Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.688
Peer-review started: January 27, 2021
First decision: March 31, 2021
Revised: April 9, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: August 24, 2021
Processing time: 208 Days and 1.7 Hours
Gastric cancer (GC) is a highly heterogeneous disease, and the identification of molecular subtyping of gastric adenocarcinoma emerged as a promising option to define therapeutic strategies and prognostic subgroups. However, the costs and technical complexity of molecular methodologies remains an obstacle to its adop
To evaluate the clinicopathological characteristics and long-term survival of GC based on the subgroups of molecular classification by immunohistochemistry (IHC) and in situ hybridization (ISH).
We retrospectively evaluated all patients who underwent D2-gastrectomy between 2009 and 2016 in a Western cohort of GC patients treated with curative intent. Microsatellite instability (MSI) status, E-cadherin, and p53 expression were analyzed by IHC, and Epstein-Barr virus (EBV) by ISH. Tissue microarrays were constructed for analysis. Clinicopathological characteristics and survival of GC were evaluated according to subtypes defined by The Cancer Genome Atlas (TCGA) Research Network Group and Asian Cancer Research Group (ACRG) classification systems.
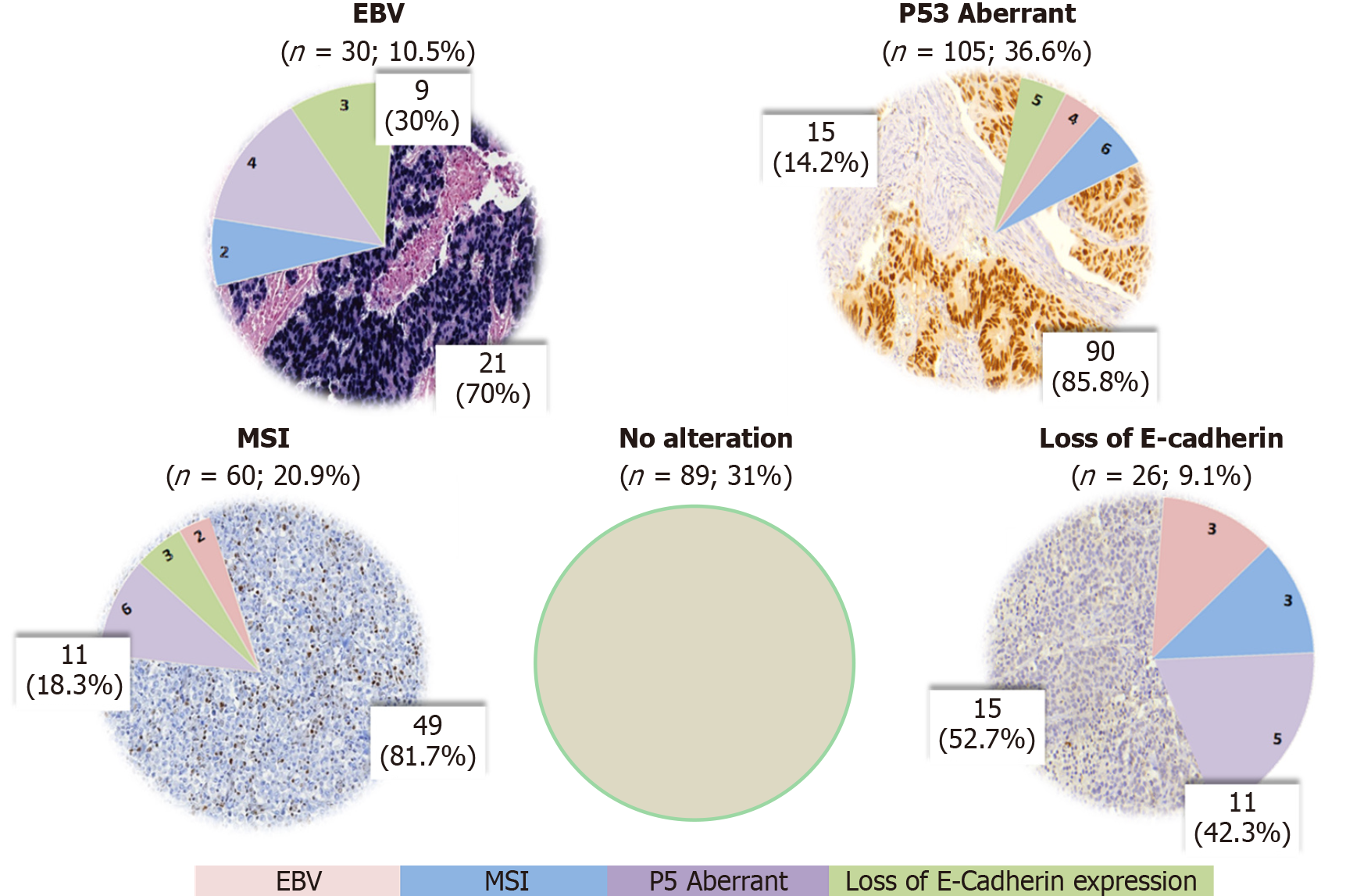
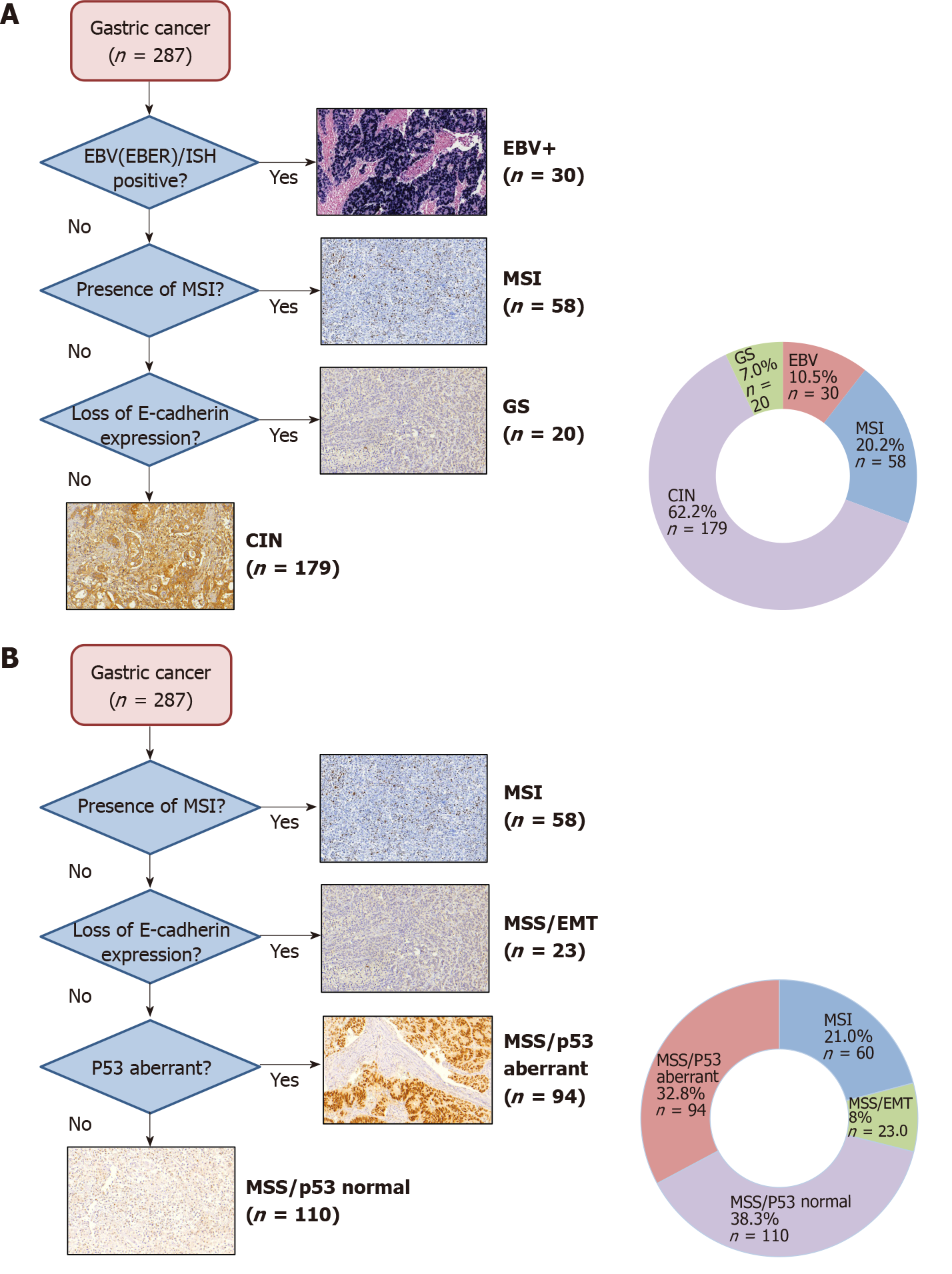
A total of 287 GC patients were included. Based on IHC and ISH analysis, five profiles were defined as follows: E-cadherin aberrant (9.1%), MSI (20.9%), p53 aberrant (36.6%), EBV positivity (10.5%), and p53 normal (31%), which corresponded to tumors that showed no alteration in another profile. A flowchart according to the TCGA and ACRG classifications were used to define the sub
The IHC/ISH analysis was able to distinguish immunophenotypic groups of GC with distinct characteristics and prognosis, resembling the subtypes of the mo
Core Tip: In this study, patients with gastric cancer (GC) were retrospectively evaluated based on subgroups of molecular classification by immunohistochemistry and in situ hybridization. Microsatellite instability status, e-cadherin, p53 expression, and Epstein-Barr virus were evaluated in a Western cohort of GC patients treated with curative intent, where it was possible to obtain subgroups with different clinicopathological characteristics and prognosis. Thus, our findings demonstrate that through techniques used in the routine pathological evaluation it is possible to identify immunophenotypic groups of GC similar to those determined by the molecular classification.
- Citation: Ramos MFKP, Pereira MA, de Mello ES, Cirqueira CDS, Zilberstein B, Alves VAF, Ribeiro-Junior U, Cecconello I. Gastric cancer molecular classification based on immunohistochemistry and in situ hybridization: Analysis in western patients after curative-intent surgery. World J Clin Oncol 2021; 12(8): 688-701
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/688.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.688
Gastric cancer (GC) remains one of the most common cancers and the 3rd cause of cancer-related mortality globally[1]. In addition to this important burden on public health, GC management also represents a challenge due to its great heterogeneity in presentation and behavior. Tumors that appear to have similar characteristics may have extremely different outcomes. In order to better characterize GC, different classifications using clinical and histological aspects have been employed[2-4]. However, these parameters are not able to contemplate the whole disease spectrum.
Recently, classifications based on the molecular profile of GC were proposed[5,6]. The major molecular classification systems published by The Cancer Genome Atlas (TCGA) and by the Asian Cancer Research Group (ACRG) established several subtypes of GC related to distinct clinical and molecular characteristics, providing a molecular subtyping structure, as well as a guide to target agents.
Through complex analyses of molecular data, a TCGA study identified four distinct molecular subtypes: Epstein-Barr virus (EBV)-positive; microsatellite instability (MSI); genomically stable (GS); and chromosomal instability (CIN)[5]. Meanwhile, the ACRG proposed a different classification based on tumor protein p53 (TP53) status, constituted by the subtypes: MSI, microsatellite stable/epithelial to mesenchymal transition (MSS/EMT), MSS/TP53+, and MSS/TP53-[6].
Despite some differences, molecular classifications can be defined by four major signatures: EBV-positivity, alteration of mismatch repair (MMR) proteins, TP53 gene, and cellular cohesion. Although the current molecular classifications have expanded our insight regarding the complex nature of GC, the technologies used to achieve such knowledge have not yet reached full clinical utility. The cost and complexity remain significant obstacles to translate these methodologies into daily clinical practice.
To overcome the complexity of molecular analysis, the search for other methods that could reproduce those findings led to the emergence of different strategies to define molecular subtypes. Techniques such as immunohistochemistry (IHC) and in situ hybridization (ISH) were employed successfully to define the molecular subtypes[7-9]. Indeed, the assessments of EBV status by ISH and MSI by immunostaining for DNA MMR proteins (MutL homolog 1, MLH1; MutS homolog 2, MSH2; MutS homolog 6, MSH6; PMS1 homolog 2, PMS2) are already well-defined methods in diagnostic practice. With regard to the other GC subtypes, p53 staining can be a simple and feasible method to detect gene alterations indirectly, common in the CIN and MSS/TP53+ subtypes. While e-cadherin staining is easily assessable, and the loss of staining is associated with diffuse-type histology and mesenchymal-like morphologic features, which are characteristic of the GS and MSS/EMT subtypes[5-10].
Thus, the aim of this study was (1) to define GC subtypes based on MSI, E-cadherin, and p53 expression determined by IHC and EBV status by ISH; and (2) to evaluate the clinicopathological characteristics and survival of these subtypes according to TCGA and ACRG classification in a western cohort of GC patients treated with curative intent.
This study included all patients with GC who underwent potentially curative gas
Abdominal and pelvis computed tomography, endoscopy, and laboratory tests were used for preoperative staging. Tumor stage was classified according to the 8th edition of the TNM[11]. Chemotherapy (CMT) was indicated for locally advanced GC (N+ and/or T3-4) with potentially resectable tumor. Lymph node (LN) dissection was performed based on the guidelines of the Japanese GC Association[3].
Postoperative follow-up was performed every 3 mo in the first year-and every 6 mo in the following years. Follow-up exams for recurrence detection were performed based on the presence of symptoms. Clinical outcomes were evaluated, including disease-free survival (DFS) and overall survival (OS). The study was approved by the hospital ethics committee and registered online (https://plataformabrasil.saude.gov.br/; CAAE: 37009120.0.0000.0068).
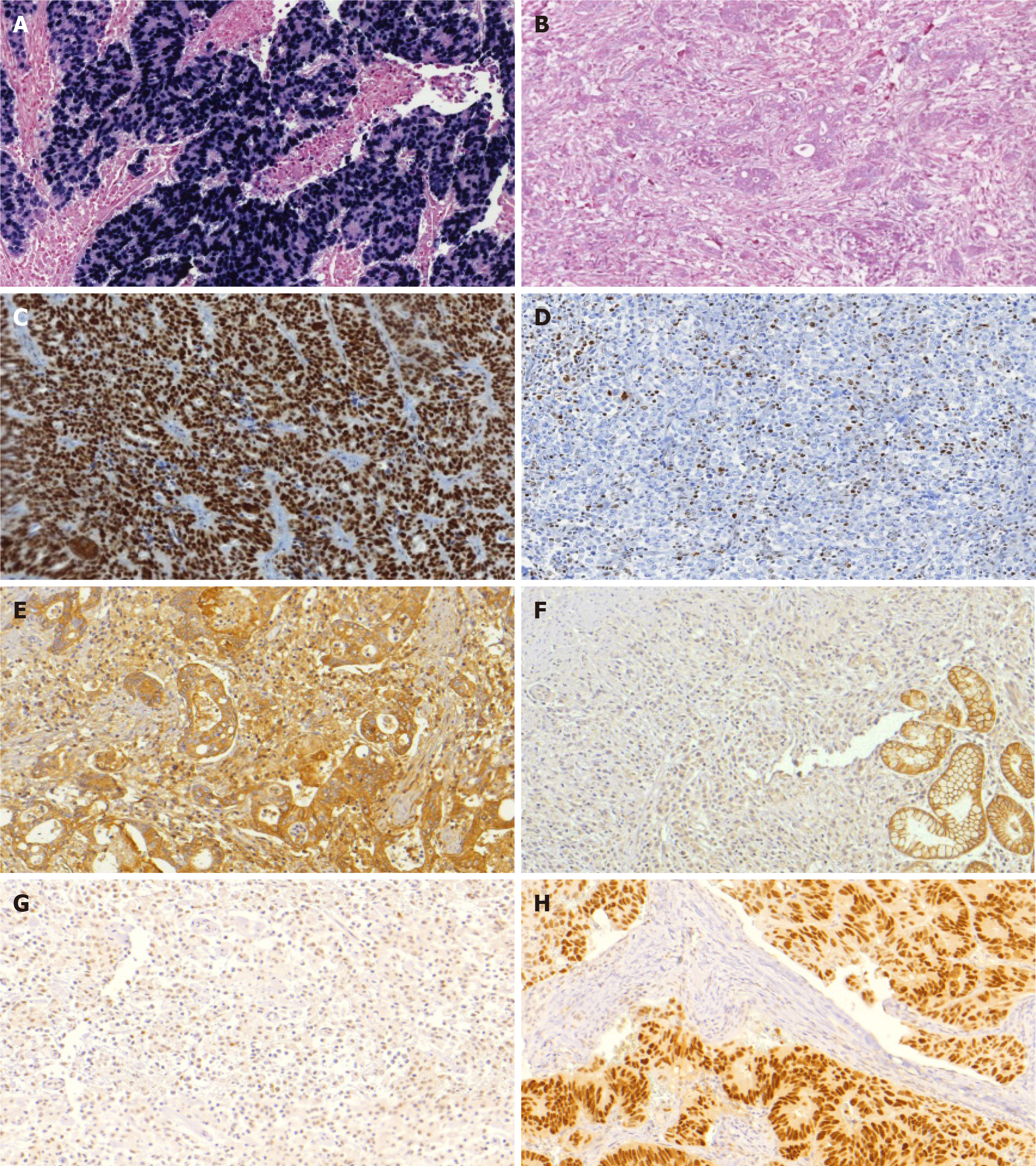
Hematoxylin and eosin (HE)-stained histologic sections from all patients included in the study were reviewed to tissue microarray (TMA) construction. Representative tumor areas (3 tumor and 2 adjacent non-tumoral mucosa tissue cores) were selected from each case, and punched out from individual FFPE tumor blocks using a precision mechanized system. TMA blocks were sectioned at 4 μm thick, and histological sections were submitted to HE staining, IHC and ISH.
For IHC reactions, all sections were de-paraffinized in xylene and rehydrated through graded ethanol. IHC to MSI status was performed with Ventana automated staining system (BenchMark Ultra), according to the manufacturer’s instructions. The following monoclonal antibodies were used for MMR proteins: Anti-MLH1 (clone M1), anti-MSH2 (clone G219-1129), anti-MSH6 (clone-44), anti-PMS2 (clone EPR 3947), all ready to use. The reactions were qualitatively interpreted according to the deposition of the chromogen product on the nuclei of the cancer cells. GC was considered negative only if there was a complete absence of tumor cells staining. GCs negative for MLH1, MSH2, PMS2, or MSH6 expression were considered deficient for MMR (dMMR) proteins, constituting the MSI profile. Tumors that maintained the expressions of all markers were defined as microsatellite stable (MSS status).
For E-cadherin (clone 36B5) and p53 (clone DO-7) staining, peroxidase activity was blocked with hydrogen peroxide and antigen retrieved by heat induction using citrate buffer (pH 6.0) Then, slides were incubated with the primary antibody (4 °C). Avidin-biotin free short polymer-based peroxidase amplification system and diaminobenzydine as chromogen were used for development of reaction products. After the reactions, sections were counterstained with hematoxylin.
A scoring system was used for the evaluation of E-cadherin expression. Scores 0 and 1 (0 = complete loss; and 1 = cytoplasmic expression) were considered aberrant expression. Scores 2 and 3 were defined as normal status (2 = cytoplasmic and membrane labeling; and 3 = membrane labeling)[12]. For p53, two different patterns were considered as aberrant expression: p53 overexpression, in which a strong nuclear staining was observed in at least 70% of tumor cells; and tumors with complete loss of p53 expression that include tumors with no staining or less than 5% staining. Stromal cells and benign epithelium served as control[13]. EBV status was determined by ISH, with probes against Epstein-Barr encoded RNA 1 (EBER1-Y5200). Tumor cell nuclei with dark-blue staining were considered positive.
All analyses were performed by two pathologists. If there was difference between these two observers, these slides were re-evaluated by both investigators.
Categorical and continuous variables were compared using the Chi-square or Fisher Exact Tests and ANOVA, respectively. Survival curves were estimated according to the Kaplan-Meier method and compared using the log-rank test. Alive patients were censored at the date of last contact. Postoperative mortality (defined as 30-d mortality in post-operative or during hospitalization) was omitted from the DFS analysis. Cox regression analyses, including hazard ratios with 95% confidence intervals, were performed to evaluate the prognostic association of clinicopathological characteristics with survival. P value < 0.05 was considered statistically significant. SPSS software (version 20.0) was used for statistical analysis.
A total of 287 patients were enrolled in the study, consisting of 168 (58.5%) men and 119 (41.5%) women. The mean age was 61.5 years. Most of the tumors had a distal location and subtotal gastrectomy was performed in 60.6% of cases. The most common macroscopic configuration was type III (48.4%) and intestinal Laurén (47.4%) the predominant histological type. The mean number of LN retrieved was 41.8, and LN metastases were presented in 56.4% of cases. Stage III was the predominant final stage, which occurred in 43.6% of the cases. Surgical mortality was 4.2%, neoadjuvant therapy was performed in 11.8% of cases, and 55.1% of patients received adjuvant therapy (Supplementary Table 1).
After IHC and ISH analysis for the 4 profiles evaluated (EBV, MSI, p53, E-cadherin), abnormal expression for a single profile was observed in 175 cases (60.9%), and 23 (8.1%) had alteration in two or more profiles. The remaining 89 cases (31%) showed no alteration in the assessed expression profile. Aberrant expression of p53 was observed in 105 cases (36.6%) (negative expression in 33 cases and strong expression in 92 cases), MSI in 60 cases (20.9%), and reduced or abnormal E-cadherin expression in 26 cases (9.1%). EBV positivity was present in 30 cases (10.5%). The protein expression and ISH results are summarized in Figure 1 and the microscopic findings in Figure 2.
The flowchart with the distribution of the subtypes according to TCGA and ACRG classifications are shown in Figure 3. According to TCGA classification, GCs were classified in 4 subtypes as follows: 30 (10.5%) EBV-positive; 58 (20.2%) MSI, 179 (62.2%) CIN; and 20 (7%) GS.
Clinicopathological characteristics of GC according to the TCGA subtypes are demonstrated in Table 1. Proximal location (P < 0.001), total gastrectomy (P = 0.001) and increased inflammatory infiltrate (P < 0.001) were related to EBV subtype. MSI subtype was predominantly associated to advanced age (P = 0.017) and higher Charlson comorbidity index (CCI) (P = 0.011). While poorly differentiated histology (P < 0.001), Laurén diffuse type (P < 0.001), and advanced stage (P = 0.029) were characteristics related to GS subtype. Clinicopathological characteristics of GC based on ACRG classification subtypes are demonstrated in Supplementary Table 2. Unlike TCGA, ACRG subtypes were also significantly different regarding category pN (pathological lymph node metastasis), lymphatic, and venous invasion. Tumor size and inflammatory infiltrate were characteristics significant only in the TCGA.
| Variables | EBV | MSI | CIN | GS | P value | |
| n = 30 (10.5%) | n = 58 (20.2%) | n = 179 (62.2%) | n = 20 (7%) | |||
| Age (yr) | 0.017 | |||||
| mean (± SD) | 61.4 (11.2) | 65.9 (12.5) | 60.1 (11.7) | 60.6 (12.8) | ||
| Sex | 0.101 | |||||
| Male | 23 (76.7) | 29 (50) | 103 (57.5) | 13 (65) | ||
| Female | 7 (23.3) | 29 (50) | 76 (42.5) | 7 (35) | ||
| ASA Classification | 0.753 | |||||
| I/II | 27 (90) | 50 (85.2) | 160 (89.4) | 19 (95) | ||
| III/IV | 3 (10) | 8 (13.8) | 19 (10.6) | 1 (5) | ||
| Charlson–Deyo comorbidity index | 0.011 | |||||
| < 5 | 19 (63.3) | 23 (39.7) | 112 (62.6) | 14 (70) | ||
| ≥ 5 | 11 (36.7) | 35 (60.3) | 67 (37.4) | 6 (30) | ||
| Tumor site | 0.004 | |||||
| Upper | 8 (26.7) | 6 (10.3) | 18 (10.1) | 2 (10) | ||
| Middle | 7 (23.3) | 8 (13.8) | 34 (19) | 3 (15) | ||
| Lower | 11 (36.7) | 44 (75.9) | 123 (68.7) | 13 (65) | ||
| Entire | 4 (13.3) | 0 (0) | 4 (2.2) | 2 (10) | ||
| Type of resection | < 0.001 | |||||
| Subtotal | 8 (26.7) | 45 (77.6) | 110 (61.5) | 11 (55) | ||
| Total | 22 (73.3) | 12 (22.4) | 69 (38.5) | 9 (45) | ||
| Macroscopic type | 0.244 | |||||
| I /II | 9 (34.6) | 19 (34.5) | 37 (22.7) | 4 (21.1) | ||
| III/IV | 17 (65.4) | 36 (65.5) | 126 (77.3) | 15 (78.9) | ||
| Tumor size (cm) | 0.029 | |||||
| mean (± SD) | 6.3 (4.4) | 5.3 (2.9) | 4.5 (3.0) | 5.2 (2.9) | ||
| Histological differentiation | 0.001 | |||||
| Well/moderately | 8 (26.7) | 28 (48.3) | 89 (49.7) | 2 (10) | ||
| Poorly differentiated | 22 (73.3) | 30 (51.7) | 90 (50.3) | 18 (90) | ||
| Laurén type | < 0.001 | |||||
| Intestinal | 11 (36.7) | 34 (58.6) | 88 (49.2) | 3 (15) | ||
| Diffuse/mixed | 12 (40) | 22 (37.9) | 88 (49.2) | 16 (80) | ||
| Undetermined | 7 (23.3) | 2 (3.4) | 3 (1.7) | 1 (5) | ||
| Tumor invasion | 0.277 | |||||
| pT1/pT2 | 10 (33.3) | 29 (50) | 70 (39.1) | 6 (30) | ||
| pT3/pT4 | 20 (66.7) | 29 (50) | 109 (60.9) | 15 (70) | ||
| pN status | 0.147 | |||||
| pN negative | 14 (46.7) | 32 (55.2) | 73 (40.8) | 6 (30) | ||
| pN positive | 16 (53.3) | 26 (44.8) | 106 (59.2) | 14 (70) | ||
| Lymphatic invasion | 0.156 | |||||
| Absent | 16 (53.3) | 22 (37.9) | 96 (53.6) | 8 (40) | ||
| Present | 14 (46.7) | 36 (62.1) | 83 (46.4) | 12 (60) | ||
| Venous Invasion | 0.300 | |||||
| Absent | 22 (73.3) | 35 (60.3) | 125 (69.7) | 11 (55) | ||
| Present | 8 (26.7) | 23 (39.7) | 54 (30.3) | 9 (45) | ||
| Perineural invasion | 0.267 | |||||
| Absent | 14 (46.7) | 36 (62.1) | 91 (50.8) | 8 (40) | ||
| Present | 16 (53.3) | 22 (37.9) | 88 (49.2) | 12 (60) | ||
| Peritumoral inflammatory infiltrate | < 0.001 | |||||
| Absent/mild | 8 (26.7) | 35 (60.3) | 131 (73.2) | 17 (85) | ||
| moderate/intense | 22 (73.3) | 23 (39.7) | 48 (26.8) | 3 (15) | ||
| pTNM stage | 0.014 | |||||
| I/II | 18 (60) | 40 (69) | 93 (52) | 6 (30) | ||
| III/IV | 12 (40) | 18 (31) | 86 (48) | 14 (70) | ||
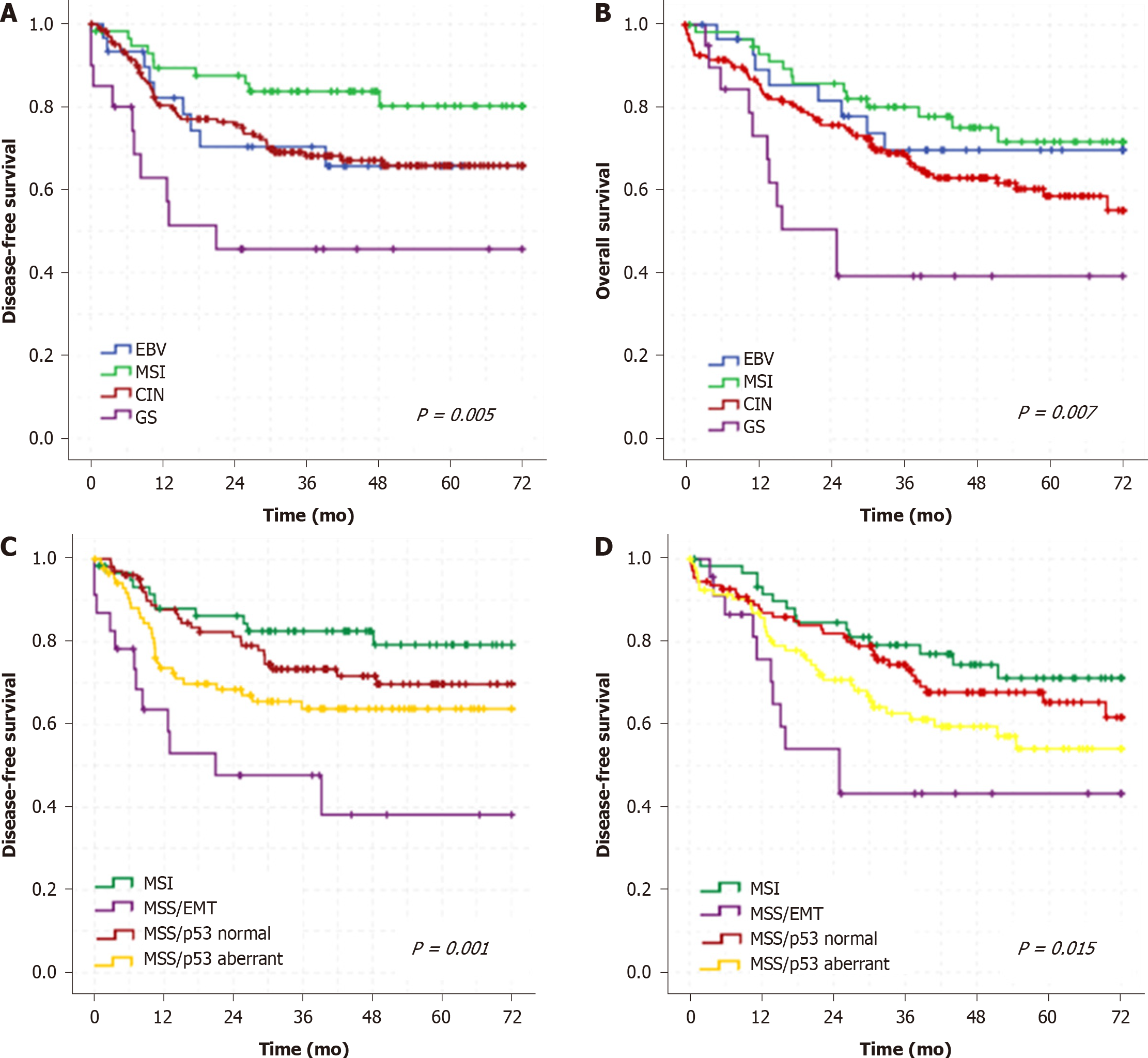
In a mean follow-up period of 37.9 mo, there were 79 recurrences and 95 deaths. GS and MSS/EMT subtype showed worse DFS and OS than the other subtypes. Conversely, MSI subtype had better survival in both classifications (Figure 4).
In multivariate analysis, type of gastrectomy, category pT, and the TCGA Classification subtypes were independent factors associated to DFS and OS (Table 2). Category pN were associated only to DFS. Regarding ACRG classification, using the EMT subtype as reference, only the MSI/p53 normal had a significant better DFS and OS (Supplementary Table 3).
| Disease-free survival | Univariate | Multivariate | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Male (vs female) | 0.93 | 0.59-1.46 | 0.745 | ─ | ─ | ─ |
| Age > 65 (vs < 65 yr) | 1.52 | 0.95-2.45 | 0.082 | ─ | ─ | ─ |
| Charlson < 5 (vs ≥ 5) | 0.65 | 0.40-1.05 | 0.076 | ─ | ─ | ─ |
| Total Gastrectomy (vs subtotal) | 2.33 | 1.49-3.62 | < 0.001 | 1.8 | 1.12-2.88 | 0.015 |
| Diffuse/mixed (vs others) | 1.80 | 1.15-2.83 | 0.011 | 1.24 | 0.78-1.97 | 0.367 |
| pT3/pT4 status (vs pT1/pT2) | 10.87 | 4.72-24.98 | < 0.001 | 5.64 | 2.33-13.64 | < 0.001 |
| pN+ (vs pN0) | 7.56 | 3.77-15.15 | < 0.001 | 3.25 | 1.54-6.80 | 0.002 |
| CMT (vs none) | 1.28 | 0.82-1.98 | 0.280 | ─ | ─ | ─ |
| TCGA Classification | ||||||
| GS | 1 | ─ | ─ | ─ | ─ | ─ |
| EBV | 0.42 | 0.17-1.06 | 0.068 | 0.37 | 0.14-0.94 | 0.037 |
| MSI | 0.21 | 0.09-0.52 | 0.001 | 0.31 | 0.13-0.76 | 0.010 |
| CIN | 0.43 | 0.21-0.84 | 0.014 | 0.43 | 0.22-0.87 | 0.018 |
| Overall survival | Univariate | Multivariate | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Male (vs female) | 1.08 | 0.72-1.63 | 0.708 | ─ | ─ | ─ |
| Age > 65 (vs < 65 yr) | 1.16 | 0.77-1.74 | 0.483 | ─ | ─ | ─ |
| Charlson < 5 (vs ≥ 5) | 1.16 | 0.84-1.88 | 0.273 | ─ | ─ | ─ |
| Total Gastrectomy (vs subtotal) | 1.90 | 1.27-2.84 | 0.002 | 1.7 | 1.11-2.60 | 0.014 |
| Diffuse/mixed (vs others) | 1.56 | 1.04-2.34 | 0.032 | 1.16 | 0.77-1.77 | 0.765 |
| pT3/pT4 status (vs pT1/pT2) | 4.50 | 2.59-7.82 | < 0.001 | 3.23 | 1.74-5.97 | < 0.001 |
| pN+ (vs pN0) | 3.29 | 2.02-5.35 | < 0.001 | 1.69 | 0.98-2.92 | 0.061 |
| CMT (vs none) | 0.96 | 0.64-1.44 | 0.841 | ─ | ─ | ─ |
| TCGA Classification | ||||||
| GS | 1 | ─ | ─ | ─ | ─ | ─ |
| EBV | 0.33 | 0.13-0.82 | 0.017 | 0.27 | 0.10-0.68 | 0.006 |
| MSI | 0.27 | 0.12-0.61 | 0.001 | 0.35 | 0.15-0.78 | 0.01 |
| CIN | 0.46 | 0.24-0.88 | 0.019 | 0.47 | 0.25-0.91 | 0.025 |
The results of this study demonstrated that IHC and ISH techniques represent a feasible strategy to reproduce the GC molecular classification and can be applied in the daily diagnostic routine. We show that through the evaluation of the four main profiles (EVB, MSI, E-cadherin and p53), it was possible to define subtypes of tumors with distinct clinicopathological characteristics, similar to the findings of previous molecular classifications. Furthermore, we demonstrate the relationship between both TCGA and ACRG subtypes with long term survival submitted to curative D2 resection.
Considering the subtypes determined by both classification systems, EBV associated tumors represent the major point of disagreement between TCGA and ACRG. In the TCGA Classification, it is the first group to be discriminated[5,7]. Instead, ACRG Classification does not contemplate EBV GC as a subtype separately[6]. Importantly, EBV positive GC has been frequently associated to high programmed death-ligand 1 (PD-L1) expression. Since this subtype is a potential candidate for immunotherapy[10,14], it becomes clinically interesting to maintain EBV positive tumors as a distinct subtype. Proximal location, larger diameter lesions, and predominance of Laurén's undetermined and poorly differentiated tumors are also features commonly associated with positive EBV tumors, and also found in our study. We also observed that there was a significantly higher frequency of total gastrectomy in this subtype, reinforcing its proximal predilection and larger dimensions. In relation to morphological aspects, the EBV subtype exhibited a predominance of intense inflammatory infiltrate, defined as gastric “lymphoepithelioma-like” carcinoma, which have a solid pattern and are classified as poorly differentiated[15].
Conversely, MSI status represents a profile considered in both classifications. MMR proteins assessment by immunoexpression is a well-established and efficient technique to determine MSI profile. It is commonly used in pathological routine evaluation for colorectal tumors, since The American College of Gastroenterology recommends screening for DNA dMMR in all newly diagnosed cases of colorectal cancer[16]. Compared to polymerase chain reaction-based analysis, IHC demonstrated sensitivity of 91.1% and specificity of 98.5% in the detection of MSI phenotype in GC[17].
GCs with MSI have been associated to some particular characteristic, including advanced age, intestinal histological type, location in the middle and distal third, and less advanced stages[18]. Similar to literature, we found that patients were older and consequently presented more comorbidity according to CCI. Tumors in our cohort were more frequently localized in the distal third of the stomach and more likely to undergo subtotal resections, with predominance of the Laurén intestinal type. MSI GC was the subtype that presented better survival rates. The reason why tumors associated to MSI usually have a better prognosis is not yet fully elucidated. It is believed that in these patients the lymphocytic peritumoral infiltrate, in response to peptides generated by MSI tumors, plays an important role in the anti-tumor response by inducing tumor cell apoptosis through cytokine stimulation[19]. The best prognosis can also be attributed to some characteristics related to these tumors, as the lower LN involvement, which is a hallmark of this subtype. Even in cases with pN+ status, metastases usually affect a smaller number of LNs and stations, besides being located closer to the primary tumor[20].
An additional, noteworthy aspect is related to the efficacy of fluopyrimidine/platin-based CMT in locally advanced GC with MSI. The CMT in these patients did not provide survival benefit[16,21]. Also, MSI GC patients who received perioperative CMT were associated with worse OS, further suggesting a deleterious effect with the addition of treatment in this subgroup of tumors. On the other hand, the immunotherapy for MSI-associated tumors has been recently approved, regardless of tumor site, and has shown a positive impact on the survival of these patients. Thus, the availability of the evaluation in the current diagnostic practice, as well as its impact on the CMT regime, makes the identification of the MSI subtype the main candidate for incorporation into the routine evaluation.
On the other hand, define the aberrant expression of p53 by IHC is a greater challenge. Different criteria have already been employed[22]. Nuclear localization is essential for p53 activity, and its nuclear accumulation may result from increased regulation of wild-type expression or decreased degradation in response to cellular stresses, including DNA damage. The presence of wild-type protein expression is a normal physiological response to decrease the G1 cell cycle and allow the repair of damaged DNA. Therefore, low levels of the wild-type p53 protein can be detected in the nucleus by IHC. In contrast, mutations in TP53 result in the production of proteins with a prolonged half-life that accumulates in the nucleus, causing its overexpression. Conversely, some missense or point mutations can result in a truncated protein that is not stable enough to cause any detectable nuclear accumulation. Thus, the aberrant expression of p53 can be defined either by the absence or exacerbated nuclear expre
Finally, the subtype of GC associated with E-cadherin aberrant expression showed poorly differentiated histology, predominance of diffuse tumors, LN metastasis at more advanced stages, and worse survival[6,9]. E-cadherin is an essential adhesion molecule for the consolidation of epithelial architecture, maintenance of cell polarity, and differentiation during fetal development and in adult life. It acts as a broad-acting tumor suppressor. It is suggested that loss of E-cadherin expression is the phenotypic expression of CDH1 and RHOA mutations[23]. IHC expression of E-cadherin is evaluated by the presence of continuous and linear membrane staining at the boun
It is important to emphasize that the association of E-cadherin expression and alterations in the CDH1 gene is not yet clearly defined. Only a minority of GC cases actually have a mutation in the CDH1 gene. Somatic mutation has been reported in about 3% to 50% of GC with diffuse histology[25]. It is estimated that 70% of patients with absence of E-cadherin expression do not have a CDH1 mutation. This fact su
In summary, the analysis through IHC and ISH techniques has proven to be feasible, but is also subject to technical limitations. It involves the use of different antibodies and probes, tissue preparation, and evaluation criteria. In addition, TMA technique allows only the representation of some areas of the tumor, which may lead to a variation in results considering the tumor heterogeneity[28]. One of the main aspects that must be considered is that the same tumor may present alterations that correspond to two or more profiles[21,29]. Thus, the definition of which subtype belongs to the patient with more than one profile will depend on the chosen molecular classification flowchart.
As we only analyze the four main profiles related to the subtypes of GC, some cases may be misclassified, since GCs may have other alteration in markers not evaluated, such as RHOA, related to GS subtype, and tyrosine kinase receptor, related to CIN subtype. Also, limitations in relation to the technique and immunohistochemical markers may lead to the misclassification some GC in the subtypes, particularly in terms of the heterogeneous staining pattern. Taking into account the importance and the usefulness of IHC markers, validating those already identified is necessary to integrate surgical and molecular pathology with clinical medicine. Another limitation of the classification based on protein expression is the impossibility to determine if the change in expression pattern is due to epigenetic factors (such as gene silencing, frequent in MLH1, for example) or genetic factors. Furthermore, we cannot define if a genetic alteration is somatic or germinative, an important issue in the context of tumors with aberrant E-cadherin, which may be related to HDGC.
As strengths, in the present study we used data collected prospectively from a database research project, minimizing the possibility of classification bias of the variables. In order to make the studied population more homogeneous in relation to surgical treatment and to reduce selection bias, all consecutive patients submitted to resection with curative intent and D2 Lymphadenectomy were included in the selected period, which provided a better prognostic prediction. Accordingly, the results obtained in this study add evidence to the literature that the effectiveness of both ISH and IHC staining to define GC subtypes will allow the adoption of a more individualized therapeutic approach[30]. The ToGA trial represented a major milestone in this regard, after demonstrating improved survival of GC HER2-positive treated with Trastuzumab with palliative intent[31]. Recently, Pembrolizumab was approved by the FDA for patients with unresectable, metastatic, or locally recurrent GC with MSI profile and/or PD-L1 positive[32-34]. Certainly, new targeted therapy drugs for specific tumoral markers and immune checkpoints will emerge in the near future, keeping GC molecular classifications as a major issue of interest and investigation.
The IHC/ISH analysis was able to distinguish immunophenotypic groups of GC with distinct clinicopathological characteristics and prognosis, resembling the subtypes of the molecular classifications. TCGA subtypes had different outcomes, where MSI subtype was associated with a better prognosis and GS had worse survival outcomes. Accordingly, this method of classification may allow an easier definition of GC subtypes in daily clinical practice, contributing to the therapeutic planning, driving target therapy and individualized treatment.
Molecular classification of gastric cancer (GC) emerged as a promising option to define therapeutic strategies and prognostic subgroups. However, the costs and technical complexity of molecular methodologies remains an obstacle to its adoption. Thus, their clinical significance by other approaches needs further evidence, especially in the western GC.
Although molecular classifications as proposed by The Cancer Genome Atlas (TCGA) Research Network Group and Asian Cancer Research Group (ACRG) subtypes provided a guide for patient stratification and trials of targeted therapy, the clinical utility of these classifications is still limited due to the technical complexity, as indicated by the fact there is little evidence of its application in a real-world setting.
The purpose of this study was to determine the subgroups of molecular classification by immunohistochemistry (IHC) and in situ hybridization (ISH), and to evaluate its characteristics and impact on long-term survival in a Western cohort of GC patients treated with curative intent.
We retrospectively evaluated GC patients who underwent D2-gastrectomy between 2009 and 2016 by tissue microarray. Cases were assessed for microsatellite instability (MSI) status, E-cadherin and p53 expression by IHC and for Epstein-Barr virus (EBV) by ISH. Clinicopathological characteristics and survival of GC were evaluated according to TCGA and ACRG classification system.
A total of 287 GC patients were included. Based on IHC and ISH analysis, 5 profiles were defined as follows: E-cadherin aberrant (9.1%), MSI (20.9%), p53 aberrant (36.6%), EBV positivity (10.5%), and p53 normal (31%), which corresponded to tumors that showed no alteration in other profiles. Flowchart according to the TCGA and ACRG classifications were used to define the subtypes, where clinical and pathological characteristics associated with GC subtypes were evidenced. In both classifications, the MSI group had better survival. While the subtype represented by the loss of e-cadherin expression (subtype GS and microsatellite stable/epithelial to mesenchymal transition) was related to a worse prognosis. The classification proposed by the TCGA was an independent factor associated with survival in patients with GC.
The IHC/ISH analysis was able to distinguish immunophenotypic groups of GC with distinct characteristics and prognosis, and is a viable option for use in a clinical setting.
The identification of gastric adenocarcinoma subtypes through techniques available in clinical practice and the development of integrated approaches for diagnostic applications, such as prognosis prediction or response to therapy intervening, emerge as an essential phase toward personalized medicine in GC treatment.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang YF S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55817] [Article Influence: 7973.9] [Reference Citation Analysis (132)] |
| 2. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. IARC. 2010;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 4. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4323] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4850] [Article Influence: 440.9] [Reference Citation Analysis (2)] |
| 6. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1579] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 7. | Ahn S, Lee SJ, Kim Y, Kim A, Shin N, Choi KU, Lee CH, Huh GY, Kim KM, Setia N, Lauwers GY, Park DY. High-throughput Protein and mRNA Expression-based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications. Am J Surg Pathol. 2017;41:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Birkman EM, Mansuri N, Kurki S, Ålgars A, Lintunen M, Ristamäki R, Sundström J, Carpén O. Gastric cancer: immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch. 2018;472:369-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Setia N, Agoston AT, Han HS, Mullen JT, Duda DG, Clark JW, Deshpande V, Mino-Kenudson M, Srivastava A, Lennerz JK, Hong TS, Kwak EL, Lauwers GY. A protein and mRNA expression-based classification of gastric cancer. Mod Pathol. 2016;29:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Pereira MA, Ramos MFKP, Faraj SF, Dias AR, Yagi OK, Zilberstein B, Cecconello I, Alves VAF, de Mello ES, Ribeiro U Jr. Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. J Surg Oncol. 2018;117:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Ajani JA, In H, Sano T, Gaspar LE, Erasmus JJ, Tang LH, Washington MK, Gerdes H. American Joint Committee on Cancer (AJCC). Springer, 2017: 203. [DOI] [Full Text] |
| 12. | Carpenter PM, Al-Kuran RA, Theuer CP. Paranuaclear E-cadherin in gastric adenocarcinoma. Am J Clin Pathol. 2002;118:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A. TP53 and gastric carcinoma: a review. Hum Mutat. 2003;21:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Almhanna K, Antonia S. PD-L1 Antibodies for EBV-Positive Gastric Cancer, Going Beyond PD-L1 Expression and Microsatellite Instability. J Natl Cancer Inst. 2018;110:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Cheng N, Hui DY, Liu Y, Zhang NN, Jiang Y, Han J, Li HG, Ding YG, Du H, Chen JN, Shao CK. Is gastric lymphoepithelioma-like carcinoma a special subtype of EBV-associated gastric carcinoma? Gastric Cancer. 2015;18:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, Fassan M, Rugge M, Valeri N, Okines A, Hewish M, Allum W, Stenning S, Nankivell M, Langley R, Cunningham D. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017;3:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 391] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 17. | Bae YS, Kim H, Noh SH. Usefulness of Immunohistochemistry for Microsatellite Instability Screening in Gastric Cancer. Gut Liver. 2015;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, Tan P, Roviello F. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 19. | Mathiak M, Warneke VS, Behrens HM, Haag J, Böger C, Krüger S, Röcken C. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl Immunohistochem Mol Morphol. 2017;25:12-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Roviello F, Polom K, D’Ignazio A, Pascale V, Marrelli D. Potential impact of molecular classification on tailored lymphadenectomy for gastric cancer. Eur J Cancer. 2018;S8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Shin SJ, Beom SH, Jung M, Choi YY, Son T, Kim HI, Cheong JH, Hyung WJ, Noh SH, Chung H, Park JC, Shin SK, Lee SK, Lee YC, Koom WS, Lim JS, Chung HC, Rha SY. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapy. Oncotarget. 2016;44608. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Ando K, Oki E, Saeki H, Yan Z, Tsuda Y, Hidaka G, Kasagi Y, Otsu H, Kawano H, Kitao H, Morita M, Maehara Y. Discrimination of p53 immunohistochemistry-positive tumors by its staining pattern in gastric cancer. Cancer Med. 2015;4:75-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Carneiro P, Fernandes MS, Figueiredo J, Caldeira J, Carvalho J, Pinheiro H, Leite M, Melo S, Oliveira P, Simões-Correia J, Oliveira MJ, Carneiro F, Figueiredo C, Paredes J, Oliveira C, Seruca R. E-cadherin dysfunction in gastric cancer--cellular consequences, clinical applications and open questions. FEBS Lett. 2012;586:2981-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Li T, Chen J, Liu QL, Huo ZH, Wang ZW. Meta-analysis: E-cadherin immunoexpression as a potential prognosis biomarker related to gastric cancer metastasis in Asian patients. Eur Rev Med Pharmacol Sci. 2014;18:2693-2703. [PubMed] |
| 25. | Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, Roviello F, Oliveira C. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Carvalho J, van Grieken NC, Pereira PM, Sousa S, Tijssen M, Buffart TE, Diosdado B, Grabsch H, Santos MA, Meijer G, Seruca R, Carvalho B, Oliveira C. Lack of microRNA-101 causes E-cadherin functional deregulation through EZH2 up-regulation in intestinal gastric cancer. J Pathol. 2012;228:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Park JW, Jang SH, Park DM, Lim NJ, Deng C, Kim DY, Green JE, Kim HK. Cooperativity of E-cadherin and Smad4 Loss to promote diffuse-type gastric adenocarcinoma and metastasis. Mol Cancer Res. 2014;12:1088-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ilyas M, Grabsch H, Ellis IO, Womack C, Brown R, Berney D, Fennell D, Salto-Tellez M, Jenkins M, Landberg G, Byers R, Treanor D, Harrison D, Green AR, Ball G, Hamilton P; National Cancer Research Institute (UK) Biomarker and Imaging Clinical Studies Group. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology. 2013;62:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Gonzalez RS, Messing S, Tu X, McMahon LA, Whitney-Miller CL. Immunohistochemistry as a surrogate for molecular subtyping of gastric adenocarcinoma. Hum Pathol. 2016;56:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Chen T, Xu XY, Zhou PH. Emerging molecular classifications and therapeutic implications for gastric cancer. Chin J Cancer. 2016;35:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5322] [Article Influence: 354.8] [Reference Citation Analysis (3)] |
| 32. | Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist. 2019;24:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 33. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1573] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 34. | Mehta R, Shah A, Almhanna K. Pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction cancer: an evidence-based review of place in therapy. Onco Targets Ther. 2018;11:6525-6537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |