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 19. | Moreno F, Reyes C, Pineda CA, Castellanos G, Cálix F, Calderón J, Vasquez-Bonilla WO. Anaplastic thyroid carcinoma with unusual long-term survival: a case report. J Med Case Rep. 2022;16:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Matrone A, De Napoli L, Torregrossa L, Aghababyan A, Papini P, Ambrosini CE, Cervelli R, Ugolini C, Basolo F, Molinaro E, Elisei R, Materazzi G. Core Needle Biopsy Can Early and Precisely Identify Large Thyroid Masses. Front Oncol. 2022;12:854755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, Hong SJ. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016;26:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Sonavane SN, Basu S. Role of PET/Computed Tomography in Elderly Thyroid Cancer: Tumor Biology and Clinical Management. PET Clin. 2023;18:81-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Adnan A, Raju S, Kumar R, Basu S. An Appraisal and Update of Fluorodeoxyglucose and Non-Fluorodeoxyglucose-PET Tracers in Thyroid and Non-Thyroid Endocrine Neoplasms. PET Clin. 2022;17:343-367. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Ferrari SM, Elia G, Ragusa F, Ruffilli I, La Motta C, Paparo SR, Patrizio A, Vita R, Benvenga S, Materazzi G, Fallahi P, Antonelli A. Novel treatments for anaplastic thyroid carcinoma. Gland Surg. 2020;9:S28-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Nasrpour Navaei Z, Taghehchian N, Zangouei AS, Abbaszadegan MR, Moghbeli M. MicroRNA-506 as a tumor suppressor in anaplastic thyroid carcinoma by regulation of WNT and NOTCH signaling pathways. Iran J Basic Med Sci. 2023;26:594-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Romano C, Martorana F, Pennisi MS, Stella S, Massimino M, Tirrò E, Vitale SR, Di Gregorio S, Puma A, Tomarchio C, Manzella L. Opportunities and Challenges of Liquid Biopsy in Thyroid Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Khatami F, Tavangar SM. Liquid Biopsy in Thyroid Cancer: New Insight. Int J Hematol Oncol Stem Cell Res. 2018;12:235-248. [PubMed] |
| 28. | Huang P, Tang N, Mao LF, Zhang Y, Tang XF, Zhou RY, Wei B, Tan HL, Shi QM, Lin J, Li ZC, Chang S. Nanoclay Drug-Delivery System Loading Potassium Iodide Promotes Endocytosis and Targeted Therapy in Anaplastic Thyroid Cancer. Nano Lett. 2023;23:8013-8021. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Uppalapati SS, Guha L, Kumar H, Mandoli A. Nanotechnological Advancements For The Theranostic Intervention In Anaplastic Thyroid Cancer: Current Perspectives And Future Direction. Curr Cancer Drug Targets. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhou J, Ma L, Li Z, Chen B, Wu Y, Meng X. Synthesis of lenvatinib-loaded upconversion@polydopamine nanocomposites for upconversion luminescence imaging-guided chemo-photothermal synergistic therapy of anaplastic thyroid cancer. RSC Adv. 2023;13:26925-26932. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wächter S, Roth S, Gercke N, Schötz U, Dikomey E, Engenhart-Cabillic R, Maurer E, Bartsch DK, Di Fazio P. Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells. Life (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 32. | Xu T, Zhu C, Chen J, Song F, Ren X, Wang S, Yi X, Zhang Y, Zhang W, Hu Q, Qin H, Liu Y, Zhang S, Tan Z, Pan Z, Huang P, Ge M. ISG15 and ISGylation modulates cancer stem cell-like characteristics in promoting tumor growth of anaplastic thyroid carcinoma. J Exp Clin Cancer Res. 2023;42:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Mahdiannasser M, Khazaei S, Akhavan Rahnama M, Soufi-Zomorrod M, Soutodeh F, Parichehreh-Dizaji S, Rakhsh-Khorshid H, Samimi H, Haghpanah V. Illuminating the role of lncRNAs ROR and MALAT1 in cancer stemness state of anaplastic thyroid cancer: An exploratory study. Noncoding RNA Res. 2023;8:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Hirokawa M, Niioka H, Suzuki A, Abe M, Arai Y, Nagahara H, Miyauchi A, Akamizu T. Application of deep learning as an ancillary diagnostic tool for thyroid FNA cytology. Cancer Cytopathol. 2023;131:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 35. | Dong W, Okamoto T, Ji X, Xiang J, Zhang D, Zhang P, Zhang H. Conditional Survival Rate Estimates for Anaplastic Thyroid Cancer Beyond the First Year: An Analysis of SEER Data (2004-2019). Thyroid. 2023;33:523-526. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Oliinyk D, Augustin T, Rauch J, Koehler VF, Belka C, Spitzweg C, Käsmann L. Role of surgery to the primary tumor in metastatic anaplastic thyroid carcinoma: pooled analysis and SEER-based study. J Cancer Res Clin Oncol. 2023;149:3527-3547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Cui H, Wang R, Zhao X, Wang S, Shi X, Sang J. Development and validation of a nomogram for predicting the early death of anaplastic thyroid cancer: a SEER population-based study. J Cancer Res Clin Oncol. 2023;149:16001-16013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Shaha AR. Anaplastic Thyroid Cancer: Shifting Paradigms-A Ray of Hope. Thyroid. 2023;33:402-403. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1856-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 683] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 40. | Gao X, Hong C, Xie Y, Zeng X. Immunotherapy or targeted therapy: What will be the future treatment for anaplastic thyroid carcinoma? Front Oncol. 2023;13:1103147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 41. | Hamidi S, Maniakas A. Recent advances in anaplastic thyroid cancer management. Curr Opin Endocrinol Diabetes Obes. 2023;30:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Wu SS, Lamarre ED, Yalamanchali A, Brauer PR, Hong H, Reddy CA, Yilmaz E, Woody N, Ku JA, Prendes B, Burkey B, Nasr C, Skugor M, Heiden K, Chute DJ, Knauf JA, Campbell SR, Koyfman SA, Geiger JL, Scharpf J. Association of Treatment Strategies and Tumor Characteristics With Overall Survival Among Patients With Anaplastic Thyroid Cancer: A Single-Institution 21-Year Experience. JAMA Otolaryngol Head Neck Surg. 2023;149:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Moyer KF, Marcadis AR, Shaha AR. Airway management, symptom relief and best supportive care in anaplastic thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2020;28:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Ginzberg SP, Gasior JA, Passman JE, Ballester JMS, Finn CB, Karakousis GC, Kelz RR, Wachtel H. Disparities in Presentation, Treatment, and Survival in Anaplastic Thyroid Cancer. Ann Surg Oncol. 2023;30:6788-6798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 45. | Vander Poorten V, Goedseels N, Triantafyllou A, Sanabria A, Clement PM, Cohen O, Golusinski P, Guntinas-Lichius O, Piazza C, Randolph GW, Rinaldo A, Ronen O, Cabanillas ME, Shaha AR, Teng Y, Tufano RP, Williams MD, Zafereo M, Ferlito A. Effectiveness of core needle biopsy in the diagnosis of thyroid lymphoma and anaplastic thyroid carcinoma: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:971249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 46. | Pavlidis ET, Pavlidis TE. A Review of Primary Thyroid Lymphoma: Molecular Factors, Diagnosis and Management. J Invest Surg. 2019;32:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Al-Mulki K, Smith RV. What is the best treatment for Anaplastic Thyroid Cancer? Laryngoscope. 2023;133:2044-2045. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Alobuia W, Gillis A, Kebebew E. Contemporary Management of Anaplastic Thyroid Cancer. Curr Treat Options Oncol. 2020;21:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | de la Fouchardière C, Wassermann J, Calcagno F, Bardet S, Al Ghuzlan A, Borget I, Borson Chazot F, Do Cao C, Buffet C, Zerdoud S, Decaussin-Petrucci M, Godbert Y, Leboulleux S. [Molecular genotyping in refractory thyroid cancers in 2021: When, how and why? A review from the TUTHYREF network]. Bull Cancer. 2021;108:1044-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Silver Karcioglu A, Iwata AJ, Pusztaszeri M, Abdelhamid Ahmed AH, Randolph GW. The American Thyroid Association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): Update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022;130:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Wang X, Ying T, Yuan J, Wang Y, Su X, Chen S, Zhao Y, Sheng J, Teng L, Luo C, Wang W. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 52. | Papanikolaou V, Kyrodimos E, Mastronikolis N, Asimakopoulos AD, Papanastasiou G, Tsiambas E, Spyropoulou D, Katsinis S, Manoli A, Papouliakos S, Pantos P, Ragos V, Peschos D, Chrysovergis A. Anti-EGFR/BRAF-Tyrosine Kinase Inhibitors in Thyroid Carcinoma. Cancer Diagn Progn. 2023;3:151-156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Cabanillas ME, Ahmed S, Wang JR. Management of Anaplastic and Recurrent Differentiated Thyroid Cancer: Indications for Surgical Resection, Molecular Testing, and Systemic Therapy. Neuroimaging Clin N Am. 2021;31:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Gong Y, Xu F, Deng L, Peng L. Recognition of Key Genes in Human Anaplastic Thyroid Cancer via the Weighing Gene Coexpression Network. Biomed Res Int. 2022;2022:2244228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Bikas A, Ahmadi S, Pappa T, Marqusee E, Wong K, Nehs MA, Cho NL, Haase J, Doherty GM, Sehgal K, Barletta JA, Alexander EK, Landa I. Additional Oncogenic Alterations in RAS-Driven Differentiated Thyroid Cancers Associate with Worse Clinicopathologic Outcomes. Clin Cancer Res. 2023;29:2678-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Haddad R, Elisei R, Hoff AO, Liu Z, Pitoia F, Pruneri G, Sadow PM, Soares F, Turk A, Williams MD, Wirth LJ, Cabanillas ME. Diagnosis and Management of Tropomyosin Receptor Kinase Fusion-Positive Thyroid Carcinomas: A Review. JAMA Oncol. 2023;9:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 57. | Zhang P, Tao C, Shimura T, Huang AC, Kong N, Dai Y, Yao S, Xi Y, Wang X, Fang J, Moses MA, Guo P. ICAM1 antibody drug conjugates exert potent antitumor activity in papillary and anaplastic thyroid carcinoma. iScience. 2023;26:107272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Chen Y, Jia L, Zhao K, Chen Z, Han Y, He X. CTHRC1 promotes anaplastic thyroid cancer progression by upregulating the proliferation, migration, and invasion of tumor cells. PeerJ. 2023;11:e15458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Boruah M, Gaddam P, Agarwal S, Mir RA, Gupta R, Sharma MC, S Deo SV, Nilima N. PD-L1 expression in rare and aggressive thyroid cancers: A preliminary investigation for a role of immunotherapy. J Cancer Res Ther. 2023;19:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 60. | Manjusha P, Kandathil JP, Nayanar SK, Jones J, Sajith B. Clinicopathological Spectrum of Anaplastic Carcinoma of Thyroid - 5 Year Experience from a Tertiary Cancer Centre. Gulf J Oncolog. 2018;1:17-22. [PubMed] |
| 61. | Onoda N, Sugitani I, Ito KI, Suzuki A, Higashiyama T, Fukumori T, Suganuma N, Masudo K, Nakayama H, Uno A, Yane K, Yoshimoto S, Ebina A, Kawasaki Y, Maeda S, Iwadate M, Suzuki S. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Li W, Li Y, Li J, Pang H. Combination of Novel Therapies and New Attempts in Anaplastic Thyroid Cancer. Technol Cancer Res Treat. 2023;22:15330338231169870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Lee H, Kim SY, Kim SM, Chang HJ, Lee YS, Park CS, Chang HS. Long-term survival of patients with anaplastic thyroid cancer after multimodal treatment. Transl Cancer Res. 2020;9:5430-5436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Song T, Chen L, Zhang H, Lu Y, Yu K, Zhan W, Fang M. Multimodal treatment based on thyroidectomy improves survival in patients with metastatic anaplastic thyroid carcinoma: a SEER analysis from 1998 to 2015. Gland Surg. 2020;9:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Jonker PKC, Turchini J, Kruijff S, Lin JF, Gill AJ, Eade T, Aniss A, Clifton-Bligh R, Learoyd D, Robinson B, Tsang V, Glover A, Sidhu S, Sywak M. Multimodality Treatment Improves Locoregional Control, Progression-Free and Overall Survival in Patients with Anaplastic Thyroid Cancer: A Retrospective Cohort Study Comparing Oncological Outcomes and Morbidity between Multimodality Treatment and Limited Treatment. Ann Surg Oncol. 2021;28:7520-7530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Lee JH, Ahn HK, Seok JY, Lee KC, Chun YS, Chung YS, Lee YD. Optimal combination of treatment modality to increase survival in patients with anaplastic thyroid carcinoma: A STROBE compliant retrospective study. Medicine (Baltimore). 2018;97:e11037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Kasemsiri P, Chaisakgreenon P, Vatanasapt P, Laohasiriwong S, Teeramatwanich W, Thongrong C, Ratanaanekchai T, Suetrong S. Survival Benefit of Intervention Treatment in Advanced Anaplastic Thyroid Cancer. Int J Surg Oncol. 2021;2021:5545127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, Campbell M, Dickson P, Duh QY, Ehya H, Goldner WS, Guo T, Haymart M, Holt S, Hunt JP, Iagaru A, Kandeel F, Lamonica DM, Mandel S, Markovina S, McIver B, Raeburn CD, Rezaee R, Ridge JA, Roth MY, Scheri RP, Shah JP, Sipos JA, Sippel R, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Yeh M, Cassara CJ, Darlow S. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:925-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 215] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 69. | Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021;31:337-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 374] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 70. | Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, McIver B, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sippel R, Smallridge RC, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Johnson-Chilla A, Hoffmann KG, Gurski LA. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw. 2018;16:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 71. | Koda K, Katoh M, Yasuhara K. Management of anaplastic thyroid cancer and proposed treatment guidelines-A 5-year case series study. Cancer Rep (Hoboken). 2022;5:e1727. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Saini S, Tulla K, Maker AV, Burman KD, Prabhakar BS. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer. 2018;17:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 73. | Zhou W, Yue Y, Zhang X. Radiotherapy Plus Chemotherapy Leads to Prolonged Survival in Patients With Anaplastic Thyroid Cancer Compared With Radiotherapy Alone Regardless of Surgical Resection and Distant Metastasis: A Retrospective Population Study. Front Endocrinol (Lausanne). 2021;12:748023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Sherman EJ, Harris J, Bible KC, Xia P, Ghossein RA, Chung CH, Riaz N, Gunn GB, Foote RL, Yom SS, Wong SJ, Koyfman SA, Dzeda MF, Clump DA, Khan SA, Shah MH, Redmond K, Torres-Saavedra PA, Le QT, Lee NY. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023;24:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Zheng X, Wang J, Ye T, Tang W, Pan X, Wang S, Liu J. Efficacy and safety of anlotinib-based chemotherapy for locally advanced or metastatic anaplastic thyroid carcinoma. Endocrine. 2023;81:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 76. | Wu J, Liang J, Liu R, Lv T, Fu K, Jiang L, Ma W, Pan Y, Tan Z, Liu Q, Qiu W, Ge M, Wang J. Autophagic blockade potentiates anlotinib-mediated ferroptosis in anaplastic thyroid cancer. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 77. | Jacob J, Vordermark D, Lorenz K, Medenwald D. Prognostic factors in radiotherapy of anaplastic thyroid carcinoma: a single center study over 31 years. Radiat Oncol. 2023;18:71. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Zhang L, Feng Q, Wang J, Tan Z, Li Q, Ge M. Molecular basis and targeted therapy in thyroid cancer: Progress and opportunities. Biochim Biophys Acta Rev Cancer. 2023;1878:188928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 79. | Miller KC, Chintakuntlawar AV. Molecular-Driven Therapy in Advanced Thyroid Cancer. Curr Treat Options Oncol. 2021;22:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 80. | Nylén C, Mechera R, Maréchal-Ross I, Tsang V, Chou A, Gill AJ, Clifton-Bligh RJ, Robinson BG, Sywak MS, Sidhu SB, Glover AR. Molecular Markers Guiding Thyroid Cancer Management. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 81. | Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid. 2019;29:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 82. | Gouda MA, Subbiah V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am Soc Clin Oncol Educ Book. 2023;43:e404770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 83. | Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, Wen PY, Dietrich S, de Jonge MJA, Blay JY, Italiano A, Yonemori K, Cho DC, de Vos FYFL, Moreau P, Fernandez EE, Schellens JHM, Zielinski CC, Redhu S, Boran A, Passos VQ, Ilankumaran P, Bang YJ. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023;29:1103-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 84. | Lang M, Longerich T, Anamaterou C. Targeted therapy with vemurafenib in BRAF(V600E)-mutated anaplastic thyroid cancer. Thyroid Res. 2023;16:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 85. | Iesato A, Li S, Sadow PM, Abbasian M, Nazarian A, Lawler J, Nucera C. The Tyrosine Kinase Inhibitor Lenvatinib Inhibits Anaplastic Thyroid Carcinoma Growth by Targeting Pericytes in the Tumor Microenvironment. Thyroid. 2023;33:835-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Iwasaki H, Toda S, Takahashi A, Masudo K. Outcome of initial lenvatinib treatment in patients with unresectable anaplastic thyroid cancer. Oncol Lett. 2023;26:416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 87. | Zhu W, Xie B. PLK4 inhibitor exhibits antitumor effect and synergizes sorafenib via arresting cell cycle and inactivating Wnt/β-catenin pathway in anaplastic thyroid cancer. Cancer Biol Ther. 2023;24:2223383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 88. | Hicks HM, Pozdeyev N, Sams SB, Pugazhenthi U, Bales ES, Hofmann MC, McKenna LR, Schweppe RE. Fibronectin Contributes to a BRAF Inhibitor-driven Invasive Phenotype in Thyroid Cancer through EGR1, Which Can Be Blocked by Inhibition of ERK1/2. Mol Cancer Res. 2023;21:867-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Aprile M, Cataldi S, Perfetto C, Federico A, Ciccodicola A, Costa V. Targeting metabolism by B-raf inhibitors and diclofenac restrains the viability of BRAF-mutated thyroid carcinomas with Hif-1α-mediated glycolytic phenotype. Br J Cancer. 2023;129:249-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 90. | de Mello DC, Saito KC, Cristovão MM, Kimura ET, Fuziwara CS. Modulation of EZH2 Activity Induces an Antitumoral Effect and Cell Redifferentiation in Anaplastic Thyroid Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Iyer PC, Dadu R, Gule-Monroe M, Busaidy NL, Ferrarotto R, Habra MA, Zafereo M, Williams MD, Gunn GB, Grosu H, Skinner HD, Sturgis EM, Gross N, Cabanillas ME. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 92. | Gunda V, Ghosh C, Hu J, Zhang L, Zhang YQ, Shen M, Kebebew E. Combination BRAF(V600E) Inhibition with the Multitargeting Tyrosine Kinase Inhibitor Axitinib Shows Additive Anticancer Activity in BRAF(V600E)-Mutant Anaplastic Thyroid Cancer. Thyroid. 2023;33:1201-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Sukari A, Kukreja G, Nagasaka M, Shukairy MK, Yoo G, Lin HS, Hotaling J, Kim H. The role of immune checkpoint inhibitors in anaplastic thyroid cancer (Case Series). Oral Oncol. 2020;109:104744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Chintakuntlawar AV, Foote RL, Kasperbauer JL, Bible KC. Diagnosis and Management of Anaplastic Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:269-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 95. | Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA, Fasolo A, Führer D, Hütter-Krönke ML, Forde PM, Wrona A, Santoro A, Sadow PM, Szpakowski S, Wu H, Bostel G, Faris J, Cameron S, Varga A, Taylor M. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol. 2020;38:2620-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 96. | Hatashima A, Archambeau B, Armbruster H, Xu M, Shah M, Konda B, Lott Limbach A, Sukrithan V. An Evaluation of Clinical Efficacy of Immune Checkpoint Inhibitors for Patients with Anaplastic Thyroid Carcinoma. Thyroid. 2022;32:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 97. | Xu Z, Shin HS, Kim YH, Ha SY, Won JK, Kim SJ, Park YJ, Parangi S, Cho SW, Lee KE. Modeling the tumor microenvironment of anaplastic thyroid cancer: an orthotopic tumor model in C57BL/6 mice. Front Immunol. 2023;14:1187388. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 98. | Hu H, Ng TSC, Kang M, Scott E, Li R, Quintana JM, Matvey D, Vantaku VR, Weissleder R, Parangi S, Miller MA. Thyroid Cancers Exhibit Oncogene-Enhanced Macropinocytosis that Is Restrained by IGF1R and Promote Albumin-Drug Conjugate Response. Clin Cancer Res. 2023;29:3457-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Turkmen E, Sogutlu F, Erdogan M, Biray Avci C. Evaluation of the anticancer effect of telomerase inhibitor BIBR1532 in anaplastic thyroid cancer in terms of apoptosis, migration and cell cycle. Med Oncol. 2023;40:196. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (3)] |
| 100. | Bellini MI, Biffoni M, Patrone R, Borcea MC, Costanzo ML, Garritano T, Melcarne R, Menditto R, Metere A, Scorziello C, Summa M, Ventrone L, D'Andrea V, Giacomelli L. Poorly Differentiated Thyroid Carcinoma: Single Centre Experience and Review of the Literature. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Farahati J, Mäder U, Gilman E, Görges R, Maric I, Binse I, Hänscheid H, Herrmann K, Buck A, Bockisch A. Changing trends of incidence and prognosis of thyroid carcinoma. Nuklearmedizin. 2019;58:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Walczyk A, Kopczyński J, Gąsior-Perczak D, Pałyga I, Kowalik A, Chrapek M, Hejnold M, Góźdź S, Kowalska A. Poorly differentiated thyroid cancer in the context of the revised 2015 American Thyroid Association Guidelines and the Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System (eighth edition). Clin Endocrinol (Oxf). 2019;91:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Roche AM, Fedewa SA, Shi LL, Chen AY. Treatment and survival vary by race/ethnicity in patients with anaplastic thyroid cancer. Cancer. 2018;124:1780-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, Williams MD, Gunn GB, Hofmann MC, Cote G, Sperling J, Gross ND, Sturgis EM, Goepfert RP, Lai SY, Cabanillas ME, Zafereo M. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol. 2020;6:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 231] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 105. | Yu AC, Han AY, Cronkite DA, Sajed D, St John MA. Anaplastic Transformation of Differentiated Thyroid Carcinoma. Laryngoscope. 2023;133:437-442. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 106. | Marchand-Crety C, Pascard M, Debreuve-Theresette A, Ettalhaoui L, Schvartz C, Zalzali M, Brugel M, Bellefqih S, Servagi-Vernat S. Prognostic Factors and Survival Score for Patients With Anaplastic Thyroid Carcinoma: A Retrospective Study from a Regional Registry. Anticancer Res. 2021;41:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Hussain SA, Subbiah S, Bharathiraja MS. Anaplastic Carcinoma Thyroid - A Review on the Management of this Aggressive Cancer. Gulf J Oncolog. 2018;1:37-41. [PubMed] |
| 108. | Cho S, Kim H, Oh YL, Hahn SY, Kim TH, Shin JH. Comparison of clinicopathological characteristics and survival between symptomatic and asymptomatic anaplastic thyroid carcinoma. Sci Rep. 2023;13:3264. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 109. | Jin S, Liu X, Peng D, Li D, Ye YN. Differences Between Cancer-Specific Survival of Patients With Anaplastic and Primary Squamous Cell Thyroid Carcinoma and Factors Influencing Prognosis: A SEER Database Analysis. Front Endocrinol (Lausanne). 2022;13:830760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Wang M, Wei T, Gong R, Zhu J, Li Z. Risk stratification in patients with anaplastic thyroid carcinoma: role of age. Endocrine. 2022;77:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |