Published online Nov 24, 2023. doi: 10.5306/wjco.v14.i11.518
Peer-review started: August 2, 2023
First decision: August 16, 2023
Revised: September 14, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 24, 2023
Processing time: 111 Days and 21.8 Hours
The development of cancer is thought to involve the dynamic crosstalk between the tumor cells and the microenvironment they inhabit. Such crosstalk is thought to involve mechanotransduction, a process whereby the cells sense mechanical cues such as stiffness, and translate these into biochemical signals, which have an impact on the subsequent cellular activities. Bibliometric analysis is a statistical method that involves investigating different aspects (including authors’ names and affiliations, article keywords, journals and citations) of large volumes of literature. Despite an increase in mechanotransduction-related research in recent years, there are currently no bibliometric studies that describe the global status and trends of mechanotransduction-related research in the cancer field.
To investigate the global research status and trends of mechanotransduction in cancer from a bibliometric viewpoint.
Literature on mechanotransduction in cancer published from January 1, 1900 to December 31, 2022 was retrieved from the Web of Science Core Collection. Excel and GraphPad software carried out the statistical analysis of the relevant author, journal, organization, and country information. The co-authorship, keyword co-occurrence, and keyword burst analysis were visualized with VOSviewer and CiteSpace.
Of 597 publications from 745 institutions in 45 countries were published in 268 journals with 35510 citation times. With 270 articles, the United States is a well-established global leader in this field, and the University of California system, the most productive (n = 36) and influential institution (n = 4705 citations), is the most highly active in collaborating with other organizations. Cancers was the most frequent publisher with the highest H-index. The most productive researcher was Valerie M. Weaver, with 10 publications. The combined analysis of concurrent and burst keywords revealed that the future research hotspots of mechanotransduction in cancer were related to the plasma membrane, autophagy, piezo1/2, heterogeneity, cancer diagnosis, and post-transcriptional modifications.
Mechanotransduction-related cancer research remains a hot topic. The United States is in the leading position of global research on mechano-oncology after almost 30 years of investigations. Research group cooperations exist but remain largely domestic, lacking cross-national communications. The next big topic in this field is to explore how the plasma membrane and its localized mechanosensor can transduce mechanical force through post-transcriptional modifications and thereby participate in cellular activity regulations and cancer development.
Core Tip: Through bibliometric analysis, we found that mechanotransduction-related cancer research remains a hot topic, with approximately 100 papers and 5000 citations generated per year in the past three years. Additionally, the United States is a well-established global leader of this field, and the University of California system is the most influential organization in this field. We predict that investigating how the plasma membrane and its localized mechanosensors transduce mechanical forces via post-transcriptional modifications and thereby participate in the regulation of cellular activity will be the next big research topic in the cancer field.
- Citation: Zhang YZ, Li MZ, Wang GX, Wang DW. Bibliometric analysis of the global research status and trends of mechanotransduction in cancer. World J Clin Oncol 2023; 14(11): 518-534
- URL: https://www.wjgnet.com/2218-4333/full/v14/i11/518.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i11.518
Cancer is a devastating disease characterized by the transduction of abnormal cell signals, which leads to oncogenic cellular behaviors such as uncontrolled proliferation and resistance to death[1]. According to the latest statistics, cancer is the second most common cause of death worldwide after cardiovascular disease[2]. Therefore, it is crucial to determine the pathogenesis of this disease so that effective therapeutic approaches can be identified. Although cancer is primarily regarded as being a genetic disease caused by the stepwise accumulation of gene mutations, an increasing number of studies suggest that environmental factors might also have a significant influence on the development of this disease[3]. The tumor microenvironment (TME) consists of cancer-associated fibroblasts (CAFs), endothelial cells, immune cells, and the extracellular matrix (ECM), and together these provide the biochemical and mechanical signals required to stimulate the occurrence, survival, and development of cancer[4]. While the importance of biochemical stimuli (such as small molecules, growth factors, and cytokines) in cancer progression is well-established, the role of mechanical force is still relatively underexplored although recent investigations suggest that it is on par with the chemical factors[5-7].
In cancerous tissues, mechanical forces such as shear stress, tension, and compression, are suggested to be generated during cell-TME and cell-cell contact events[8]. These forces are then translated into biochemical signaling cascades with the help of various mechanoreceptors, including G protein-coupled receptors, ion channels, and cell junction proteins[9,10]. This process of transducing specific mechanosignals into distinct intracellular biochemical signals is called mechanotransduction[5]. Abnormal mechanical signals (generated by the environment or cells), can alter the expression of genes and transduction of signaling pathways. This leads to the dysregulated cell behaviors that are associated with many diseases, including cancer[11]. For example, when compared with normal tissues, cancerous tissues have a more rigid ECM, which contributes to the aberrant mechanotransduction observed[12]. This leads to an increase in the expression of many oncogenic transcription factors (such as YAP/TAZ, Twist1, and β-catenin), which enhances cell proliferation, the epithelial-mesenchymal transition (EMT), and/or cell migration in cancer cells[13]. Interestingly, YAP/TAZ has also been reported to contribute to tissue stiffness by increasing the expression of the crucial ECM modifiers, CTGF and CYR61[14-17]. Therefore, a positive mechanotransductive feedback loop between cancer cells and the ECM is regarded as one of the major culprits for cancer malignancy[17]. In addition, when cancer cells are surrounded by a rigid, crosslinked ECM, then this impedes immune cell invasion and drug distribution, which leads to immune surveillance escape and drug resistance[6,18].
The concept of mechanotransduction also helps to explain the mechanisms of cancer initiation, invasion, and metastasis from a new perspective. Considering the fundamental role of mechanotransduction in cancer development, researchers are now developing new cancer therapeutics based on the specific mechanical properties of and mechano-signal transducers generated by tumors. For example, Simtuzumab and Simvastatin, which reduce ECM stiffness and inhibit YAP/TAZ hyperactivation, respectively, have been evaluated for their anti-cancer efficacy in preclinical studies[18-20]. Mechanotransduction is therefore already showing great promise for clinical applications, and so further comprehensive investigations in this field will likely reveal more therapeutic strategies for cancer treatment. Thus, gaining a better understanding of the current research status of mechanotransduction in cancer is likely to shed light on important research questions and directions for future study.
Bibliometric analysis is a useful quantitative method to comprehensively analyze publications from many different aspects (including the authors and their affiliations, journal title and article keywords), to reveal collaborative networks and emerging trends in specific research areas[21-23]. Although increasing numbers of research papers now focus on the role of mechanotransduction in cancer, to date, no bibliometric analysis has been conducted to quantify the situation. To this end, here we present the updated bibliometric analysis of research conducted on mechanotransduction in cancer and reveal the current research being conducted in this field. In this way, we aim to provide a better understanding of the present status of this study area and predict key topics of future investigation in this promising research field.
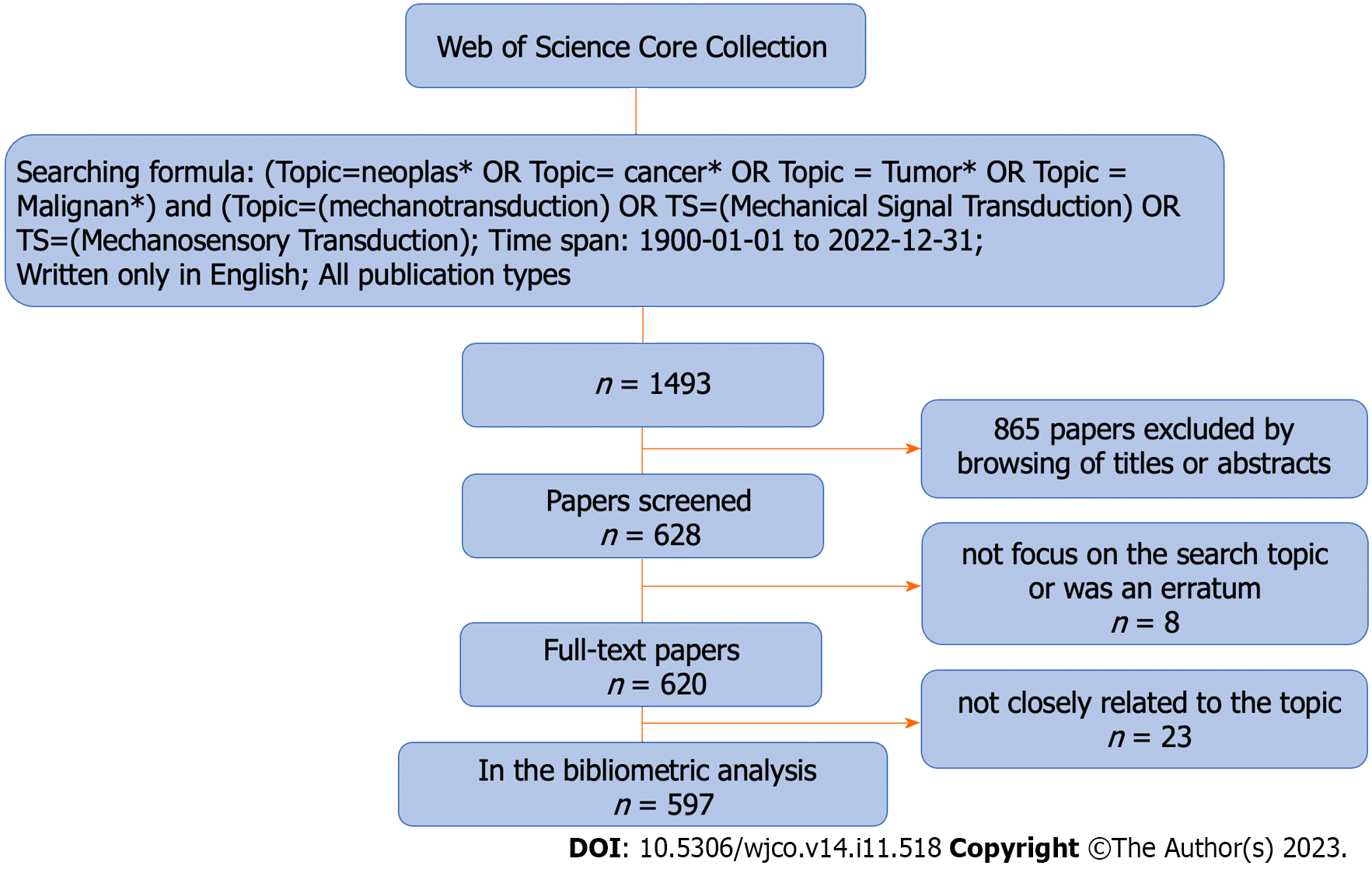
We retrieved literature on ‘mechanotransduction in cancer’ research from the Web of Science Core Collection (WoSCC). The publication period was set from January 1, 1900 to December 31, 2022, and only English publications were included. The search strategy is illustrated in Figure 1. All recorded data, including the authors’ names, institutions, and countries, as well as keywords, were downloaded from the WoSCC and normalized to a standard format. To avoid ambiguity, we cross-checked duplicate authors among the documents. Meeting abstracts, editorial materials, corrections, and retractions were excluded from our research.
The keywords were extracted from the keyword section of articles. To avoid potential deviations, similar or same keywords with different expressions were manually standardized to correct and/or group similarities as previously suggested[23-25], before VOSviewer or CiteSpace analysis. Burst keywords were assessed using CiteSpace (V6.2R4 SE) with the following parameters: time slicing (from January 1994 to December 2022), years per slice (1), node type (keyword), the minimum burst duration (1 year), γ (0.39) and others (default). A keyword co-occurrence analysis was conducted with VOSviewer (version 1.6.18) with the following parameters: Type of analysis (co-occurrence), unit of analysis (keywords), counting method (full counting), minimum number of occurrences of a keyword (3).
The number of publications and citations in the indicated time was presented using GraphPad Prism 8. Visualization of bibliometric information, including co-authorship analysis, keyword co-occurrence, and burst analysis, was conducted with VOSviewer (version 1.6.18) and CiteSpace (V6.2R4 SE).
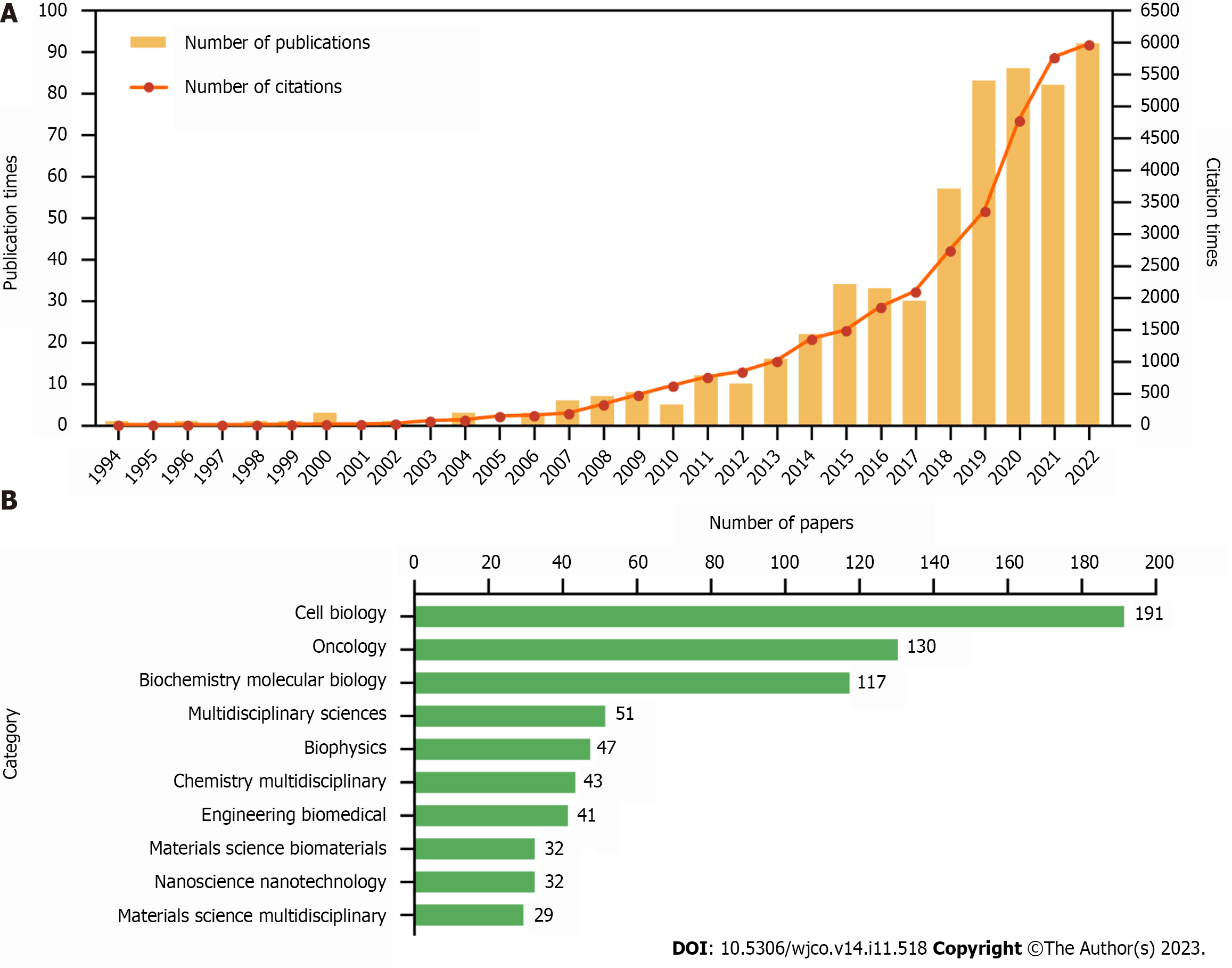
A total of 597 publications comprising 388 research and 209 review articles, were extracted for deep analysis. Although the concept of mechanotransduction was established progressively from the 1950s to the 1980s[26-28], the first paper describing mechanotransduction in cancer was only published in 1994[29]. Since then, this topic has gradually gained more attention from researchers in the cancer research field. Based on the number of publications and citations analysed in our bibliometric study on mechanotransduction-related cancer research, we prepared a growth curve comprising three clear stages. The first stage described the period from 1994 to 2010, during which time < 10 papers were published each year. In the second stage, which ran from 2012 to 2017, a slow growth rate was observed, and in the third stage (2018-2022), the growth rate started to accelerate, with almost 100 articles being published in the final year (Figure 2A).
In the 597 articles that were published, there was an average of 59.48 citations per paper. The top 10 most cited papers included six reviews and four research articles; these are listed in Supplementary Table 1 and they are ranked by the number of times they were cited. The most highly cited review article (n = 2882 citations) was published by Chambers et al[30], and the most highly cited research article (n = 1021 citations) was contributed by Aragona et al[31].
During our exploration of mechanotransduction in cancer, we sub-divided the publications into 56 research categories to indicate the multidisciplinary crossovers that occurred. After the top 10 categories were generated (Figure 2B), we found that cell biology (n = 191), oncology (n = 130), and biochemistry molecular biology (n = 117), accounted for 73.37% of all the publications. This indicates that molecular cytology is a key contributor to research on the role of mechanical force in cancer development.
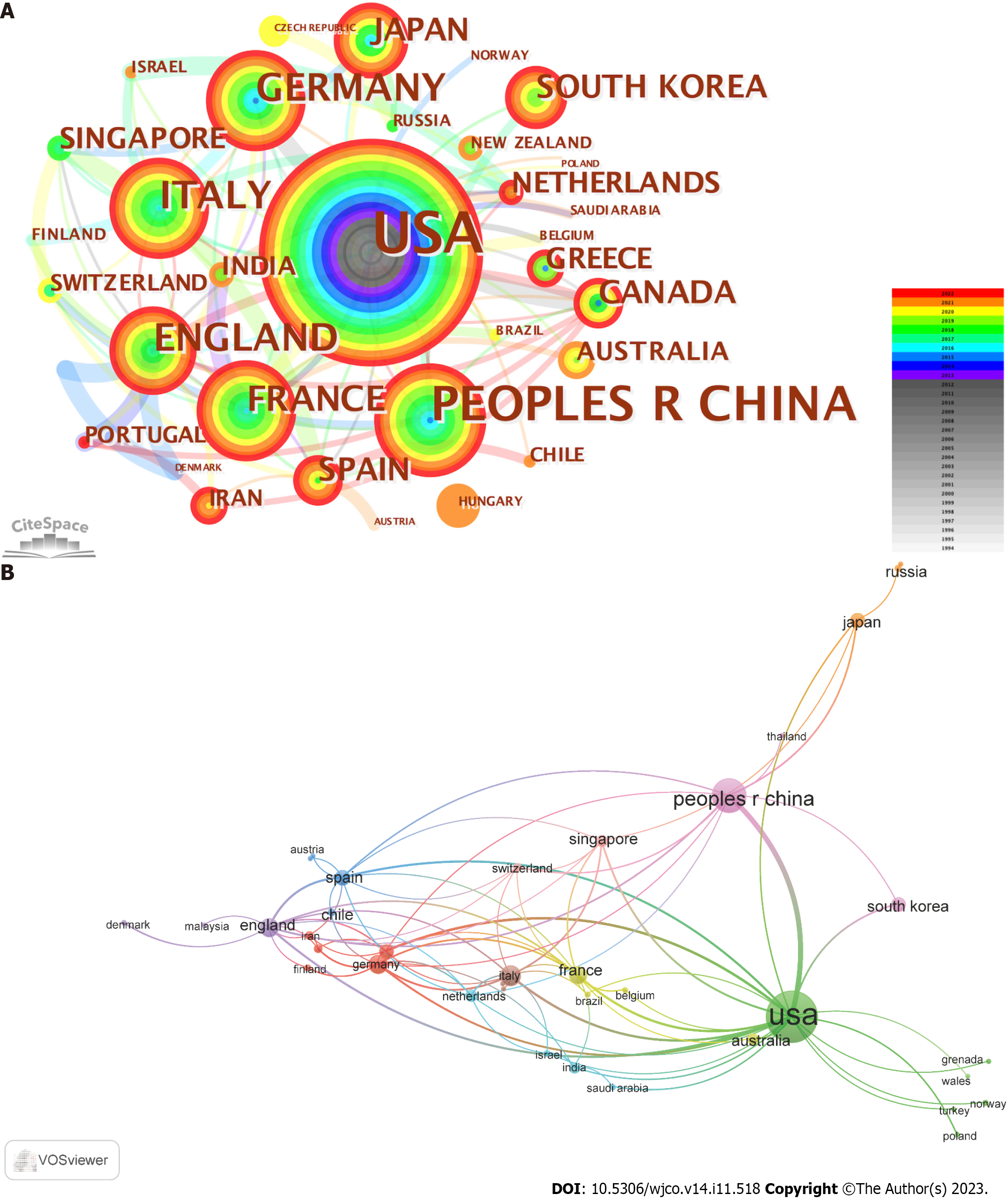
Although a total of 45 countries was found to contribute publications on the topic of mechanotransduction in cancer, the top 10 most productive countries generated 87.6% of all the papers. Of these, the United States was the most productive as it was responsible for 270 (i.e., 45.23%) of the publications. China (n = 119, 19.93%) and Italy (n = 41, 6.87%) held the second and third positions, respectively, but both lagged behind the United States (Table 1). However, when we ranked the countries in terms of the average citation times, Canada occupied the first place with an average of 197.33 citations per paper. This was followed by Italy and Spain with values of 109.17 and 93.09, respectively. In addition, according to this ranking approach, the United States (73.74 times) held a middle position, whereas China (20.34 times) was at the bottom of the ranking list. To evaluate the cooperation between different countries, a co-authorship network was established with a criterion of at least three publications in each country. As shown in Figure 3, the United States (with the most extended research history in this field), collaborated most with other countries, followed by the United Kingdom. In addition, it was interesting to find that although 37.78% (i.e., 17/45) of the countries only published one or two papers, most of this work was conducted in the recent five years. This indicates that more countries are stepping into this research field.
| Rank | Country | Publications | % | Total citations | Average citations | Connections | H-index |
| 1 | United States | 270 | 45.23 | 19911 | 73.74 | 22 | 69 |
| 2 | China | 119 | 19.93 | 2421 | 20.34 | 10 | 25 |
| 3 | Italy | 41 | 6.87 | 4476 | 109.17 | 12 | 22 |
| 4 | Germany | 37 | 6.20 | 2072 | 56.00 | 10 | 18 |
| 5 | United Kingdom | 34 | 7.54 | 2098 | 61.71 | 15 | 20 |
| 6 | France | 31 | 5.19 | 2466 | 79.55 | 12 | 19 |
| 7 | Spain | 22 | 3.69 | 2048 | 93.09 | 11 | 14 |
| 8 | Canada | 21 | 3.52 | 4144 | 197.33 | 8 | 13 |
| 9 | Japan | 20 | 3.35 | 761 | 38.05 | 4 | 14 |
| 10 | South Korea | 20 | 3.35 | 355 | 17.75 | 2 | 9 |
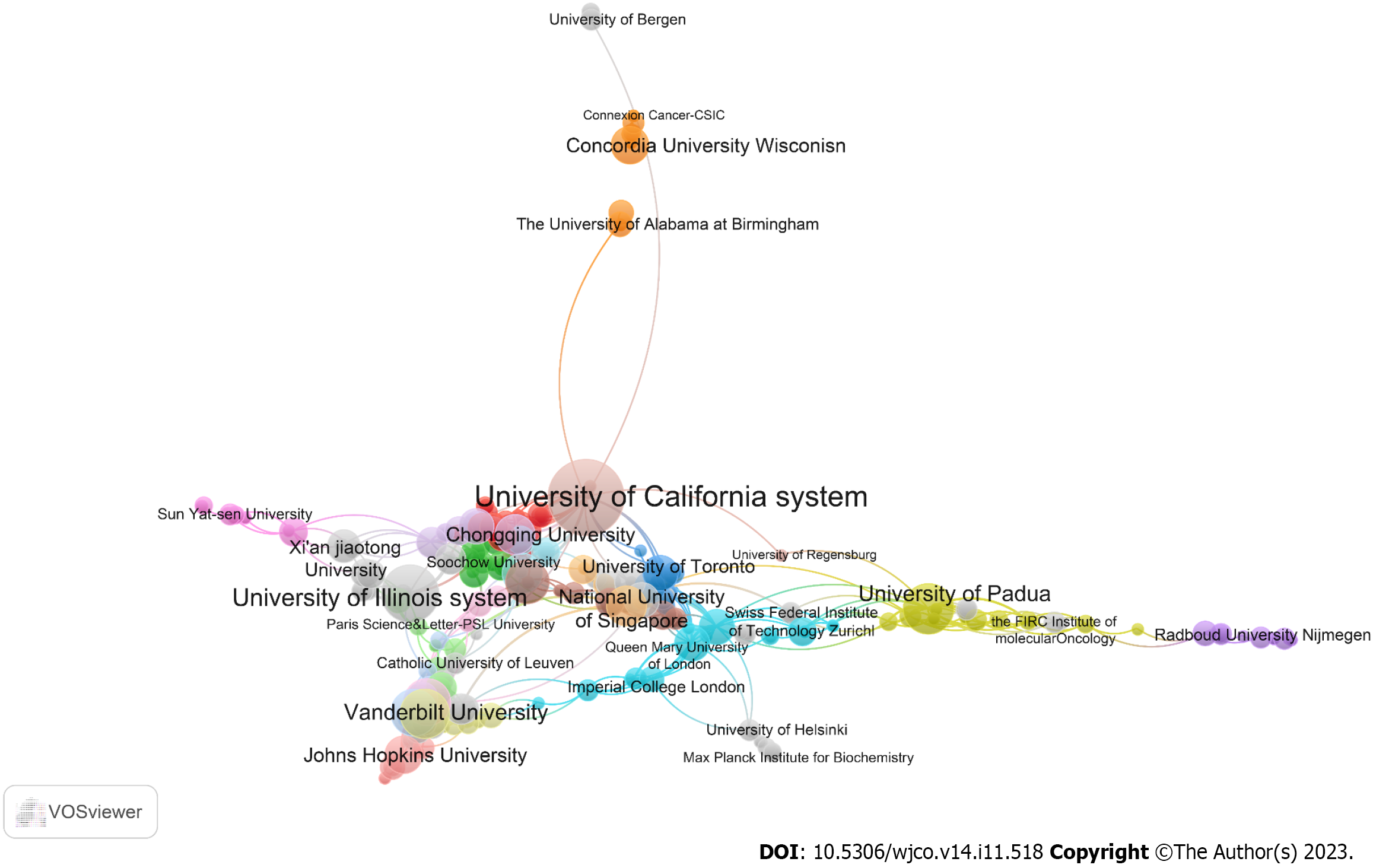
Our data analysis also showed that 745 organizations were involved in research related to mechanotransduction in cancer. After ranking these according to the number of publications, we found that the top 10 organizations accounted for 35.85% (214/597) of all papers. The University of California system ranked first with 36 articles, and this was followed sequentially by UDICE-French Research Universities (n = 27), Centre National de la Recherche Scientifique (n = 25), the University of Illinois system (n = 21), and Institut National de la Sante et de la Recherche Medicale (Inserm). It was striking to find that three institutions from France occupied three of the top five positions with a total of 31 publications (Table 2). Another analysis of the global inter-institutional network showed that the University of California system collaborated most with other organizations, followed sequentially by the University of Padua, Harvard University, and University College London (Figure 4).
| Rank | Organization | Publications | Citations | Average citation | Country | % | H-index |
| 1 | University of California System | 36 | 4705 | 130.69 | United States | 6.03 | 21 |
| 2 | UDICE-French Research Universities | 27 | 1875 | 69.44 | France | 4.52 | 17 |
| 3 | Centre National de la Recherche Scientifique | 25 | 1843 | 73.72 | France | 4.19 | 16 |
| 4 | University of Illinois System | 21 | 1013 | 48.24 | United States | 3.52 | 14 |
| 5 | Institut National de la Sante et de la Recherche Medicale | 20 | 1845 | 92.25 | France | 3.35 | 13 |
| 6 | University of Texas System | 20 | 1619 | 80.95 | United States | 3.35 | 15 |
| 7 | University of London | 18 | 1388 | 77.11 | United Kingdom | 3.02 | 13 |
| 8 | University of Padua | 16 | 3399 | 212.44 | Italy | 2.68 | 12 |
| 9 | Harvard University | 16 | 2463 | 153.94 | United States | 2.68 | 13 |
| 10 | Vanderbilt University | 15 | 691 | 46.07 | United States | 2.51 | 10 |
Articles related to the field of mechanotransductive cancer research were distributed among 268 different academic journals. The top 10 journals to publish papers in this field accounted for 22.61% (135/597) of all the articles (Supplementary Table 2), but 162 journals (60.45%) only published one paper in this research area. Cancers ranked first with 29 (4.86%) publications, followed by the International Journal of Molecular Sciences (n = 20, 3.35%) and the Journal of Cell Science (n = 17, 2.85%).
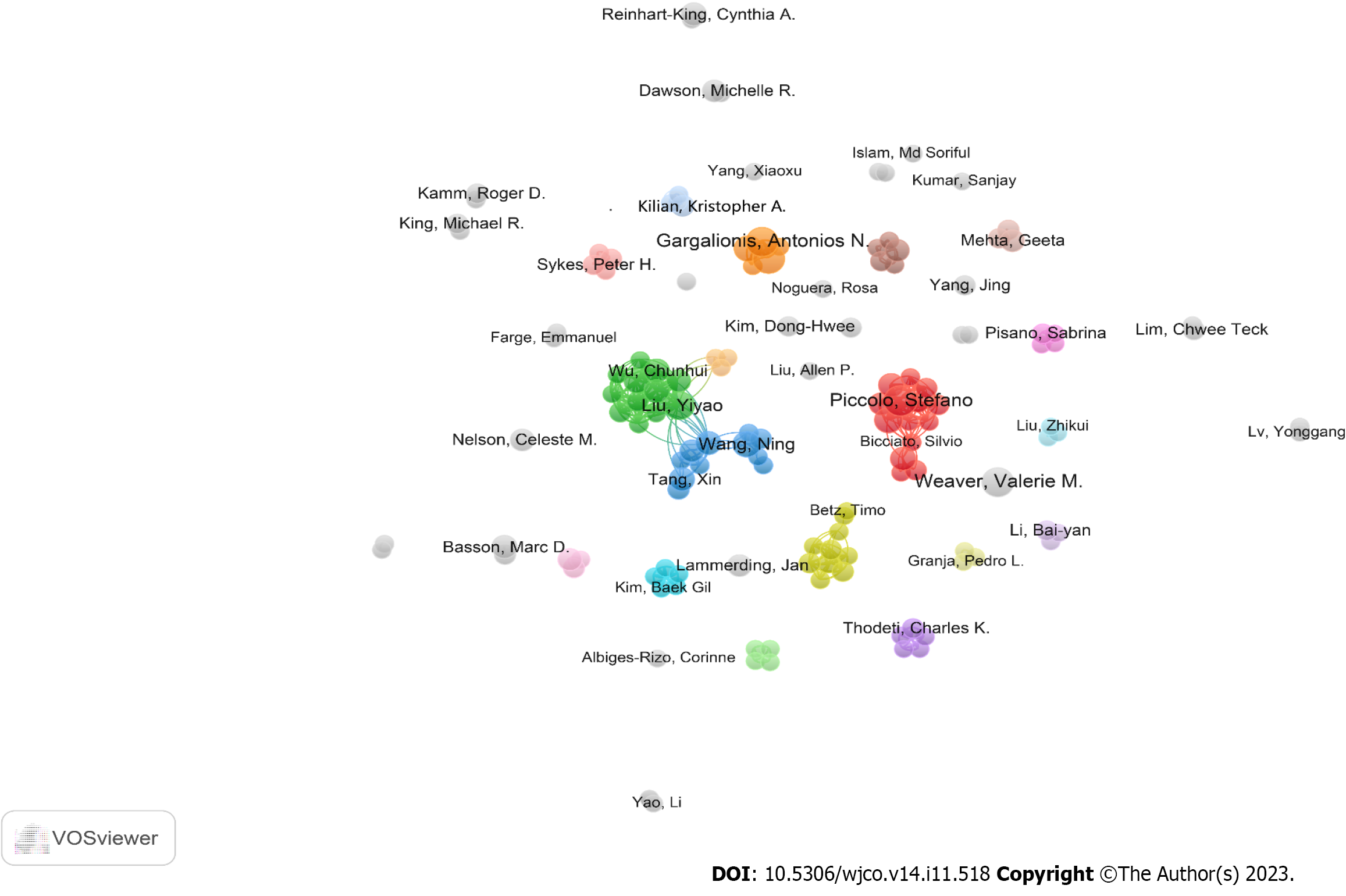
We also found that a total of 3001 authors contributed to these 597 publications. After analyzing the top 10 authors in terms of the number of papers (Supplementary Table 3), we found that Valerie M. Weaver from the University of California system was the most productive researcher with n = 10 publications. She was followed by Stefano Piccolo from the University of Padua (n = 9), Antonios Gargalionis from the National & Kapodistrian University of Athens (n = 9), and Marc D. Basson from John D. Dingell Veterans Affairs Medical Center (n = 8). Notably, according to the total (T) and average (A) citation times of the papers generated from each author, Stefano Piccolo (T = 3167; A = 351.89), Tito Panciera (T = 2076, A = 296.57), Valerie M. Weaver (T = 2495, A = 249.5), Michelangelo Cordenonsi (T = 1983, A = 330.5), and Patricia J. Keely (T = 1478, A = 246.33) ranked the top 5, indicating that they were in positions of authority. To investigate if there were any collaborations between the various authors in this field, an authorship network analysis was performed by identifying those with at least three publications. As shown in Figure 5, the authors were distributed into 43 clusters, with 4 clusters containing at least ten authors. This indicates that there were partial connections between the different groups in this research area.
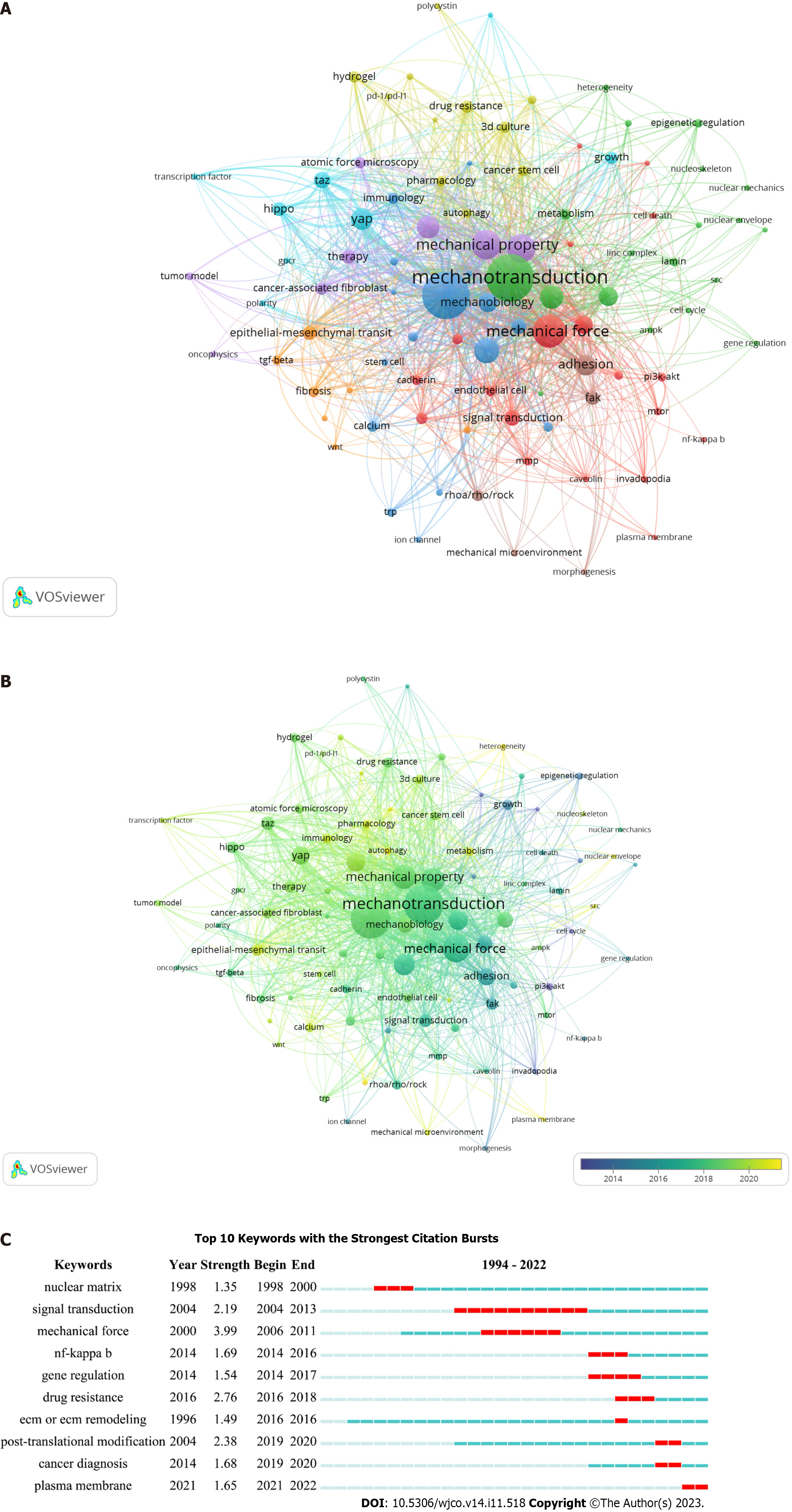
To explore the current research themes and discover hot topics in mechanotransduction-related cancer research, we acquired 666 keywords by collating those with the same meaning or category from all 597 papers. Among them, the top 4 co-occurrent keywords that were consistent with our research topic, were: Mechanotransduction, cancer, mechanical force, and mechanical property. However, when these keywords were restricted to at least 3 co-occurrences, only 93 items could satisfy this criterion. We next established a network based on these 93 keywords and found that they could be further subdivided into eight clusters (Figure 6A and Supplementary Table 4). We found that 19 keywords were included in cluster 1 (red) as follows: Angiogenesis, cadherin, caveolin, cell death, endothelial cell, epithelial cell, growth factor/receptor, hypoxia, integrin, invadopodia, mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK), mechanical force, matrix metalloproteinases (MMPs), mechanistic target of rapamycin (mTOR), NF-κB, phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), plasma membrane, signal transduction, and traction force microscopy. Cluster 2 (green) also contained 19 items. These were: AMP-activated protein kinase, cell cycle, cell morphology, cytoskeleton or cytoskeleton remodeling, epigenetic regulation, gene regulation, heterogeneity, lamin, linc complex, mechanotransduction, metabolism, microfluidics, migration, nuclear envelope, nuclear mechanics, nucleoskeleton, nucleus, pancreatic stellate cell, and SRC. Cluster 3 (blue) contained the following 16 items: Calcium, cancer, DNA damage, dormancy, immunology, invasion, ion channel, mechanobiology, metastasis, motility, oxidative stress, piezo1/2, post-translational modification, SRF, stem cell, and TRP. Cluster 4 (yellow) contained 11 items: 3D culture, autophagy, cancer diagnosis, cancer stem cell (CSC), drug resistance, hydrogel, immunotherapy, organoid, programmed cell death protein 1/programmed cell death ligand 1, pharmacology, and polycystin. Cluster 5 (purple) contained 8 items: Atomic force microscopy, CAF, ECM or ECM remodeling, mechanical property, oncophysics, therapy, TME, and tumor model. Cluster 6 (cyan) contained 8 items: Contact inhibition, G protein-coupled receptor (GPCR), growth, Hippo, polarity, TAZ, transcription factor, and YAP. Cluster 7 (orange) contained 7 items: Catenin, EMT, fibrosis, noncoding RNA, transforming growth factor-beta (TGF-β), transcription, and Wnt, and cluster 8 (brown) had 5 items: Adhesion, focal adhesion kinase (FAK), mechanical microenvironment, morphogenesis, and RhoA/Rho/ROCK. These grouped keywords were further marked according to the average publication year to reflect their yearly development. Figure 6B shows that the keywords in clusters 1 and 8 were mainly labeled in purple, suggesting that they appeared relatively early on in this field. In contrast, there were more keywords labeled in yellow in clusters 4 and 5, indicating that the topics in these two clusters gained more attention in recent years. Of note, some keywords exhibited a relatively late mean publication year and low mean frequency of occurrence, and so we suggest that these might be the next big topics to be investigated in this area. These include “plasma membrane [average appearing year (AAY) = 2021.00]”, “autophagy (AAY = 2021.00)”, “piezo1/2 (AAY = 2021.17)”, and “heterogeneity (AAY = 2021.25)”.
In addition to our analysis of co-occurring keywords, a burst keyword analysis (which identifies keywords that were frequently used over a certain period), was carried out with the CiteSpace software. We found that “mechanical force” was the most potent keyword; this appeared in 2006 with a burst strength of 3.99. In addition, the longest burst duration occurred for “signal transduction” (with a burst strength of 2.19); this started in 2004 and lasted for 9 years. Apart from these two keywords, “cancer diagnosis”, “post-transcriptional modification” and “plasma membrane” were shown to have the highest more recent burst time (Figure 6C), indicating possible new research topics in mechanotransduction-associated cancer studies.
The concept of mechanotransduction developed gradually between the 1950s and 1980s as researchers studied how stretching forces influenced membrane depolarization in excitable cells such as nerves[26-28]. The fact that mechanotransduction might play a role in cancer was first suggested in 1994, and since then it has helped to explain many puzzles in the cancer research field[29]. However, compared to biochemical signal transduction, the role of mechanotransduction in cancer development was largely overlooked from 1994 to 2006. The field then gradually received more attention, and more than 40 papers were published between 2006 and 2016, probably due to the boom in research on the Hippo pathway[32]. Notably, there was then a slight reduction in the number of publications in 2017, followed by a substantial increase in 2018. Since then, this field has become far more popular among cancer researchers, such that in the last three years, > 80 papers were published per year. As the number of publications increased, so did the number of times that the publications were cited. Indeed, the average citation time reached 59.48 citations per paper, suggesting the high quality of research conducted in this field. Furthermore, Drs David Julius and Ardem Patapoutian were awarded the 2021 Nobel Prize in Physiology or medicine for their work on mechanosensitive ion channels[33]. Therefore, this might encourage more groups to conduct mechanotransduction-related cancer research.
By ranking the top 10 productive countries in this area, we found that all except China are advanced countries. The United States has established the lead position of global research on mechano-oncology after nearly 30 years of investigations. However, the number of publications from China indicates that they are in a catch-up position, although the average citation time for their papers is still relatively low. This might be due to the relatively low quality of the publications so far, even though the quantity is high. In addition, the low number of publications in the past from China and the more recent fast growth rate might inherently overly inflate the contribution of highly cited papers to the average citation time. To address this phenomenon, the Chinese government should provide more financial and political support for this research field and encourage original research. For Chinese scholars, discovering new research frontiers as early as possible and carrying out in-depth research is indispensable for improving their international influence and academic standing. It is also important to note that although Canada published 21 articles and is ranked only eighth according to the number of publications, it has the highest average citation rate. One reason for this might be that a review paper published by a Canadian oncology group (Chambers et al[30]), systematically describes the role of mechanical factors on the various physiological stages of cancer metastasis, and this attracts the highest citation frequency in this field.
We also found that cooperations existed between each country, with China and the United States having the most exchanges and collaborations. At the institutional level, we found that cooperations existed in developed countries such as the United States, France, and the United Kingdom. However, in other countries mutual partnerships tended to be insufficient, especially in undeveloped countries, including China. In addition, although several connections between different research groups were found on our authorship network map, these group cooperations largely remained domestic, lacking cross-national communications. Furthermore, three of the top productive and influential scientists in this field (i.e., Valerie M. Weaver, Stefano Piccolo, and Antonios Gargalionis), have relatively few collaborations with other researchers. The study of mechanotransduction in cancer not only requires an in-depth exploration of molecular mechanisms but also necessitates a large number of clinical samples or populations for clinical validation or translation. At this point, inter-country or inter-institutional collaboration should be advocated, either by sharing clinical databases or by dividing the project into concrete tasks based on the respective expertise. With this approach, significant breakthroughs in this field might be achieved at the earliest time. In addition to collaboration between research groups, interdisciplinary collaborations are also essential for a research field to flourish. Mechanotransduction-related cancer research involves various different disciplines, including biology, physics, and medicine, and so interdisciplinary exchanges are beneficial for the diversity of research and to create new perspectives and questions. For example, in vivo mechanosensing is based on force-dependent protein deformation and reorganisation[34]. However, due to a lack of molecular resolution in cellular imaging techniques, the intracellular mechanisms are unknown. Recently, with the development of super-resolution microscopy and molecular force sensors, it is now possible to gain molecular insights into mechanosensing in living cells[35]. Moreover, the development of novel imaging techniques has helped to advance our knowledge of the molecular mechanisms involved in mechanotransduction[36].
The 597 papers we found were published in almost 300 journals. This suggests that this field is widely recognized by publishers. Notably, some highly prestigious and influential journals, such as Nature, Cell, and Cancer Cell, have accepted relevant articles in recent years, which indicates that this research direction is significant and holds great potential for future investigations. We also found that there was a positive correlation between the average number of times that papers published in the top 10 most productive journals were cited, and the impact factor of the journal. This reflects the vibrancy and attractiveness of this research field. However, except for the Proceedings of the National Academy of Science of the United States of America and Cancer Research, we found that most of these journals such as Cancers and International Journal of Molecular Sciences are less highly qualified; therefore, more in-depth explorations are required in the future.
Eight clusters were enriched through a keywords co-occurrence analysis. Cluster 1, with 19 keywords, appeared to elucidate the mechanisms of tumor angiogenesis from the viewpoint of mechanotransduction. Indeed, by providing oxygen and nutrition, tumor angiogenesis, which is initiated by vascular endothelial cell activation, is one of the most fundamental factors for tumor growth and metastasis[37,38]. Hypoxia and growth factors plus their receptors (including vascular endothelial-derived growth factor/vascular endothelial-derived growth factor receptor and fibroblast growth factor/fibroblast growth factor receptor) are known to induce endothelial cell proliferation via various signal transduction pathways such as via hypoxia-inducible factor-1, PI3K/Akt/mTOR, MAPK/ERK, integrin, and NF-κB signaling[39,40]. In addition, MMPs facilitate endothelial cell invasion by degrading the surrounding ECM components[41,42]. As well as these biochemical pathways, the shear force of the blood flow and stiffness of the tumor tissues are also considered to be essential parameters that influence angiogenesis by regulating endothelial (tip and stalk) cell migration and vessel stabilization[43,44]. For example, it has been demonstrated that caveolin-1 and caveolae act as mechanosensors to respond to altered shear forces for endothelial cell stimulation[45,46]. The mechanical forces exerted on cells can be measured using traction force microscopy, and so this technique might help researchers discover more plasma membrane-localized mechanosensors during angiogenesis[47,48].
Clusters 2 and 3 emphasize the molecular mechanisms of mechanotransduction in cancer metastasis from the perspective of cytoskeletal and nucleoskeletal remodeling, and ion channels, respectively. During metastasis, cancer cells detach from the site of the primary tumor. They then migrate and invade the surrounding microenvironments, and after intravasation into vessels and escaping immune surveillance, they seed and colonize distant sites[49]. As essential initial steps during metastasis, the migration and invasion processes subject cancer cells to different mechanical forces. These are generated due to contact with the TME, and are characterized by increased solid stress, fluid pressure, and tissue stiffness when compared with their counterparts in normal tissue[17]. Although the rigid TME imposes compressive stress on migrating cancer cells, which can cause DNA damage, these mechanical forces can in turn activate the DNA repair system, which limits genotoxicity and maintains regular cellular activity[50]. Various ion channels, such as TRP, polycystin, and piezo1/2, are involved in this process as mechanosensors. For example, in the presence of membrane tension, TRP or piezo1/2 undergo conformational changes, which leads to their activation and an influx of extracellular Ca2+[51-53]. Such alterations in the Ca2+ influx often result in cytoskeleton rearrangements, which directly affect downstream signaling by changing the affinity of cytoskeletal protein binding and regulatory proteins[54]. Then, with the assistance of the linker of the nucleoskeleton and cytoskeleton complex, the mechanical forces are transferred to the nucleoskeleton, which distorts the nuclear envelope, impacts the epigenetic state and regulates gene expression[55].
Clusters 4 and 5 highlight the contributing factors that determine the mechanical properties during cancer progression and so these might be useful for the development of relevant pharmaceutical interventions and therapeutical applications. CSCs are the main culprits for the heterogeneity of cancer as they are responsible for cancer initiation, invasive front formation, and drug resistance. It is suggested that the mechanical forces induced by the dense ECM might activate the autophagy and Hippo pathways to promote CSC proliferation and stemness[56,57]. Thus, an alternative explanation for the generation of heterogenous CSC populations (except for their intrinsic differences) might be that the heterogenous TME exerts differential mechanical forces on these cells[58]. CAFs are a key component of the TME in most solid tumor tissues, and the secretomes that originate from these cells contribute to the formation of the ECM[59]. During cancer development, CAFs and cancer cells secrete proteolytic enzymes, cytokines, growth factors, and/or other ECM components, which result in high ECM deposition and increased stiffness[60]. The upregulation of programmed cell death ligand 1 under these stiffer ECM conditions enables cancer cells to evade the immune system; a characteristic that is positively correlated with increased malignancy[61]. As expected, these unique cancer ECM characteristics have already been applied to cancer diagnosis[62]. The three-way biochemical and mechanical crosstalk between CAFs, cancer cells, and the ECM facilitate the remodeling of the latter. The resulting altered mechanical properties of the ECM are favorable for cancer survival, proliferation, drug resistance, and metastasis[63]. Therefore, mechanistic investigations of mechanotransduction during oncogenesis are crucial for the development of relevant pharmaceutical interventions and therapeutical applications[64]. Due to the development of 3D cell culture systems and atomic force microscopy, the mechanical properties can now be mimicked and evaluated to reflect the TME and physical cell-matrix interactions
Cluster 6 shows how cancer cells can override contact inhibition from the viewpoint of mechanotransduction. In cell culture conditions, when the density of normal cells reaches a high enough level, the close physical contact between the cells leads to cell cycle arrest and the suppression of proliferation[67]. Several GPCRs can sense and transduce the higher pressure resulting from the increased cell contacts, to downstream signals. This leads to the cytoplasmic translocation of YAP and TAZ, which nullifies their transcriptional activation[68]. In addition to an inhibition of proliferation in cell dense regions, cell migration is also affected when two normal cells collide. Indeed, cell repolarisation is necessary to separate cells after a collision, and RACK1-dependent cytoskeletal reorganization at the migratory front is also crucial for this process[69,70]. However, these events do not occur during carcinogenesis, and so cancer outgrowth and metastasis is the result[71]. The hyperactivation of YAP and TAZ (caused by a loss of E-cadherin or spectrin), helps to explain the reduced contact inhibition that occurs in cancer from the proliferation perspective[72,73]. However, the reasons for the loss of contact inhibition from the cell migration viewpoint, remain elusive and require further investigation.
Cluster 7 describes the role of Wnt signaling in the mechanical force-driven EMT. The EMT is a process by which epithelial cells are transformed into mesenchymal cells, which then migrate and secrete more ECM components, and so it is crucial for cancer metastasis and drug resistance[74,75]. The activation of the WNT/β-catenin signaling pathway contributes to the transcription of EMT regulators such as Snail and Slug, both of which inhibit the expression of E-cadherin, and therefore reduce intercellular adhesion and increase cell motility[76]. In addition, TGF-β and some long noncoding RNAs respond to the increase in ECM stiffness, and in this way, they regulate WNT/β-catenin activity and participate in the EMT[18,77].
Finally, cluster 8 demonstrates the impact of the mechanical microenvironment on cell adhesion. The three main characteristics of tumor mechanical microenvironments are an increase in matrix stiffness, solid stress, and interstitial fluid pressure, and these are proposed to activate FAK, which drives focal adhesion formation and primes the RhoA/ROCK signaling cascade[78,79]. This signaling pathway is involved in regulating the organization of the actin cytoskeleton and, therefore, it enables cells to alter their shape and migrate from their primary sites[80].
After performing co-occurring and burst keyword analyses, we found that the keywords “plasma membrane”, “autophagy”, “piezo1/2”, “heterogeneity”, “cancer diagnosis”, and “post-transcriptional modification” are likely to be the next topics of interest in this field. Interestingly, both keyword analysis methods indicated that the plasma membrane, a mechanosensing structure that transduces the mechanical stimulus to downstream biochemical signal transduction pathways, is a popular topic for future research. Contrary to the increase in substrate stiffness that occurs in cancerous tissues, the plasma membrane of cancer cells is softer than in normal cells, which means that they can migrate more easily[81]. Moreover, the softer plasma membrane along with the underlying cytoskeleton, are more likely to undergo alterations in configuration in response to external mechanical stimuli, and this affects the subsequent transmission of biochemical signals[82]. Since membrane tension is closely associated with cancer cell behavior, this characteristic has recently been used for the diagnosis and prognosis of a low-grade glioma via the establishment of a membrane tension-related gene signature[83]. In addition, many cell membrane-localized proteins such as ion channels and other mechanosensitive proteins are reported to be highly expressed in cancers and act as mechanosensors, which respond to the rigid TME by changing their conformation[84,85]. Of note, the 2021 Nobel Prize was awarded for the discovery of piezo1/2 as mechanosensitive ion channel proteins, and this has initiated a burst of related studies, especially in the cancer research field[33]. Indeed, piezo proteins are closely associated with several cancers and so their potential as diagnostic and prognostic cancer biomarkers is indisputable. In addition, due to the contribution of mechanical force from the TME in the regulation of tumor heterogeneity, identifying the mechanical properties of areas surrounding a tumor and developing therapeutics to counter the mechanical forces with carcinogenic impact would be favorable for precise cancer diagnosis and treatment[86].
The main biochemical signals generated from the mechanical forces, are transduced in the form of phosphorylations. Therefore, identifying more post-transcriptional modification types under different mechanical stimuli might provide some novel perspectives for determining how extracellular cues influence intracellular activities[87,88]. For example, it is now accepted that autophagy is activated by mechanical stress and plays a role in tumorigenesis[89-91]. Therefore, further investigations to explore how the plasma membrane and its localized mechanosensors transduce mechanical forces through post-transcriptional modifications (and thereby participate in the regulation of cellular activity), will not only help to reveal the reasons behind tumor heterogeneity but will also benefit the diagnosis, treatment, and prognosis of cancer. For example, the increase in stiffness is a well-recognized feature of cancer mechanics that has been used previously for cancer diagnosis and prognosis[12]. The continued development and validation of mechanobiological biomarkers that reflect the mechanical properties of tissue microenvironments are likely to facilitate the clinical application of mechano-oncology. Moreover, the mechanosensitivity of cancer cells is suggested to promote malignant cell behaviors[92,93], and mechanical abnormalities are the main culprit that drives cancer chemoresistance via the activation of cellular drug efflux or DNA repair systems[94]. Therefore, deciphering the detailed signaling pathways such as autophagy and post-transcriptional modifications involved in mechanotransduction might allow the development of new drugs that can be used in combination with current cancer therapies. This would increase the likelihood of therapeutic success and minimize the chance of developing drug resistance, which is advantageous for the prognosis of cancer patients.
Here, we used a bibliometric approach to analyze the trends and important issues regarding mechanotransduction studies in cancer research. While this analysis provides a relatively complete and understandable picture of the state of research today, there are several inevitable limitations. First, while the WoSCC used in this study, is regarded as being a reliable database for bibliometric analysis, the use of additional database sources would provide a more comprehensive view of the situation. Second, some papers that are already included in the associated databases might be delayed being included in the WoSCC, leading to statistical bias and a loss of precision. Finally, analyzing and summarizing research trends based on keywords alone, might be subjective and therefore lack a depth of exploration when compared with traditional literature reviews.
Our results show that mechanotransduction-related cancer research is an increasingly popular topic in the world today. The United States is in the leading position of global research on mechano-oncology after almost 30 years of investigations, and the University of California system (with the largest number of collaborators), is the most influential organization based on its publication and citation times. Research group cooperations exist but remain largely domestic, lacking cross-national communications. Our findings suggest that investigations exploring how the plasma membrane and its localized mechanosensors might transduce mechanical force through post-transcriptional modifications and thereby participate in the cellular activity regulations and cancer development, will be the next big topic in this field.
Mechanical stimuli, generated by the contact between cells (both tumor and non-tumur) or with the non-cellular microenvironment, have been demonstrated to play a significant role in the development of cancer. Unlike biochemical transduction, which depends on small molecules, growth factors, and cytokines, mechanotransduction is a process whereby cells sense mechanical cues in their external environment and translate them into biochemical signals to impact their intracellular activities. Indeed, in recent investigations, the importance of mechanical stimulation in cancer development is described as being on par with biochemical factors. Drs David Julius and Ardem Patapoutian were awarded the 2021 Nobel Prize in Physiology or Medicine for their work on mechanosensitive ion channels, which has already acted as a new catalyst for the increasing numbers of researchers to conduct mechanotransduction-related cancer research. Bibliometrics is a useful quantitative method to comprehensively analyze publications in multiple aspects, including the authors, organizations, countries, journals, and keywords, to uncover collaboration conditions and emerging trends in specific research areas. Although increasing numbers of research papers are now starting to focus on the role of mechanotransduction in cancer, to date no bibliometric analysis has been conducted to quantify the situation.
The deep understanding of mechanotransduction in cancer will not only help determine the reasons behind the tumor heterogeneity, but also facilitate the development of more versatile approaches to cancer diagnosis and therapy.
To provides an objective evaluation of the dynamics and emerging trends of mechanotransduction-related cancer research.
We present the first bibliometric analysis of research conducted on mechanotransduction in cancer and reveal the current trends and hot topics in this field.
This study showed that mechanotransduction-related cancer research remains a hot topic, with approximately 100 papers and 5000 citations generated per year in the past three years. Additionally, the United States is a well-established global leader of this field, and the University of California system is the most influential organization in this field. The keywords “plasma membrane”, “autophagy”, “piezo1/2”, “heterogeneity”, “cancer diagnosis”, and “post-transcriptional modification” are likely to be the next topics of interest in this field.
Our results found that mechanotransduction-related cancer research is an increasingly popular topic in the world today. The United States is in the leading position of global research on mechano-oncology after almost 30 years of investigations, and the University of California system (with the largest number of collaborators), is the most influential organization based on its publication and citation times. Research group cooperations exist but remain largely domestic, lacking cross-national communications.
We predict that the next ‘hot’ topic in cancer research will be investigating how localized mechanosensors in the plasma membrane transduce mechanical forces via post-transcriptional modifications to participate in the regulation of cellular activity.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Fu L, China; Jeong KY, South Korea; Safarzadeh Kozani P, Iran; Gupta MK, Germany S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Vaghari-Tabari M, Ferns GA, Qujeq D, Andevari AN, Sabahi Z, Moein S. Signaling, metabolism, and cancer: An important relationship for therapeutic intervention. J Cell Physiol. 2021;236:5512-5532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, Ababneh E, Abbasi-Kangevari M, Abbastabar H, Abd-Elsalam SM, Abdoli A, Abedi A, Abidi H, Abolhassani H, Adedeji IA, Adnani QES, Advani SM, Afzal MS, Aghaali M, Ahinkorah BO, Ahmad S, Ahmad T, Ahmadi A, Ahmadi S, Ahmed Rashid T, Ahmed Salih Y, Akalu GT, Aklilu A, Akram T, Akunna CJ, Al Hamad H, Alahdab F, Al-Aly Z, Ali S, Alimohamadi Y, Alipour V, Aljunid SM, Alkhayyat M, Almasi-Hashiani A, Almasri NA, Al-Maweri SAA, Almustanyir S, Alonso N, Alvis-Guzman N, Amu H, Anbesu EW, Ancuceanu R, Ansari F, Ansari-Moghaddam A, Antwi MH, Anvari D, Anyasodor AE, Aqeel M, Arabloo J, Arab-Zozani M, Aremu O, Ariffin H, Aripov T, Arshad M, Artaman A, Arulappan J, Asemi Z, Asghari Jafarabadi M, Ashraf T, Atorkey P, Aujayeb A, Ausloos M, Awedew AF, Ayala Quintanilla BP, Ayenew T, Azab MA, Azadnajafabad S, Azari Jafari A, Azarian G, Azzam AY, Badiye AD, Bahadory S, Baig AA, Baker JL, Balakrishnan S, Banach M, Bärnighausen TW, Barone-Adesi F, Barra F, Barrow A, Behzadifar M, Belgaumi UI, Bezabhe WMM, Bezabih YM, Bhagat DS, Bhagavathula AS, Bhardwaj N, Bhardwaj P, Bhaskar S, Bhattacharyya K, Bhojaraja VS, Bibi S, Bijani A, Biondi A, Bisignano C, Bjørge T, Bleyer A, Blyuss O, Bolarinwa OA, Bolla SR, Braithwaite D, Brar A, Brenner H, Bustamante-Teixeira MT, Butt NS, Butt ZA, Caetano Dos Santos FL, Cao Y, Carreras G, Catalá-López F, Cembranel F, Cerin E, Cernigliaro A, Chakinala RC, Chattu SK, Chattu VK, Chaturvedi P, Chimed-Ochir O, Cho DY, Christopher DJ, Chu DT, Chung MT, Conde J, Cortés S, Cortesi PA, Costa VM, Cunha AR, Dadras O, Dagnew AB, Dahlawi SMA, Dai X, Dandona L, Dandona R, Darwesh AM, das Neves J, De la Hoz FP, Demis AB, Denova-Gutiérrez E, Dhamnetiya D, Dhimal ML, Dhimal M, Dianatinasab M, Diaz D, Djalalinia S, Do HP, Doaei S, Dorostkar F, Dos Santos Figueiredo FW, Driscoll TR, Ebrahimi H, Eftekharzadeh S, El Tantawi M, El-Abid H, Elbarazi I, Elhabashy HR, Elhadi M, El-Jaafary SI, Eshrati B, Eskandarieh S, Esmaeilzadeh F, Etemadi A, Ezzikouri S, Faisaluddin M, Faraon EJA, Fares J, Farzadfar F, Feroze AH, Ferrero S, Ferro Desideri L, Filip I, Fischer F, Fisher JL, Foroutan M, Fukumoto T, Gaal PA, Gad MM, Gadanya MA, Gallus S, Gaspar Fonseca M, Getachew Obsa A, Ghafourifard M, Ghashghaee A, Ghith N, Gholamalizadeh M, Gilani SA, Ginindza TG, Gizaw ATT, Glasbey JC, Golechha M, Goleij P, Gomez RS, Gopalani SV, Gorini G, Goudarzi H, Grosso G, Gubari MIM, Guerra MR, Guha A, Gunasekera DS, Gupta B, Gupta VB, Gupta VK, Gutiérrez RA, Hafezi-Nejad N, Haider MR, Haj-Mirzaian A, Halwani R, Hamadeh RR, Hameed S, Hamidi S, Hanif A, Haque S, Harlianto NI, Haro JM, Hasaballah AI, Hassanipour S, Hay RJ, Hay SI, Hayat K, Heidari G, Heidari M, Herrera-Serna BY, Herteliu C, Hezam K, Holla R, Hossain MM, Hossain MBH, Hosseini MS, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hsairi M, Huang J, Hugo FN, Hussain R, Hussein NR, Hwang BF, Iavicoli I, Ibitoye SE, Ida F, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Irham LM, Islam JY, Islam RM, Islam SMS, Ismail NE, Isola G, Iwagami M, Jacob L, Jain V, Jakovljevic MB, Javaheri T, Jayaram S, Jazayeri SB, Jha RP, Jonas JB, Joo T, Joseph N, Joukar F, Jürisson M, Kabir A, Kahrizi D, Kalankesh LR, Kalhor R, Kaliyadan F, Kalkonde Y, Kamath A, Kameran Al-Salihi N, Kandel H, Kapoor N, Karch A, Kasa AS, Katikireddi SV, Kauppila JH, Kavetskyy T, Kebede SA, Keshavarz P, Keykhaei M, Khader YS, Khalilov R, Khan G, Khan M, Khan MN, Khan MAB, Khang YH, Khater AM, Khayamzadeh M, Kim GR, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Kopec JA, Koteeswaran R, Koul PA, Koulmane Laxminarayana SL, Koyanagi A, Kucuk Bicer B, Kugbey N, Kumar GA, Kumar N, Kurmi OP, Kutluk T, La Vecchia C, Lami FH, Landires I, Lauriola P, Lee SW, Lee SWH, Lee WC, Lee YH, Leigh J, Leong E, Li J, Li MC, Liu X, Loureiro JA, Lunevicius R, Magdy Abd El Razek M, Majeed A, Makki A, Male S, Malik AA, Mansournia MA, Martini S, Masoumi SZ, Mathur P, McKee M, Mehrotra R, Mendoza W, Menezes RG, Mengesha EW, Mesregah MK, Mestrovic T, Miao Jonasson J, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirzaei H, Mirzaei HR, Misra S, Mithra P, Moghadaszadeh M, Mohammad KA, Mohammad Y, Mohammadi M, Mohammadi SM, Mohammadian-Hafshejani A, Mohammed S, Moka N, Mokdad AH, Molokhia M, Monasta L, Moni MA, Moosavi MA, Moradi Y, Moraga P, Morgado-da-Costa J, Morrison SD, Mosapour A, Mubarik S, Mwanri L, Nagarajan AJ, Nagaraju SP, Nagata C, Naimzada MD, Nangia V, Naqvi AA, Narasimha Swamy S, Ndejjo R, Nduaguba SO, Negoi I, Negru SM, Neupane Kandel S, Nguyen CT, Nguyen HLT, Niazi RK, Nnaji CA, Noor NM, Nuñez-Samudio V, Nzoputam CI, Oancea B, Ochir C, Odukoya OO, Ogbo FA, Olagunju AT, Olakunde BO, Omar E, Omar Bali A, Omonisi AEE, Ong S, Onwujekwe OE, Orru H, Ortega-Altamirano DV, Otstavnov N, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakshir K, Pana A, Panagiotakos D, Panda-Jonas S, Pardhan S, Park EC, Park EK, Pashazadeh Kan F, Patel HK, Patel JR, Pati S, Pattanshetty SM, Paudel U, Pereira DM, Pereira RB, Perianayagam A, Pillay JD, Pirouzpanah S, Pishgar F, Podder I, Postma MJ, Pourjafar H, Prashant A, Preotescu L, Rabiee M, Rabiee N, Radfar A, Radhakrishnan RA, Radhakrishnan V, Rafiee A, Rahim F, Rahimzadeh S, Rahman M, Rahman MA, Rahmani AM, Rajai N, Rajesh A, Rakovac I, Ram P, Ramezanzadeh K, Ranabhat K, Ranasinghe P, Rao CR, Rao SJ, Rawassizadeh R, Razeghinia MS, Renzaho AMN, Rezaei N, Rezapour A, Roberts TJ, Rodriguez JAB, Rohloff P, Romoli M, Ronfani L, Roshandel G, Rwegerera GM, S M, Sabour S, Saddik B, Saeed U, Sahebkar A, Sahoo H, Salehi S, Salem MR, Salimzadeh H, Samaei M, Samy AM, Sanabria J, Sankararaman S, Santric-Milicevic MM, Sardiwalla Y, Sarveazad A, Sathian B, Sawhney M, Saylan M, Schneider IJC, Sekerija M, Seylani A, Shafaat O, Shaghaghi Z, Shaikh MA, Shamsoddin E, Shannawaz M, Sharma R, Sheikh A, Sheikhbahaei S, Shetty A, Shetty JK, Shetty PH, Shibuya K, Shirkoohi R, Shivakumar KM, Shivarov V, Siabani S, Siddappa Malleshappa SK, Silva DAS, Singh JA, Sintayehu Y, Skryabin VY, Skryabina AA, Soeberg MJ, Sofi-Mahmudi A, Sotoudeh H, Steiropoulos P, Straif K, Subedi R, Sufiyan MB, Sultan I, Sultana S, Sur D, Szerencsés V, Szócska M, Tabarés-Seisdedos R, Tabuchi T, Tadbiri H, Taherkhani A, Takahashi K, Talaat IM, Tan KK, Tat VY, Tedla BAA, Tefera YG, Tehrani-Banihashemi A, Temsah MH, Tesfay FH, Tessema GA, Thapar R, Thavamani A, Thoguluva Chandrasekar V, Thomas N, Tohidinik HR, Touvier M, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tran MTN, Tripathy JP, Tusa BS, Ullah I, Ullah S, Umapathi KK, Unnikrishnan B, Upadhyay E, Vacante M, Vaezi M, Valadan Tahbaz S, Velazquez DZ, Veroux M, Violante FS, Vlassov V, Vo B, Volovici V, Vu GT, Waheed Y, Wamai RG, Ward P, Wen YF, Westerman R, Winkler AS, Yadav L, Yahyazadeh Jabbari SH, Yang L, Yaya S, Yazie TSY, Yeshaw Y, Yonemoto N, Younis MZ, Yousefi Z, Yu C, Yuce D, Yunusa I, Zadnik V, Zare F, Zastrozhin MS, Zastrozhina A, Zhang J, Zhong C, Zhou L, Zhu C, Ziapour A, Zimmermann IR, Fitzmaurice C, Murray CJL, Force LM. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1180] [Cited by in RCA: 1135] [Article Influence: 378.3] [Reference Citation Analysis (0)] |
| 3. | Mbemi A, Khanna S, Njiki S, Yedjou CG, Tchounwou PB. Impact of Gene-Environment Interactions on Cancer Development. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1581] [Reference Citation Analysis (0)] |
| 5. | Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez ME, Farge E. Mechanotransduction in tumor progression: The dark side of the force. J Cell Biol. 2018;217:1571-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 6. | Mierke CT. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep Prog Phys. 2014;77:076602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1638] [Cited by in RCA: 1421] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 8. | Liu Q, Luo Q, Ju Y, Song G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020;17:282-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 9. | Fang Y, Wu D, Birukov KG. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr Physiol. 2019;9:873-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Lin HH, Ng KF, Chen TC, Tseng WY. Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 945] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 12. | Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 1324] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 13. | Abdollahiyan P, Oroojalian F, Baradaran B, de la Guardia M, Mokhtarzadeh A. Advanced mechanotherapy: Biotensegrity for governing metastatic tumor cell fate via modulating the extracellular matrix. J Control Release. 2021;335:596-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 16. | Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials. 2017;146:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Mohammadi H, Sahai E. Mechanisms and impact of altered tumour mechanics. Nat Cell Biol. 2018;20:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 18. | Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 264] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 19. | Er EE, Tello-Lafoz M, Huse M. Mechanoregulation of Metastasis beyond the Matrix. Cancer Res. 2022;82:3409-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y, Li Y, Zhou A, Yao S, Zhou R, Huo J, Che L, Li J. Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation. Mol Ther. 2021;29:2995-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: An overview and guidelines. J Bus Res. 2021;133:285-296. [DOI] [Full Text] |
| 22. | Ugolini D, Puntoni R, Perera FP, Schulte PA, Bonassi S. A bibliometric analysis of scientific production in cancer molecular epidemiology. Carcinogenesis. 2007;28:1774-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Song M, Lu Q, Xu M, Li Y, Zhao Y, Gong C, Ou X. The global research and emerging trends in autophagy of pancreatic cancer: A bibliometric and visualized study. Front Oncol. 2022;12:987026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Li M, Zhang Y, Zhao J, Wang D. The global landscape and research trend of phase separation in cancer: a bibliometric analysis and visualization. Front Oncol. 2023;13:1170157. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Kong H, Li M, Deng CM, Wu YJ, He ST, Mu DL. A comprehensive overview of clinical research on dexmedetomidine in the past 2 decades: A bibliometric analysis. Front Pharmacol. 2023;14:1043956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950;111:261-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 679] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Erxleben C. Stretch-activated current through single ion channels in the abdominal stretch receptor organ of the crayfish. J Gen Physiol. 1989;94:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Weiss L. Cell adhesion molecules: a critical examination of their role in metastasis. Invasion Metastasis. 14:192-197. [PubMed] |
| 30. | Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2801] [Cited by in RCA: 2791] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 31. | Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1247] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 32. | Ma S, Meng Z, Chen R, Guan KL. The Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem. 2019;88:577-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 857] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 33. | Martinac B. 2021 Nobel Prize for mechanosensory transduction. Biophys Rev. 2022;14:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Massou S, Nunes Vicente F, Wetzel F, Mehidi A, Strehle D, Leduc C, Voituriez R, Rossier O, Nassoy P, Giannone G. Cell stretching is amplified by active actin remodelling to deform and recruit proteins in mechanosensitive structures. Nat Cell Biol. 2020;22:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Nunes Vicente F, Chen T, Rossier O, Giannone G. Novel imaging methods and force probes for molecular mechanobiology of cytoskeleton and adhesion. Trends Cell Biol. 2023;33:204-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Lavrenyuk K, Conway D, Dahl KN. Imaging methods in mechanosensing: a historical perspective and visions for the future. Mol Biol Cell. 2021;32:842-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5900] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 38. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1845] [Cited by in RCA: 1937] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 39. | Liu G, Chen T, Ding Z, Wang Y, Wei Y, Wei X. Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment. Cell Prolif. 2021;54:e13009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 40. | Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1788] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 41. | Alaseem A, Alhazzani K, Dondapati P, Alobid S, Bishayee A, Rathinavelu A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin Cancer Biol. 2019;56:100-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 42. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3948] [Cited by in RCA: 3800] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 43. | Kai F, Laklai H, Weaver VM. Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease. Trends Cell Biol. 2016;26:486-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 44. | Kretschmer M, Rüdiger D, Zahler S. Mechanical Aspects of Angiogenesis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 45. | Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP. Caveolae: A Role in Endothelial Inflammation and Mechanotransduction? Front Physiol. 2016;7:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Frank PG, Lisanti MP. Role of caveolin-1 in the regulation of the vascular shear stress response. J Clin Invest. 2006;116:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Boldock L, Wittkowske C, Perrault CM. Microfluidic traction force microscopy to study mechanotransduction in angiogenesis. Microcirculation. 2017;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Hur SS, Jeong JH, Ban MJ, Park JH, Yoon JK, Hwang Y. Traction force microscopy for understanding cellular mechanotransduction. BMB Rep. 2020;53:74-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 1376] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 50. | Montagner M, Dupont S. Mechanical Forces as Determinants of Disseminated Metastatic Cell Fate. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Pethő Z, Najder K, Bulk E, Schwab A. Mechanosensitive ion channels push cancer progression. Cell Calcium. 2019;80:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007;428:183-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Jiang Y, Yang X, Jiang J, Xiao B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem Sci. 2021;46:472-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 54. | Li X, Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci. 2020;16:2014-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 55. | Janota CS, Calero-Cuenca FJ, Gomes ER. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol. 2020;63:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 56. | Li Y, Randriantsilefisoa R, Chen J, Cuellar-Camacho JL, Liang W, Li W. Matrix Stiffness Regulates Chemosensitivity, Stemness Characteristics, and Autophagy in Breast Cancer Cells. ACS Appl Bio Mater. 2020;3:4474-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Li YR, Fang Y, Lyu Z, Zhu Y, Yang L. Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: implications for novel therapeutic strategies. J Transl Med. 2023;21:686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 58. | Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 565] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 59. | Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 422] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 60. | Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 472] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 61. | Miyazawa A, Ito S, Asano S, Tanaka I, Sato M, Kondo M, Hasegawa Y. Regulation of PD-L1 expression by matrix stiffness in lung cancer cells. Biochem Biophys Res Commun. 2018;495:2344-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Petersen EV, Chudakova DA, Skorova EY, Anikin V, Reshetov IV, Mynbaev OA. The Extracellular Matrix-Derived Biomarkers for Diagnosis, Prognosis, and Personalized Therapy of Malignant Tumors. Front Oncol. 2020;10:575569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Jang I, Beningo KA. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 64. | Gargalionis AN, Basdra EK, Papavassiliou AG. Tumor mechanosensing and its therapeutic potential. J Cell Biochem. 2018;119:4304-4308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Trache A, Xie L, Huang H, Glinsky VV, Meininger GA. Applications of Atomic Force Microscopy for Adhesion Force Measurements in Mechanotransduction. Methods Mol Biol. 2018;1814:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Zonderland J, Moroni L. Steering cell behavior through mechanobiology in 3D: A regenerative medicine perspective. Biomaterials. 2021;268:120572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 67. | McClatchey AI, Yap AS. Contact inhibition (of proliferation) redux. Curr Opin Cell Biol. 2012;24:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 68. | Luo J, Yu FX. GPCR-Hippo Signaling in Cancer. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Duff D, Long A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal. 2017;35:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 70. | Roycroft A, Mayor R. Molecular basis of contact inhibition of locomotion. Cell Mol Life Sci. 2016;73:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 71. | Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 72. | Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930-11935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 73. | Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34:940-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 74. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1892] [Article Influence: 270.3] [Reference Citation Analysis (0)] |
| 75. | Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 673] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 76. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7915] [Article Influence: 494.7] [Reference Citation Analysis (0)] |
| 77. | Xu X, Zhang Y, Wang X, Li S, Tang L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Zebda N, Dubrovskyi O, Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res. 2012;83:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 79. | Chuang HH, Zhen YY, Tsai YC, Chuang CH, Hsiao M, Huang MS, Yang CJ. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 80. | Crosas-Molist E, Samain R, Kohlhammer L, Orgaz JL, George SL, Maiques O, Barcelo J, Sanz-Moreno V. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102:455-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 81. | Nguyen HL, Man VH, Li MS, Derreumaux P, Wang J, Nguyen PH. Elastic moduli of normal and cancer cell membranes revealed by molecular dynamics simulations. Phys Chem Chem Phys. 2022;24:6225-6237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Uray IP, Uray K. Mechanotransduction at the Plasma Membrane-Cytoskeleton Interface. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 83. | Li J, Lv F, Jin T. Structuring and validating a prognostic model for low-grade gliomas based on the genes for plasma membrane tension. Front Neurol. 2022;13:1024869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 84. | Haining AW, Lieberthal TJ, Del Río Hernández A. Talin: a mechanosensitive molecule in health and disease. FASEB J. 2016;30:2073-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Yu JL, Liao HY. Piezo-type mechanosensitive ion channel component 1 (Piezo1) in human cancer. Biomed Pharmacother. 2021;140:111692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 86. | Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 919] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 87. | Buwa N, Mazumdar D, Balasubramanian N. Caveolin1 Tyrosine-14 Phosphorylation: Role in Cellular Responsiveness to Mechanical Cues. J Membr Biol. 2020;253:509-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Hernández-Cáceres MP, Munoz L, Pradenas JM, Pena F, Lagos P, Aceiton P, Owen GI, Morselli E, Criollo A, Ravasio A, Bertocchi C. Mechanobiology of Autophagy: The Unexplored Side of Cancer. Front Oncol. 2021;11:632956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 90. | Ravasio A, Morselli E, Bertocchi C. Mechanoautophagy: Synergies Between Autophagy and Cell Mechanotransduction at Adhesive Complexes. Front Cell Dev Biol. 2022;10:917662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1948] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 92. | Abidine Y, Constantinescu A, Laurent VM, Sundar Rajan V, Michel R, Laplaud V, Duperray A, Verdier C. Mechanosensitivity of Cancer Cells in Contact with Soft Substrates Using AFM. Biophys J. 2018;114:1165-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Chen X, Momin A, Wanggou S, Wang X, Min HK, Dou W, Gong Z, Chan J, Dong W, Fan JJ, Xiong Y, Talipova K, Zhao H, Chen YX, Veerasammy K, Fekete A, Kumar SA, Liu H, Yang Q, Son JE, Dou Z, Hu M, Pardis P, Juraschka K, Donovan LK, Zhang J, Ramaswamy V, Selvadurai HJ, Dirks PB, Taylor MD, Wang LY, Hui CC, Abzalimov R, He Y, Sun Y, Li X, Huang X. Mechanosensitive brain tumor cells construct blood-tumor barrier to mask chemosensitivity. Neuron. 2023;111:30-48.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 94. | Kalli M, Poskus MD, Stylianopoulos T, Zervantonakis IK. Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer. 2023;9:937-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |