Published online Oct 24, 2023. doi: 10.5306/wjco.v14.i10.400
Peer-review started: June 27, 2023
First decision: August 10, 2023
Revised: September 1, 2023
Accepted: September 22, 2023
Article in press: September 22, 2023
Published online: October 24, 2023
Processing time: 118 Days and 15.6 Hours
Radiosurgery for multiple brain metastases has been more reported recently without using whole-brain radiotherapy. Nevertheless, the sparsity of the data still claims more information about toxicity and survival and their association with both dosimetric and geometric aspects of this treatment.
To assess the toxicity and survival outcome of radiosurgery in patients with multiple (four or more lesions) brain metastases.
In a single institution, data were collected retrospectively from patients who underwent radiosurgery to treat brain metastases from diverse primary sites. Patients with 4-21 brain metastases were treated with a single fraction with a dose of 18 Gy or 20 Gy. The clinical variables collected were relevant to toxicity, survival, treatment response, planning, and dosimetric variables. The Spearman’s rank correlation coefficients, Mann-Whitney test, Kruskal-Wallis test, and Log-rank test were used according to the type of variable and outcomes.
From August 2017 to February 2020, 55 patients were evaluated. Headache was the most common complaint (38.2%). The median overall survival (OS) for patients with karnofsky performance status (KPS) > 70 was 8.9 mo, and this was 3.6 mo for those with KPS ≤ 70 (P = 0.047). Patients with treated lesions had a median progression-free survival of 7.6 mo. There were no differences in OS (19.7 vs 9.5 mo) or progression-free survival (10.6 vs 6.3 mo) based on prior irradiation. There was no correlation found between reported toxicities and planning, dosimetric, and geometric variables, implying that no additional significant toxicity risks appear to be added to the treatment of multiple (four or more) lesions.
No associations were found between the evaluated toxicities and the planning dosimetric parameters, and no differences in survival rates were detected based on previous treatment status.
Core Tip: Toxicity and survival outcome of radiosurgery in patients with multiple brain metastases (≥ 4) were evaluated. A total of 55 patients were evaluated; headache was the most common complaint, but no associations were found between the evaluated toxicities and the planning and dosimetric parameters. The median overall survival found was 10 mo and the survival of the group that did not undergo irradiation before radiosurgery was 9.5 mo. The results are equivalent to those found by authors who evaluated patients with up to four lesions. Our data demonstrate the safe use of isolated stereotactic radiosurgery to treat patients with four or more brain metastases.
- Citation: de Camargo AV, de Mattos MD, Kawasaki MK, Gomes DNS, Borges ABB, Vazquez VL, Araujo RLC. Treatment of patients with multiple brain metastases by isolated radiosurgery: Toxicity and survival. World J Clin Oncol 2023; 14(10): 400-408
- URL: https://www.wjgnet.com/2218-4333/full/v14/i10/400.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i10.400
It is estimated that 19.3 million new cancer cases and 10 million deaths occurred in 2020. Breast (11.7%) and lung (11.4%) cancers are among the most common cancer cases, causing 2.5 million deaths (24.9% of all cancer deaths)[1]. Besides being the most prevalent in the population, they are the most prevalent cancer types that evolve into brain metastasis due to their favorable microenvironment for brain metastases development[2,3].
The main radiotherapy technique used in brain metastasis is stereotactic radiosurgery (SRS) performed in a linear accelerator (LA). Thus, it is necessary to determine whether the treatment of multiple brain metastases by isolated radiotherapy is safe and non-inferior to the treatment of one or few lesions, regarding toxicity and survival[4-7] and if previous treatment, such as whole brain radiotherapy (WBRT), is beneficial before radiosurgery[8,9]. Moreover, to determine which therapy is appropriate for each patient’s prognosis, it is also important to estimate the survival rate of patients with brain metastasis through prognostic factors such as Karnofsky performance status (KPS), diagnosis-specific graded prognostic assessment (DS-GPA), score index for radiosurgery (SIR), and recursive partitioning analyses (RPA), to determine which therapy is adequate for the prognosis of each patient[10-14].
This work aimed to evaluate the toxicity of isolated radiosurgery in patients with multiple brain metastases (≥ 4 lesions). In addition, overall survival and progression-free survival were evaluated, and survival was correlated with the prognostic index.
Retrospective data were collected from 55 patients who underwent radiosurgery at Barretos Cancer Hospital from August 2017 to February 2020. Patients who presented with 4-21 brain metastases delineated with the aid of magnetic resonance (MR) were treated in a single fraction with a dose of 18 Gy or 20 Gy. Patients who met the inclusion criteria were included regardless of previous systemic and primary local treatment since all of them received radiation therapy for four or more brain lesions in palliative manners, and the main outcomes were either local or systemic toxicity. A frameless immobilization system was used for simulation and treatment. Simulation computed tomography with a slice thickness of 1.25 mm was used for all plannings.
All lesions were treated on a Varian TrueBeam® ™ STX Varian Medical Systems LA with high-definition mulitleaf (120-leaf) collimator and planned with an Eclipse® treatment planning system (Varian Medical System Inc, version 13.6). The calculation algorithm used was the anisotropic analytical algorithm with a 1.25 mm calculation grid and heterogeneity correction. VMAT (RapidArc®, Varian Medical System, Inc.) treatment technique was used for all cases with a planning target volume (PTV) margin of 1 mm from the gross target volume contour. Before treatment, a cone-beam computed tomography scan was performed. Planning was carried out by the Department of Radiation Oncology with many physicists and radiation oncologists in who followed the institutional protocol of dose constraints in the organs at risk and of coverage of targets.
The following toxicities were collected: Headache, convulsion, focal deficit, drop in the level of consciousness, fatigue, nausea or vomiting, and mental confusion. They were based on the Common Terminology Criteria for Adverse Events. The patient’s first complaint after radiosurgery was selected.
Prognostic factors were also collected: Initial KPS and that at the first follow-up after radiosurgery, DS-GPA, SIR, and RPA. In addition, age, gender, and the International Classification of Disease of the primary tumor were also surveyed.
Dosimetric variables included were V5Gy, V8Gy, V10Gy, V12Gy, V14Gy, conformity index (CI), heterogeneity index (HI), dmax, and 50% isodose CI (CI_R50). The VxGy represents the volume of the “x” Gy dose that the normal brain minus PTV received. The CI was calculated by the ratio between the volume of the prescription isodose and the volume of the PTVs: Vpresc_isodose/VPTVs. The HI was calculated as (D2%-D98%)/D50%[15]. Dmax is the maximum point dose of the plan and the CI_R50 is the ratio between the volume of the 50% isodose line and the volume of the PTVs.
The geometric variables collected were the number of lesions, total target volumes, the smallest and largest target volumes, and the distance between the isocenter and the most distant lesion. The distance between the isocenter and the most distant lesion was determined using the coordinates of the lesion center and its respective isocenter. The calculation was according to equation 1. In cases where there was more than one isocenter, the distance was measured between the isocenter and the most distant lesion that its arcs was treated, as demonstrated in the Supplementary material.
Technical variables included were the total number of arcs, coplanar or non-coplanar arcs, number of non-coplanar arcs if used, and number of isocenters. The correlations between the dosimetric, geometric, and technical variables collected for this work were previously published by our group[16].
We reported the response in treated lesions as complete response, partial response, stable disease, progressive disease, or radionecrosis. Complete response indicated complete remission of all lesions; partial response indicated that some lesions entered complete remission, while others remained stable; stable disease indicated that all lesions remained the same size; progressive disease indicated that at least one of the lesions enlarged in size; and radionecrosis indicated that at least one lesion went through necrosis due to radiation. Information on the location of new lesions was also reported as either parenchymal or meningeal.
The initial date of treatment was used for estimating the overall and progression-free survival rates and for the outcomes, respectively, the date of death or the date of the last information obtained in the medical records after the treatment, and the date of the MR in which the progression of the treated lesions was detected or the date of the MR in which the appearance of new lesions was detected were used. Survival rates were calculated based on the data of 53 patients with assessable clinical records.
In this study, radiosurgery treatment of multiple brain metastasis (≥ 4) delivered in a isolated manner and at a single dose was referred to as radiosurgery. Whenever the patient underwent radiosurgery in more than one course of treatment, we would consider the first radiosurgery with four or more lesions. To evaluate if the previous treatment influenced survival rates, patients were divided into two groups: No previous irradiation (NP) and irradiation before radiosurgery (P).
Comparisons of toxicities between categories or between different groups of patients were made using chi-square tests or Fisher exact tests, and Mann-Whitney tests for continuous variables, and the relation between prognostic factors and age was evaluated using Mann-Whitney or Kruskal-Wallis tests. The results are presented as proportions or median and interquartile when appropriate. Spearman’s correlation coefficient was used to determine if there was a correlation between toxicities and dosimetric and geometric variables. KPS comparison was made using a marginal homogeneity test. Survival was estimated by the Kaplan-Meier method and the curves of each category were compared using the Log-rank test. Statistical relevance was considered if P < 0.05. Data were collected and managed using the research electronic data capture platform[17] and analyzed using the software SSPS® (v. 20).
The descriptive characteristics of groups NP and P are displayed in Table 1. Briefly, the most prescribed dosage was 18 Gy (83.6%), and 67.3% of patients were female. Of the 55 patients who underwent radiosurgery, 32 (58.2%) declared feeling some toxicity, with headaches (38.2%) being the most frequent. Incidence rates for each toxicity are shown in Table 2.
| Characteristic | NP (n = 35) | P (n = 20) | P value |
| Age at treatment (yr)1 | 62 (51-67) | 53 (42-58) | 0.032 |
| Age (yr) | 0.162 | ||
| < 55 | 15 (42.9) | 13 (65) | |
| ≥ 55 | 20 (57.1) | 7 (35) | |
| Gender | 0.391 | ||
| Female | 22 (62.9) | 15 (75) | |
| Male | 13 (37.1) | 5 (25) | |
| Number of lesions1 | 5 (4-7) | 5 (4-7) | 0.89 |
| Target volumes1 | 5.4 (2.2-9.9) | 2.9 (1.63-4.3) | 0.08 |
| Primary site | 0.85 | ||
| Lung | 16 (45.7) | 7 (35) | |
| Breast | 8 (22.9) | 7 (35) | |
| Melanoma | 8 (22.9) | 6 (30) | |
| Others | 3 (8.6) | 0 | |
| KPS | n = 34 | n = 20 | 0.194 |
| ≤ 70 | 6 (17.6) | 7 (35) | |
| > 70 | 28 (82.4) | 13 (65) | |
| DS-GPA | n = 31 | n = 20 | 0.7 |
| 0-1 | 11 (35.5) | 8 (40) | |
| 1.5-2 | 14 (45.2) | 7 (35) | |
| 2.5-3 | 4 (12.9) | 2 (10) | |
| 3-4 | 2 (6.5) | 3 (15) | |
| RPA | n = 34 | n = 20 | 0.751 |
| Class 1 | 2 (5.9) | 1 (5) | |
| Class 2 | 28 (82.4) | 15 (75) | |
| Class 3 | 4 (11.8) | 4 (20) | |
| SIR1 | n = 34 | n = 20 | 0.104 |
| 4 (4-6) | 5 (4-6.5) | ||
| Prescription dose (Gy) | 0.133 | ||
| 20 | 8 (22.9) | 1 (5) | |
| 18 | 27 (77.1) | 19 (95) |
| Toxicity | n of patients | Percentage (%) |
| Headache | 21 | 38.2 |
| Convulsion | 4 | 7.3 |
| Focal deficit | 5 | 9.1 |
| Drop in level of consciousness | 3 | 5.5 |
| Fatigue | 8 | 14.5 |
| Nausea or vomiting | 6 | 10.9 |
| Mental confusion | 1 | 1.8 |
The number of reported cases of toxicity as a function of time after treatment is shown in Table 3. It was observed that the highest incidence (40.6%) occurred between the first and third month after treatment. To deal with the heterogeneity of patients who had nervous system irradiation more than once, they were divided into four groups: (1) Patients with ≥ 4 lesions who underwent radiosurgery only; (2) patients with previous either WBRT or SRS with less than four lesions or fractionated stereotactic radiotherapy (SRT); (3) patients that underwent irradiation after radiosurgery and reported side effects after the second irradiation; and (4) patients that were irradiated before and after radiosurgery and reported some toxicities after the last irradiation.
| Period (mo) | n of patients | Percentage |
| t < 1 | 4 | 12.5 |
| 1 ≤ t < 3 | 13 | 40.6 |
| 3 ≤ t < 6 | 6 | 18.8 |
| 6 ≤ t < 9 | 6 | 18.8 |
| 9 ≤ t < 12 | 2 | 6.2 |
| t ≥ 12 | 1 | 3.1 |
The proportion of patients per technique that had one irradiation before SRS of multiple lesions was ten for WBRT, seven for SRS with less than four lesions, and eight for SRT. Five patients did two previous irradiations before treating multiple brain metastases (≥ 4 lesions) by SRS. The proportion of patients in each group that reported toxicity was 17 of 18 patients (94.4%) in group 1; 7 of 11 (12.7%) in group 2; 3 of 17 (17.6%) in group 3; and 5 of 9 (55.6%) in group 4.
The incidence of toxicities in each patient group is presented in Table 4. Despite the higher incidence in group 1, no statistical relevance was found between the four groups regarding the seven toxicities. There was also no difference in the toxicities among the different categories of DS-GPA, RPA, and SIR.
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Headache | 10 (58.8) | 4 (57.1) | 2 (66.7) | 5 (100) |
| Convulsion | 1 (5.9) | 1 (14.3) | 1 (33.3) | 1 (20) |
| Focal deficit | 3 (17.6) | 1 (14.3) | 1 (20) | |
| Drop in level of consciousness | 2 (11.8) | 1 (33.3) | ||
| Fatigue | 6 (35.3) | 1 (14.3) | 1 (33.3) | |
| Nausea or vomiting | 5 (29.4) | 1 (14.3) | ||
| Mental confusion | 1 (5.9) | |||
| Total | 28 | 8 | 5 | 7 |
Regarding the response of treated lesions and the emergence of new lesions, there were no differences observed between the P and NP groups (P = 0.643 and P = 0.412, respectively). A single patient had a complete response, 17 had partial responses, seven were stable, 15 had progression, and six presented radionecrosis (half in each group).
Despite the higher number of patients that presented new lesions in group NP (18) compared to group P (11), there were no differences between the two groups. The number of patients with new lesions was 29, and 17 patients did not develop new lesions. According to location, new parenchymal lesions (26) were more frequent than meningeal ones (3).
Comparing the initial KPS with that evaluated in the first consult after treatment, a relevant difference was observed between them (P = 0.033). The percentage of patients whose KPS decreased after treatment was 39.6%, and 60.4% of patients improved or maintained their KPS.
No statistical correlation was observed between dosimetric and geometric variables and toxicities. The descriptive statistics of dosimetric, geometric, and technical variables have previously been published by our group[16].
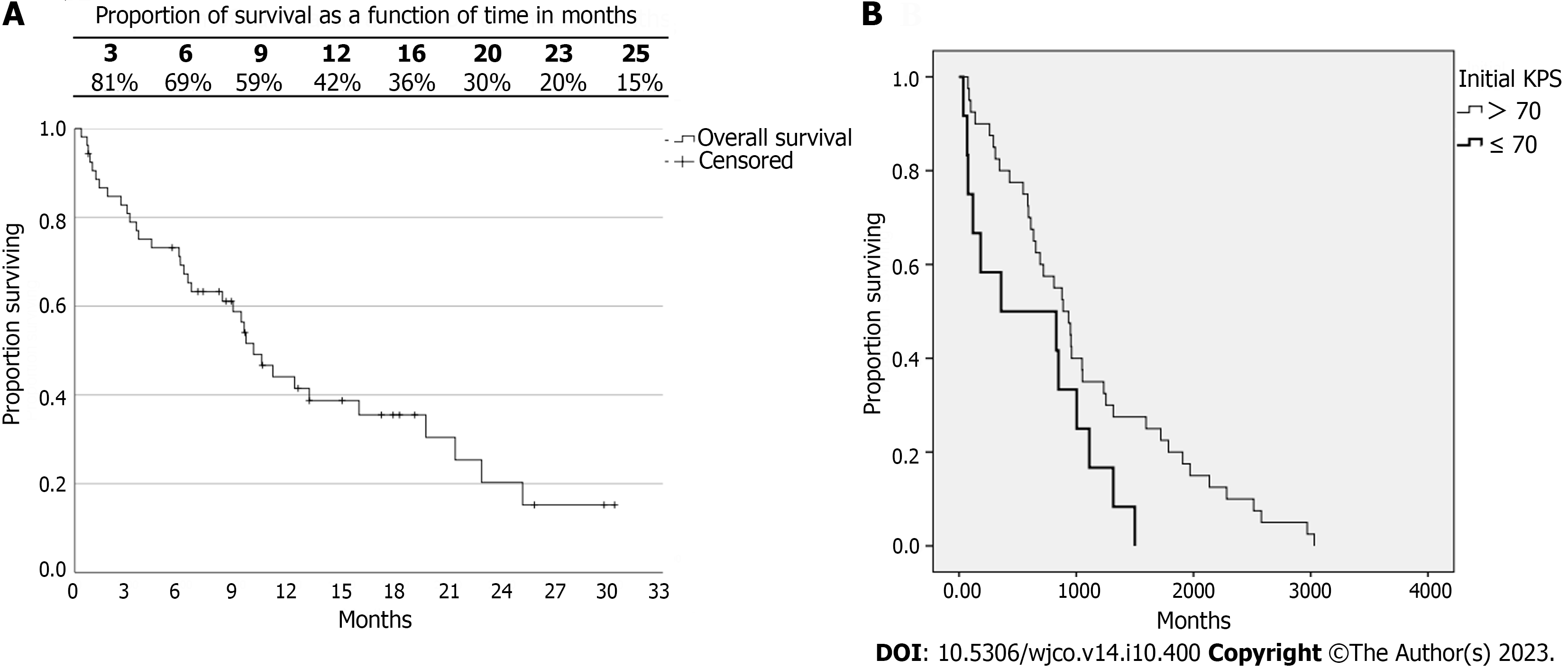
The average overall survival (OS) was 13.3 mo, and the median was 10 mo. The life expectancy over time can be observed in Figure 1. It is noteworthy that the survival rate at 12 mo was 42%. Of 53 patients, 78% died, and of those, only 10 patients (18.9%) were due to neurological causes.
No differences were observed in the OS rates between groups NP and P. The median survival in group NP was 9.5 mo, and in group P it was 19.7 mo (P = 0.110). Considering patients with KPS > 70 and KPS ≤ 70, a difference was observed in OS (P = 0.047) (Figure 1B). The median survival of the group with KPS > 70 was 8.9 mo, and in the group with KPS ≤ 70 it was 3.6 mo. No differences were observed in the survival of DS-GPA (P = 0.547), RPA (P = 0.113), and SIR categories 0 to 4 and 5 to 10 (P = 0.586).
The OS of patients was categorized into two groups for each variable for analysis. No difference between them was found: Number of lesions (P = 0.840), n < 6 (10.5 mo) and n ≥ 6 (9.3 mo); volume of targets (P = 0.786), v < 5 cc (10.5 mo) and v ≥ 5 cc (9.5 mo); V12Gy ≤ 10 cc (11.1 mo) and V12Gy > 10 cc (9.6 mo) (P = 0.693); CI_R50 ≤ 8 (13.2 mo) and CI_R50 > 8 (9.6 mo) (P = 0.655).
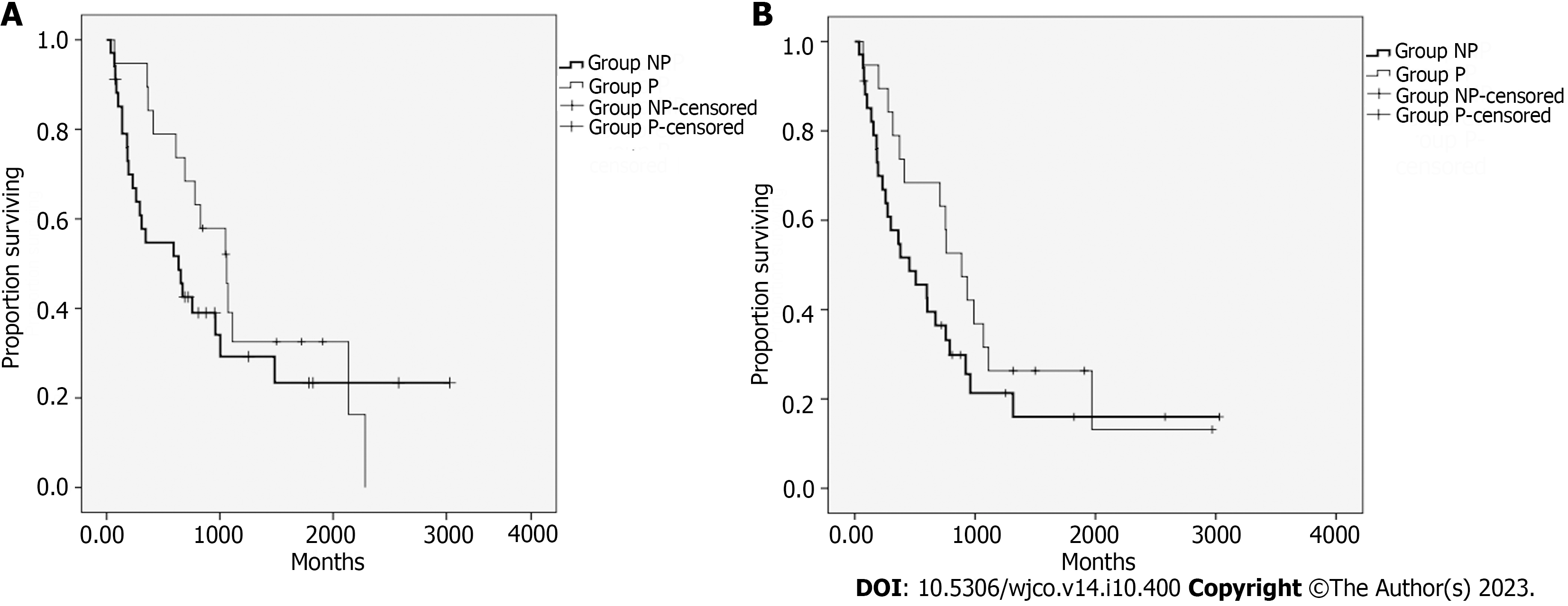
The median progression-free survival of patients with treated lesions (PFSL) was 7.6 mo. No differences (P = 0.293) were found between groups NP and P. The median PFSL of group NP was 6.3 mo, and in group P it was 10.6 mo. The curves for the PFSL of both groups are displayed in Figure 2A. The median survival free from the appearance of new lesions was 6 mo. No difference was observed between groups NP and P (P = 0.188). The median for group NP was 4.5 mo, and for group P it was 8.9 mo. The curves of survival free from the appearance of new lesions in both groups are displayed in Figure 2B.
It was observed that in group 1, a higher proportion (94.4%) of patients reported grievances and a higher number of different toxicities. Nevertheless, no difference was observed between groups when comparing their toxicity incidence. Besides, the toxicities reported varied regarding their start point. One of the patients reported a grievance a year after treatment, thus rendering it difficult to classify it as a side effect of radiosurgery.
Analyzing the responses of treated lesions, six patients developed radionecrosis. As discussed by Blonigen et al[18], V10Gy and V12Gy can be predictors of radionecrosis. The median of V10Gy and V12Gy of those six patients was 27.8 cc (9.7-45.5 cc) and 17.6 cc (6.2-27.4 cc), respectively (only a single patient had the dosage of 20Gy as prescription).
The median OS found by Chang et al[8] was 9.2 mo and the median survival of patients treated only by SRS was 15.2 mo. Aoyama et al[9] obtained a median OS of 8 mo on the arm of patients treated only by SRS. Brown et al[19] found a median OS of 13.5 mo and a median survival of 10.4 mo for patients treated only by SRS.
Sahgal et al[20] found a median OS of 10 mo for the group that only received SRS. The median time to local failure and development of new lesions was 6.6 mo and 4.7 mo, respectively. This last result matches the PFSL and the development of new lesions in this study. The four aforementioned studies compared patients who underwent SRS alone with patients treated by SRS + WBRT.
Scorsetti et al[21] observed a median OS of 16.2 mo and a 12-mo survival rate of 65.3% in the group of patients who underwent only SRS with an LA. They also indicated that 27 of the 130 patients (20.8%) included in that study presented symptomatic radionecrosis. The incidence obtained in that study was 10.9%.
Differently from the studies mentioned before, in which treated patients had up to four lesions, the current study evaluated patients with 4 to 21 lesions and, despite the underestimated OS (group P began treatment of metastasis before the studied SRS), it was observed that survival values are similar to studies with up to four lesions, especially when analyzing the global survival of all patients, and the survival of patients without previous irradiation, whose comparison is possible with the aforementioned studies.
Among the prognostic indexes, despite the predictive power of survival from DS-GPA, RPA, and SIR[11-14,22] being better than KPS for patients with brain metastasis, only KPS showed a difference in OS (P = 0.047) between patients with KPS > 70 and KPS ≤ 70. This likely occurred due to KPS considering only the clinical condition of patients, whereas other indexes also consider specific parameters of patients with brain metastasis that were not discretized in the analysis, such as the primary site of disease, number of lesions, and systemic diseases, among others.
Regarding the number of patients whose KPS decreased, it is important to note that metastatic patients have systemic diseases that worsen the clinical outcome. Therefore, we cannot contribute the decline of KPS to SRS, which is corroborated by the low death number due to neurological causes.
According to dosimetric, geometric, and technical variables, the lack of correlation with toxicities does not imply they do not impact each other, especially considering dosimetric variables used for planning approval. It is known that the volume of targets, number of lesions, distance between lesions, and the isocenter impact these plan evaluation indexes[16,23-25]. What can be observed is that the indication of isolated radiosurgery for multiple brain metastases was safe, considering that the technique achieved dosimetric values good enough not to cause collateral effects.
This study has limitations inherent to the retrospective cohort model where selection and information biases cannot be discarded. There were patients subjected to multiple irradiation techniques before the SRS in this study, and many of the patients were also under systemic treatment, which may interfere with the clinical results. In addition, all of them received some or many kinds of local and systemic treatment for many types of tumors, since in our institution the radiosurgery for four or more lesions was reserved for local control in a palliative manner and usually failed for previous treatments. Regarding the toxicities, precise graduation was not possible to obtain and therefore, they were not differentiated. Although our study had some missing data for clinical variables, they represent less than 4%, which seems acceptable for a retrospective study[26]. If factors were significantly associated with outcomes in univariate analysis, and they were not as demonstrated in Table 1, they would be entered into a multivariate analysis, but it was not possible.
The planning was performed by different personnel, with distinct dose prescriptions and, in some patients with one or more lesions (more significant volumes), planned with three fractions but, even in these cases, there were also four or more lesions treated with a single dose. Considering the prescription, we tested the difference between the doses of 18 and 20 Gy regarding geometric, dosimetric, and technical variables, and no differences were observed.
Our data demonstrate the safe use of isolated SRS to treat patients with four or more brain metastases, with no significant association between dosimetric, geometric, or clinical parameters and the related toxicities.
Radiosurgery for multiple brain metastases has been more reported recently without using whole-brain radiotherapy, but mainly for oligometastatic scenarios (up to 3-4 lesions). Nevertheless, the sparsity of the data still claims more information about toxicity and survival and their association with both dosimetric and geometric aspects of this treatment, especially for the presence of more lesions or in patients with previous irradiation.
To evaluate the toxicity of treatment offered for patients with four or more lesions.
To assess associations of toxicity and survival outcome of stereotactic radiosurgery (SRS) among patients with four or more brain lesions with or without previous brain irradiation.
Retrospective cohort.
Neither difference in toxicity nor survival was detected when comparing patients who underwent SRS for four or more brain lesions with or without previous brain irradiation.
This retrospective study did not detect differences in toxicity for this population with or without previous irradiation, suggesting that the use of SRS for four or more brain lesions with or without previous brain irradiation is safe.
This study claims for more data in larger studies in a prospective manner to better address this question.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: SSAT; IHPBA; ILLS; AHPBA; SSO.
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Bergsma H, Netherlands; Kupeli S, Turkey; Tang H, China S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55781] [Article Influence: 7968.7] [Reference Citation Analysis (132)] |
| 2. | Huh HD, Sub Y, Oh J, Kim YE, Lee JY, Kim HR, Lee S, Lee H, Pak S, Amos SE, Vahala D, Park JH, Shin JE, Park SY, Kim HS, Roh YH, Lee HW, Guan KL, Choi YS, Jeong J, Choi J, Roe JS, Gee HY, Park HW. Reprogramming anchorage dependency by adherent-to-suspension transition promotes metastatic dissemination. Mol Cancer. 2023;22:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Zou Y, Ye F, Kong Y, Hu X, Deng X, Xie J, Song C, Ou X, Wu S, Wu L, Xie Y, Tian W, Tang Y, Wong CW, Chen ZS, Xie X, Tang H. The Single-Cell Landscape of Intratumoral Heterogeneity and The Immunosuppressive Microenvironment in Liver and Brain Metastases of Breast Cancer. Adv Sci (Weinh). 2023;10:e2203699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 4. | Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 993] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 5. | Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Watanabe S, Kasuya H. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2-9 versus 10 or more tumors. J Neurosurg. 2014;121 Suppl:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 591] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 7. | Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1750] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 9. | Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1604] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 10. | Reali A, Allis S, Girardi A, Verna R, Bianco L, Redda MG. Is Karnofsky Performance Status Correlate with Better Overall Survival in Palliative Conformal Whole Brain Radiotherapy? Our Experience. Indian J Palliat Care. 2015;21:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 1070] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 12. | Nagtegaal SHJ, Claes A, Suijkerbuijk KPM, Schramel FMNH, Snijders TJ, Verhoeff JJC. Comparing survival predicted by the diagnosis-specific Graded Prognostic Assessment (DS-GPA) to actual survival in patients with 1-10 brain metastases treated with stereotactic radiosurgery. Radiother Oncol. 2019;138:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Weltman E, Brandt RA, Hanriot RM, Luz FP, Chen MJ, Cruz JC, Wajsbrot DB, Nadalin W. Validating the SIR: a better prognostic score index for patients with brain metastases treated with stereotactic radiosurgery. Int. J. Radiat. Oncol. 5:S268-S269. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1862] [Cited by in RCA: 1829] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 15. | International Commission on Radiation Units and Measurements (ICRU). Feb 16, 2021. [cited 16 February 2021]. Available from: https://icru.org/testing/reports/prescribing-recording-and-reporting-intensitymodulated-photon-beam-therapy-imrt-icru-report-83. |
| 16. | de Camargo AV, Cao M, da Silva DDCSA, de Araújo RLC. Evaluation of the correlation between dosimetric, geometric, and technical parameters of radiosurgery planning for multiple brain metastases. J Appl Clin Med Phys. 2021;22:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36737] [Article Influence: 2296.1] [Reference Citation Analysis (0)] |
| 18. | Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 19. | Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1188] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 20. | Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, Shaw P, Beyene J, Chang EL. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 21. | Scorsetti M, Navarria P, Cozzi L, Clerici E, Bellu L, Franceschini D, Marzo AM, Franzese C, Torri V, Reggiori G, Lobefalo F, Raspagliesi L, Attuati L, Pessina F, Franzini A, Picozzi P, Tomatis S. Radiosurgery of limited brain metastases from primary solid tumor: results of the randomized phase III trial (NCT02355613) comparing treatments executed with a specialized or a C-arm linac-based platform. Radiat Oncol. 2023;18:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Nieder C, Andratschke NH, Geinitz H, Grosu AL. Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit. 2012;18:CR450-CR455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Narayanasamy G, Smith A, Van Meter E, McGarry R, Molloy JA. Total target volume is a better predictor of whole brain dose from gamma stereotactic radiosurgery than the number, shape, or location of the lesions. Med Phys. 2013;40:091714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Roper J, Chanyavanich V, Betzel G, Switchenko J, Dhabaan A. Single-Isocenter Multiple-Target Stereotactic Radiosurgery: Risk of Compromised Coverage. Int J Radiat Oncol Biol Phys. 2015;93:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Selvan KT, Padma G, Revathy MK, Nambi Raj NA, Senthilnathan K, Babu PR. Dosimetric Effect of Rotational Setup Errors in Single-Isocenter Volumetric-Modulated Arc Therapy of Multiple Brain Metastases. J Med Phys. 2019;44:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5622] [Article Influence: 255.5] [Reference Citation Analysis (0)] |