Published online Nov 24, 2023. doi: 10.5306/wjco.v14.i11.544
Peer-review started: July 26, 2023
First decision: September 4, 2023
Revised: September 20, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 24, 2023
Processing time: 119 Days and 3.6 Hours
Calcitriol-induced hypercalcemia has been rarely reported in cases of lung cancer; however, it is frequently reported in cases of lymphoid malignancy and granulomatous disease. We present a rare case of hypercalcemia associated with squamo
A 61-year-old Caucasian female with severe hypercalcemia of 15 mg/dL, which led to a new diagnosis of metastatic lung cancer. Since the parathyroid hormone-related peptide (PTHrP) level was minimally elevated at 2.1 pmol/L, we believe excessive calcitriol production by tumor cells was the underlying mechanism for hypercalcemia. Calcitriol was significantly elevated at 130 pg/mL with a low 25-hydroxyvitamin D level of 25.9 ng/mL and suppressed PTH level of 8 pg/mL. Corticosteroids are generally used to treat calcitriol-induced hypercalcemia, but we successfully treated our patient with bisphosphonate, highlighting the further utility of bisphosphonates in hypercalcemia treatment.
We believe that the underlying cause of hypercalcemia, in this case of metastatic squamous cell lung carcinoma, was elevated calcitriol, which was likely produced by the tumor cells. In addition to PTHrP, calcitriol levels should be included in the workup for hypercalcemia in cases of lung cancer. However, the pathophysiology and prognostic significance of dysregulated calcitriol production in solid tumors remain unclear and warrant further research. Bisphosphonate may be used as a steroid-sparing therapy even in cases of calcitriol-induced hypercalcemia and warrants further investigation.
Core Tip: Our case report illuminates a rare mechanism of hypercalcemia in lung malignancies, characterized by elevated calcitriol. Despite its rarity, it sheds light on the pathophysiology of hypercalcemia in solid malignancies, notably lung cancers. To the best of our knowledge, this is the first documented case in medical literature to present this mechanism. Moreover, the successful management of this condition with bisphosphonates highlights the potential efficacy of this treatment approach for future cases involving similar symptoms. By emphasizing this novel observation, our report contributes to the expanding body of knowledge regarding hypercalcemia in lung cancers and paves the way for the development of novel therapeutic strategies for the treatment of such cases.
- Citation: Prakash A, Khalid F, Alalwan A, Bader H, Du D, Meghal T. Calcitriol induced hypercalcemia - a rare phenomenon in lung cancer: A case report. World J Clin Oncol 2023; 14(11): 544-548
- URL: https://www.wjgnet.com/2218-4333/full/v14/i11/544.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i11.544
Hypercalcemia associated with malignancy (HAM) is a common clinical finding and may even present as an oncologic emergency. It has been found in up to 30% of cases of malignancy[1]. The estimated yearly prevalence of hypercalcemia for all cancers is 1.46% to 2.74%[2]. Calcitriol overproduction is a rare etiology of HAM and accounts for merely 1% of cases of HAM[3]. It has been frequently reported in cases of Hodgkin and non-Hodgkin lymphoma and also in some cases of ovarian dysgerminoma, pancreatic neuroendocrine tumors, seminomas, and renal cell carcinoma[4-6]. In our extensive literature search, we came across just one case report of squamous cell lung cancer by Akai et al[7], where calcitriol overproduction was exclusively responsible for hypercalcemia and treated with tumor resection. We present a rare case of squamous cell lung carcinoma with hypercalcemia and elevated calcitriol levels, which was treated succ
Hypercalcemia on routine blood work investigation.
The patient didn’t complain of any significant symptoms at the time of presentation. However, during detailed history taking, she reported vague complaints of nausea, fatigue, and generalized weakness but denied any other symptoms like constipation, palpitation, confusion, etc.
She has a past medical history of diabetes, hyperlipidemia, and depression.
The patient has a remote history of tobacco smoking more than 15 years ago. She denies any supplementation with vitamin A, vitamin D, or calcium, frequent use of antacids, or excessive consumption of dairy products. Her current medications include atorvastatin and sertraline. Patient denies any significant family history.
Her vital signs and physical exam were within normal limits.
Three months prior to the presentation, her calcium level was noted to be within the normal range of 10.1 (range: 8.6-10.3 mg/dL). In a routine lab work performed one week before the presentation, her corrected serum calcium was noted to be elevated first time at 13 mg/dL. She was asymptomatic at that point so decision was made to monitor with serial labs. Our patient presented for repeat lab draw a week later and now her corrected serum calcium was 14.3 mg/dL, so she was instructed to visit the emergency department for further evaluation. She was admitted 12 h later on the same day and her corrected serum calcium further worsened to 15 mg/dL with an ionized calcium value of 7.5 mg/dL (range: 4.5-5.6). Her albumin was 3.4 mg/dL, creatinine 0.5 mg/dL, estimated glomerular filtration rate > 60 mL/min/1.73 m2, magnesium 1.9 mg/dL (range: 1.8-2.4), phosphorus 2.4 mg/dL (range: 2.5-4.9). 25-hydroxyvitamin D was noted to be low: 25.9 ng/mL (30-100 ng/mL), with elevated calcitriol level: 130 pg/mL (24.8-81.5 pg/mL) and suppressed intact parathyroid hormone (PTH) level: 8 pg/mL (15-65 pg/mL) and minimally elevated PTH-related peptide (PTHrP) 2.1 pmol/L (normal < 2.0 pmol/L). Complete blood count and urinalysis were within normal limits.
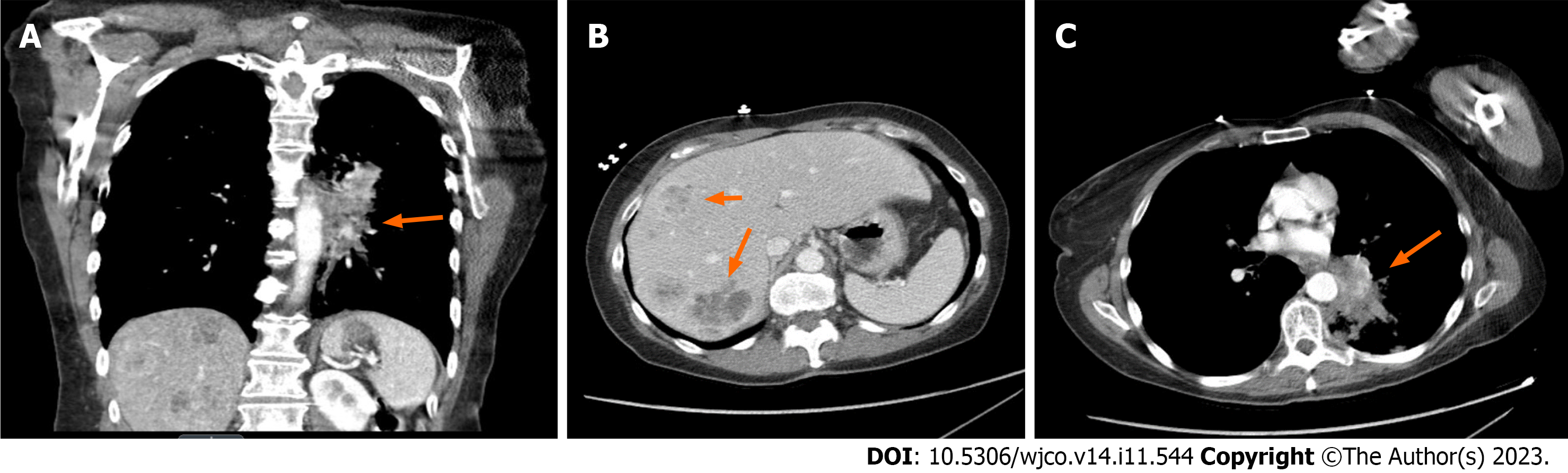
Chest X-ray reveals a left hilar opacity, which was concerning for lung neoplasm. Upon further investigation with contrasted pan-computed tomography, the patient was noted to have a left lower lobe mass with an epicenter in the left lower lobe bronchus with invasion into the mediastinum and multiple hepatic metastases. No metastasis to bones, brain or spleen was noted. A core biopsy was performed on one of the liver metastases. Histopathology findings were consis
Calcitriol induced hypercalcemia.
Our patient was paucisymptomatic but had rapid rise in serum calcium level so we decided to treat her hypercalcemia. The patient was managed with intravenous normal saline, subcutaneous calcitonin administered twice, and a single infusion of zoledronic acid 4 mg during the course of the hospital stay. Her calcium level improved rapidly to 9.8 mg/dL within 48 h and she was discharged from the hospital. Her calcium level 2-wk later was noted to be elevated again at 13 mg/dL and was given another dose of zolendronic acid. Four weeks after discharge, her calcium level was noted to be within the normal range at 9.9 mg/dL.
The patient is scheduled to receive infusion of zolendronic acid once every month to manage hypercalcemia. She is planned to undergo chemotherapy with carboplatin, paclitaxel and pembrolizumab.
Hypercalcemia results from dysregulation between normal bone formation and the degradation cycle. The patho
In the current case, calcitriol level was noted to be significantly elevated with suppressed PTH level and minimal elevation of PTHrP. The question is about what’s causing the calcitriol elevation in this case. Squamous cell cancer of the lung is usually associated with hypercalcemia driven by PTHrP elevation. In granulomatous disease such as sacrcoidosis, there is extrarenal production of calcitriol via autonomous 1-α-hydroxylase activity in tissue macrophages. PTHrP can also upregulate 1-alpha hydroxylase activity and calcitriol production in mice models, but it does not increase calcitriol production in humans[8,9]. In this patient, we tend to believe that hypercalcemia was due to a PTHrP-independent mechanism since PTHrP was minimally elevated. Additionally, extrarenal synthesis of calcitriol is dependent on its substrate 25-hydroxyvitamin D, which was low in this case, thus excluding extrarenal calcitriol production as an underlying mechanism. It’s very possible that it was being ectopically produced by tumor cells in an autonomous fashion[10]. Although staining of the biopsy sample for 1,25-dihydroxy vitamin D and 1-alpha-hydroxylase was not done to confirm calcitriol’s ectopic production, the treatment response further solidifies our hypothesis. Increased calcitriol level in cases of granulomatous disease is believed to be due to the upregulation of 1-alpha hydroxylase activity, and its autocrine regulation is sensitive to corticosteroid therapy. Hence, corticosteroid is indicated in the treatment of hypercalcemia in such cases[11,12]. However, in our case, hypercalcemia responded remarkably to treatment with bispho
The standard treatment approach for HAM is aimed at: (1) Promoting renal calcium excretion through intravenous normal saline administration and even loop diuretics, sometimes; and (2) Reducing bone absorption through the use of bisphosphonates and denosumab in refractory cases. Calcitonin can also be used as an adjunctive therapy with bisphosphonates, but tachyphylaxis develops within 48 h. Corticosteroids are the first-line agents for the treatment of calcitriol-mediated hypercalcemia by inhibiting the transcription of 25-hydroxyvitamin D-1-hydroxylase; however, it was not required in our case. That being said, bisphosphonates may have more of a role in the treatment of HAM. Bisphosphonate may inhibit the adhesion of osteoclast precursors to stromal osteoblasts through the increased expression of intercellular adhesion molecule-1, which is promoted by calcitriol[13,14]. In a case series reported by Rizzoli et al[15], bisphosphonate was more effective than steroids in the treatment of hypercalcemia, probably due to its bone anti-resorptive effect. Bisphosphonates may have additional effects, including induction of apoptosis, inhibition of invasion, and antiangiogenic properties, as seen in some preclinical studies[16]. It would be worthwhile to conduct further research to investigate the cellular effects of calcitriol and bisphosphonate in patients with lung cancer.
We believe that the underlying cause of hypercalcemia, in this case of metastatic squamous cell lung carcinoma, was elevated calcitriol, which was likely produced by the tumor cells. In addition to PTHrP, calcitriol levels should be included in the workup for hypercalcemia in cases of lung cancer. However, the pathophysiology and prognostic significance of dysregulated calcitriol production in solid tumors remain unclear and warrant further research. Bisphosphonate may be used as a steroid-sparing therapy even in cases of calcitriol-induced hypercalcemia and warrants further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Physicians, 04320738.
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Musetti C, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 2. | Jick S, Li L, Gastanaga VM, Liede A. Prevalence of hypercalcemia of malignancy among cancer patients in the UK: analysis of the Clinical Practice Research Datalink database. Cancer Epidemiol. 2015;39:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Goldner W. Cancer-Related Hypercalcemia. J Oncol Pract. 2016;12:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Rodríguez-Gutiérrez R, Zapata-Rivera MA, Quintanilla-Flores DL, Camara-Lemarroy CR, Lavalle-Gonzalez FJ, González-González JG, Villarreal-Pérez JZ. 1,25-dihydroxyvitamin D and PTHrP mediated malignant hypercalcemia in a seminoma. BMC Endocr Disord. 2014;14:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Shivnani SB, Shelton JM, Richardson JA, Maalouf NM. Hypercalcemia of malignancy with simultaneous elevation in serum parathyroid hormone--related peptide and 1,25-dihydroxyvitamin D in a patient with metastatic renal cell carcinoma. Endocr Pract. 2009;15:234-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Hibi M, Hara F, Tomishige H, Nishida Y, Kato T, Okumura N, Hashimoto T, Kato R. 1,25-dihydroxyvitamin D-mediated hypercalcemia in ovarian dysgerminoma. Pediatr Hematol Oncol. 2008;25:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Akai PS, Wong T, Chang-Poon V, Green F, Whitelaw WA, Hanley DA. Resectable bronchogenic carcinoma presenting with hypercalcemia: tumor-associated granulomatous reaction and probable production of 1,25-dihydroxyvitamin D. Clin Invest Med. 1989;12:212-216. [PubMed] |
| 8. | Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocaña A, Bisello A, Hollis BW, Rosen CJ, Wysolmerski JJ, Dann P, Gundberg C, Stewart AF. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res. 2005;20:1792-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Schilling T, Pecherstorfer M, Blind E, Leidig G, Ziegler R, Raue F. Parathyroid hormone-related protein (PTHrP) does not regulate 1,25-dihydroxyvitamin D serum levels in hypercalcemia of malignancy. J Clin Endocrinol Metab. 1993;76:801-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 10. | Chukir T, Liu Y, Hoffman K, Bilezikian JP, Farooki A. Calcitriol Elevation Is Associated with a Higher Risk of Refractory Hypercalcemia of Malignancy in Solid Tumors. J Clin Endocrinol Metab. 2020;105:e1115-e1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Fuss M, Pepersack T, Gillet C, Karmali R, Corvilain J. Calcium and vitamin D metabolism in granulomatous diseases. Clin Rheumatol. 1992;11:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kallas M, Green F, Hewison M, White C, Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. J Clin Endocrinol Metab. 2010;95:3111-3117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Mateo RCI, Ortiz R, Rosen HN. BISPHOSPHONATES FOR THE TREATMENT OF CALCITRIOL-INDUCED HYPERCALCEMIA. AACE Clin Case Rep. 2019;5:e316-e320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Okada Y, Morimoto I, Ura K, Watanabe K, Eto S, Kumegawa M, Raisz L, Pilbeam C, Tanaka Y. Cell-to-Cell adhesion via intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 pathway is involved in 1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast differentiation and bone resorption. Endocr J. 2002;49:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Rizzoli R, Stoermann C, Ammann P, Bonjour JP. Hypercalcemia and hyperosteolysis in vitamin D intoxication: effects of clodronate therapy. Bone. 1994;15:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Clezardin P. Potential anticancer properties of bisphosphonates: insights from preclinical studies. Anticancer Agents Med Chem. 2012;12:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |