Published online Dec 24, 2020. doi: 10.5306/wjco.v11.i12.1076
Peer-review started: August 21, 2020
First decision: September 13, 2020
Revised: September 27, 2020
Accepted: October 15, 2020
Article in press: October 15, 2020
Published online: December 24, 2020
Processing time: 119 Days and 6.8 Hours
To investigate the clinicopathological features of endometrial clear cell carcinoma that has invaded the right oviduct with a cooccurring ipsilateral oviduct adenomatoid tumor.
A case of endometrial clear cell carcinoma invading the right oviduct with a cooccurring ipsilateral oviduct adenomatoid tumor was collected and analyzed using pathomorphology and immunohistochemistry. Endometrial clear cell carcinoma cells were distributed in a solid nest, papillary, shoe nail-like, and glandular tube-like distribution. There was infiltrative growth, and tumor cells had clear cytoplasm and obvious nuclear heteromorphism. The cancer tissue was necrotic and mitotic. The cancer tissue invaded the right oviduct. The ipsilateral oviduct also had an adenomatoid tumor. The adenomatoid tumor was arranged in microcapsules lined with flat or cubic cells that were surrounded by smooth muscle tissue. The adenomatoid tumor cells were round in shape.
Clear cell carcinoma of the endometrium can invade the oviduct and occur simultaneously with tubal adenomatoid tumors. Upon pathological diagnosis, one should pay close attention to distinguishing whether an endometrial clear cell carcinoma is invading the oviduct or whether it is accompanied by an adenomatoid tumor of the oviduct. Immunohistochemistry is helpful to differentiate these two disease entities. Endometrial clear cell carcinomas express Napsin-A and P16 and are negative for estrogen receptor and progesterone receptor. The presence of endometrial clear cell carcinoma does not affect the expression of CK and calretinin in adenomatoid tumors.
Core Tip: In pathological diagnosis, when investigating invasion of the oviduct, one should pay close attention to distinguishing whether the endometrial clear cell carcinoma invades the oviduct or whether it is accompanied by an oviduct adenomatoid tumor. The significance of this investigation is to determine whether the endometrial adenocarcinoma has tubal metastasis. This is critical for the confirmation of T3a in tumor-node-metastasis staging.
- Citation: Hu ZX, Tan MH, Li QZ, Xu JL, Chen W, Xie ZH, Zhou YJ, Liang Q, An JH, Shen H. Endometrial clear cell carcinoma invading the right oviduct with a cooccurring ipsilateral oviduct adenomatoid tumor: A case report. World J Clin Oncol 2020; 11(12): 1076-1083
- URL: https://www.wjgnet.com/2218-4333/full/v11/i12/1076.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i12.1076
Clear cell carcinoma of the endometrium is a special pathological type of endometrial carcinoma which accounts for only 3.5% of all endometrial carcinomas[1]. Clear cell carcinoma of the endometrium is highly invasive and is associated with a poor prognosis. Adenomatoid tumors are rare benign tumors that mainly occur in the reproductive systems of both men and women[2]. In women they are common in the uterus and rarely occur in the oviduct. The coexistence of endometrial clear cell carcinoma and oviduct adenomatoid tumor is even rarer. Here we present a case of endometrial clear cell carcinoma invading the right oviduct with a cooccurring ipsilateral adenomatoid tumor which was collected at The First People’s Hospital of Zhaoqing. Pathological morphology and immunohistochemical analysis were performed to provide reference for clinical pathological diagnosis and differential diagnosis.
The patient presented with irregular vaginal bleeding for 2 years and vaginal discharge for 1 year. Additionally, her menstrual period was prolonged and disordered, and her menstrual volume was large. Occasionally during menstruation there were blood clots and flocculent tissues.
In April 2020, curettage was performed in another hospital and the pathology report of the resected tissue suggested endometrial carcinoma of the uterine cavity. The tumor was predicted to be a type ΙΙ endometrial carcinoma and surgical treatment was recommended.
For further diagnosis and treatment, the patient was admitted to The First People’s Hospital of Zhaoqing in June 2020. The patient underwent bilateral tubal ligation in 2004.
A gynecological examination concluded that the vulva was normal, the vagina was unobstructed, there was a low volume of thin secretions, the cervix was slightly inflamed, there was no hypertrophy of the cervix and no lifting pain or coloring, the uterine body was in the posterior position, the uterus was enlarged and tough to the equivalent of 2 mo of pregnancy, the activity of cervix was general, there was no tenderness in the abdomen, and no mass was palpable in bilateral appendages.
Routine blood tests showed a low hemoglobin level at 69 g/L and high levels of tumor associated antigens CA125 and CA15-3, which were 76.2 U/mL and 28.28 U/mL, respectively.
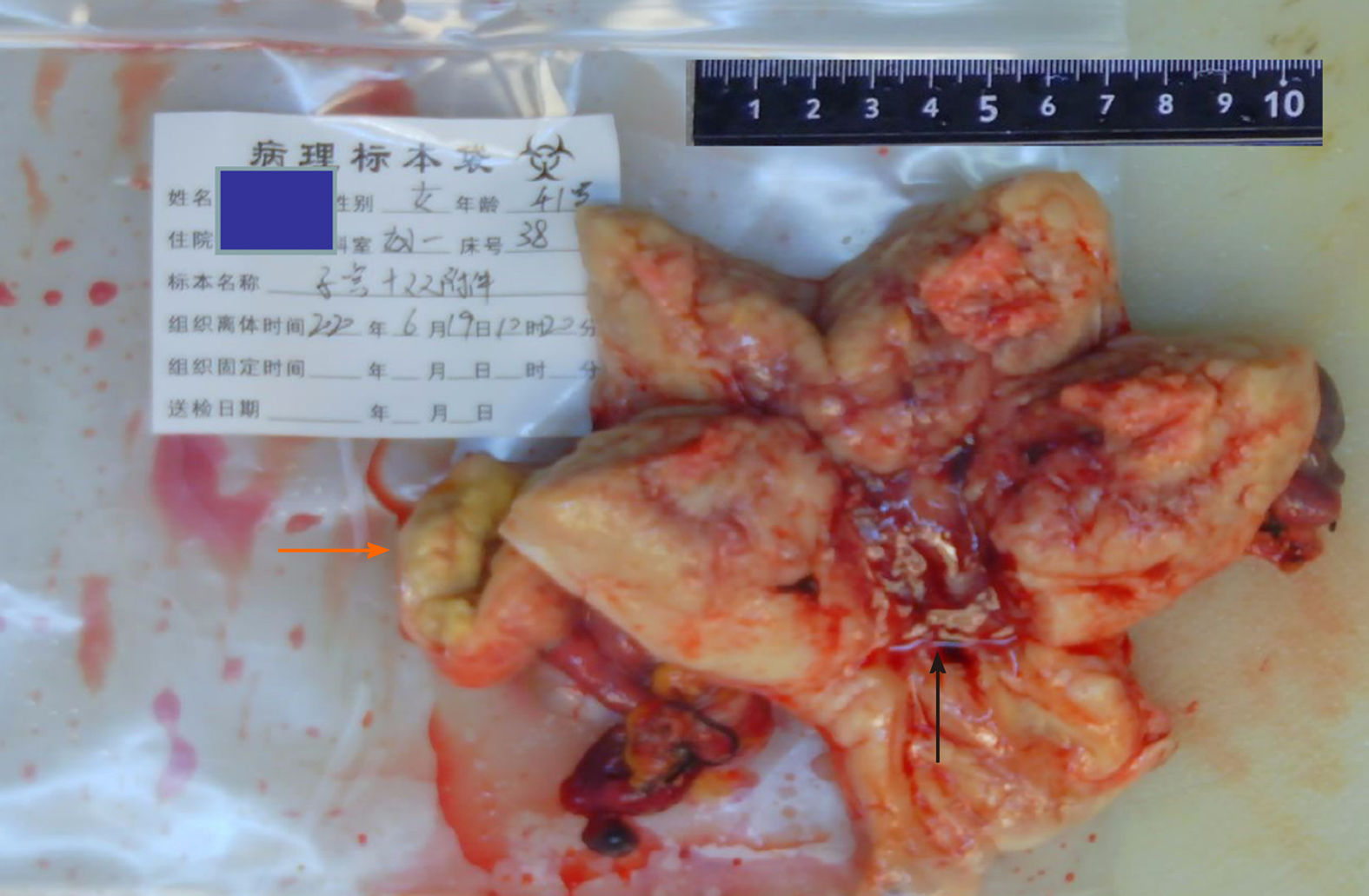
Gynecological color Doppler ultrasound showed solid lesions in the uterus and mixed lesions between the right ovary and right uterine horn. The lesions were approximately 44 mm × 27 mm in size, had an unclear boundary with the right ovary, had an uneven echo distribution, and had no change in posterior echo. The patient was treated by total abdominal hysterectomy, bilateral adnexectomy, pelvic lymph node dissection, and pelvic adhesion lysis (Figure 1).
There were three main pathological diagnostic observations: (1) The cancerous tissue was a mixed endometrial adenocarcinoma. The cancer tissue infiltrated the deep muscle layer of the uterus, and invaded the right oviduct and the ipsilateral oviduct. The mixed endometrial adenocarcinoma was accompanied by an adenomatous tumor; (2) No tumors were found in the cervix, left oviduct, or bilateral ovaries; and (3) No cancer metastasis was found in bilateral pelvic lymph nodes.
The patient presented with irregular vaginal bleeding, excessive vaginal discharge, moderate anemia, and increased tumor associated antigen expression. A B-mode ultrasound scan revealed solid intrauterine lesions and mixed lesions between the right ovary and the right cornu uteri. The pathological investigation of the tumor biopsy confirmed the presence of uterine cavity type II endometrial carcinoma and indicated that surgical intervention was required.
The patient recovered well after operation, and no complications occurred during the follow-up of 3 mo.
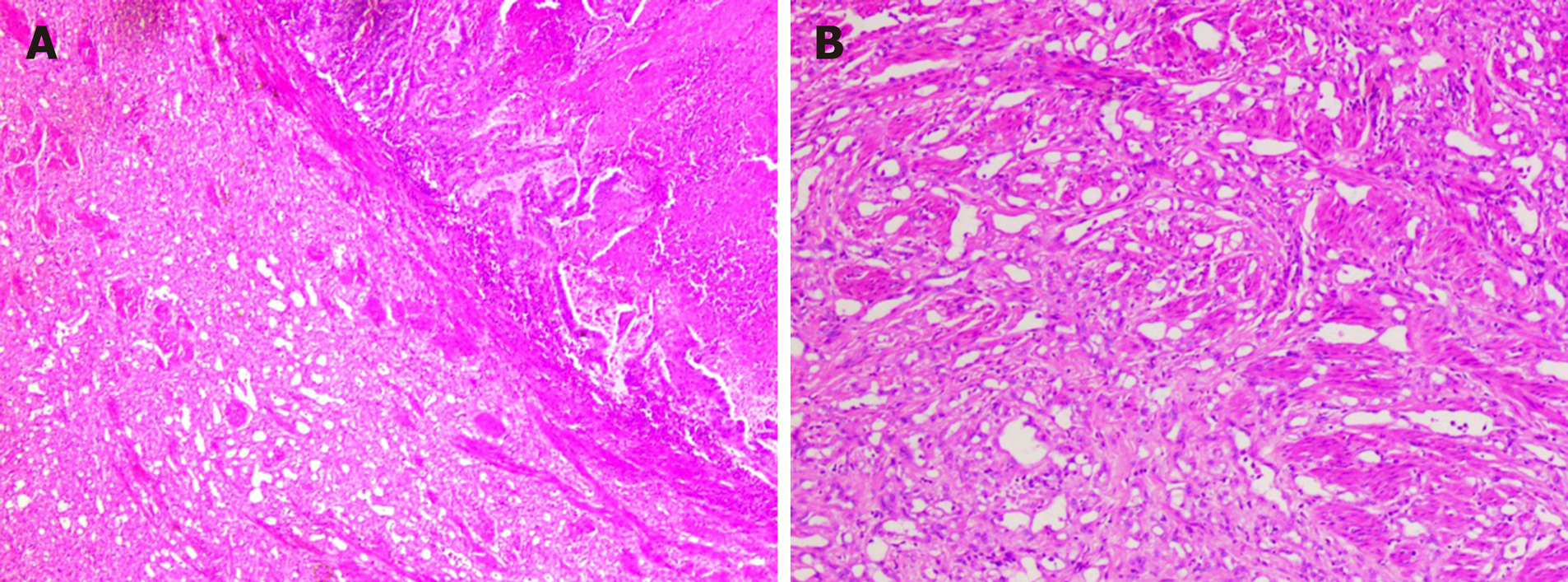
Endometrial clear cell carcinoma is a rare malignant tumor. It is a type II endometrial cancer that is highly invasive and prone to metastasis, has high recurrence and mortality rates, and has a poor prognosis[3,4]. The main clinical symptom is irregular vaginal bleeding. This patient presented with irregular vaginal bleeding and discharge. Investigative blood tests showed moderate anemia and elevated tumor-associated antigens CA125 and CA15-3. B-mode ultrasound showed multiple lesions consistent with malignant endometrial tumors which had pathological abnormalities. This was confirmed by immunohistochemical staining. Cancer tissue invaded the right oviduct, indicating that metastasis has occurred. A high proportion of tumor cells expressed Ki-67, indicating that the tumor had a high proliferation index and that the tumor was growing fast. In this case, the morphology of the endometrial clear cell carcinoma was complex. We report nest-like, solid, papillary, shoe nail-like and glandular tube-like infiltration into the deep myometrium. The cytoplasm of the cancer cells was clear or eosinophilic and the nucleoli were obvious. Necrosis and mitosis were also obvious. Mucus secretion was observed in some glandular cavities. We also observed interstitial fiber hyperplasia that is consistent with the histopathological morphology of endometrial clear cell carcinoma reported in the literature[5] (Figure 2).
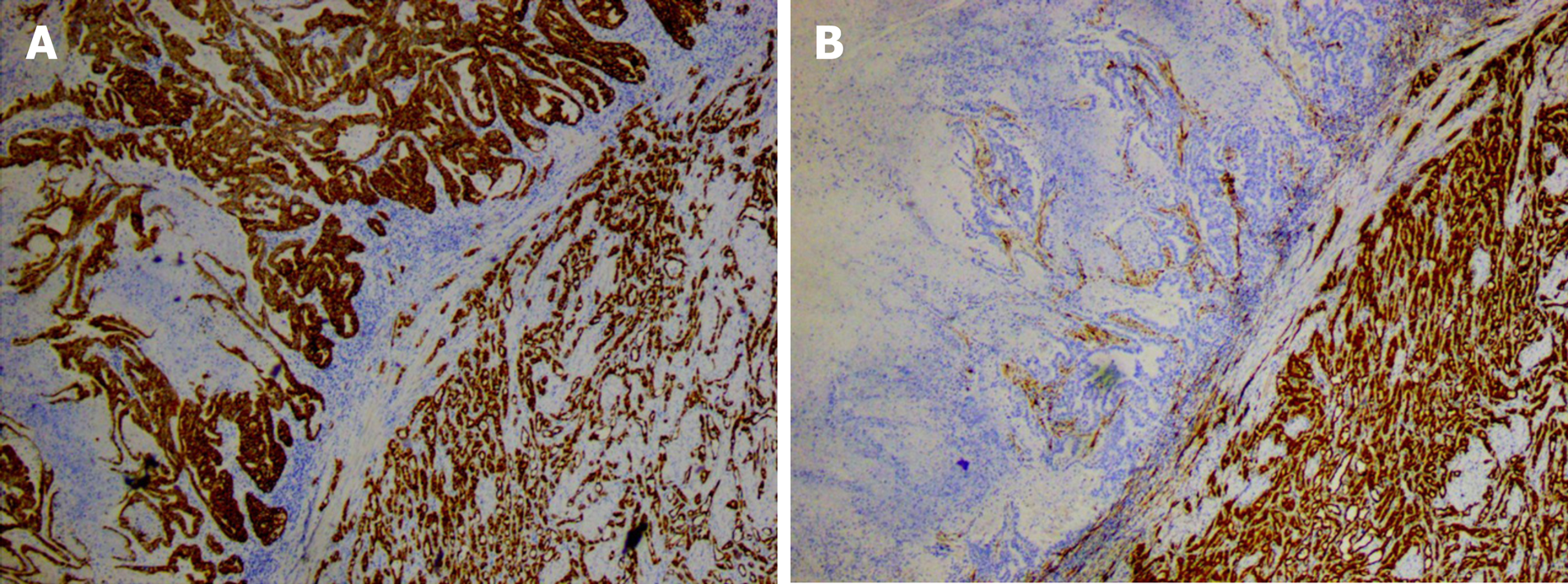
In order to make informed decisions on the best future therapeutic regimes, the tumor tissue needs to be differentiated from endometrioid carcinoma, serous carcinoma, and metastatic clear cell renal cell carcinoma (ccRCC). Here we identified a type II endometrioid carcinoma. Type II endometrioid carcinoma is often histologically tubular and cribriform, whereas papillary histology is rare. In this case, stromal cells were absent and the tumor was surrounded by complex hyperplasia of the endometrium which lacked the characteristic transparent cytoplasm of clear cell carcinoma. Occasionally in endometrial carcinoma, subnuclear vacuoles similar to secretory endometrium may appear. These express CK, CK7, vimentin, ER, PR, and P16, but do not express the endometrial marker Napsin A. Here we report an endometrial carcinoma with the typical morphology of clear cell carcinoma. This case expresses CK, CK7, P16, and Napsin A, but does not express vimentin, ER, or PR. This confirms the presence of endometrial clear cell carcinoma[6]. Ping et al[7] and others have shown that there are three expression patterns of Napsin A in endometrial clear cell carcinoma: Punctate, focal, and diffuse. In this case, we observed punctate expression of Napsin A, which supports the diagnosis of clear cell carcinoma. ER and PR are robust differential diagnostic markers which distinguish between clear cell carcinoma and endometrioid carcinoma. The lack of ER and PR expression in this case excluded the possibility of endometrioid carcinoma. Microscopically, serous carcinoma typically forms a nipple-like structure but can also be arranged in glandular tube or solid structures. The nipple has three forms: A large and wide nipple, small nipples clustered around a large nipple, or an intraductal nipple. Intraductal nipple is characterized by obvious atypia of tumor cells, high nuclear grade, serous cytoplasm, and eosinophilic cytoplasm, and some may have sand bodies. Additionally, immunohistochemical staining indicates that serous carcinoma is strongly positive for p53 and P16[8] (Figure 3).
Serous carcinoma was ruled out in this patient due to transparent cytoplasm of the tumor cells, morphological characteristics that did not resemble serous carcinoma, normal P53 expression, mottled positive expression of P16, and Napsin A positive cells. Additionally, there was an absence of Wilms’ tumor 1 (WT-1), a tumor suppressor gene that is widely expressed in ovarian serous carcinoma and less than 20%-30% in endometrial serous carcinomas[9]. The lack of WT-1 expression in this case further eliminates the diagnosis of serous carcinoma.
Metastatic ccRCC is associated with similar symptoms to a renal tumor, such as a hematuria. Imaging examination of ccRCC can find renal space-occupying lesions. Common metastatic sites are the lung, bone, liver, and brain. Morphologically, in addition to clear cells, blood sinus-like structures can also be observed. ccRCC tumors express CD10 and vimentin and have a low Ki-67 index.
In this patient, vimentin was not expressed whereas Ki-67 was highly expressed; we therefore excluded metastatic ccRCC. Furthermore, CA125 was localized to the margin of the cavity, which supports the diagnosis of a primary uterine tumor. However, the possibility of a mixed endometrial carcinoma could not be ruled out.
Adenomatous tumors are benign tumors that are exclusive to the reproductive system. They can occur in the uterus, oviducts, ovaries, and ovarian crowns[10]. It is now clear that adenomatoid tumors originate from mesothelial cells[11,12]. The pathological diagnosis of an adenomatoid tumor is mainly based on the characteristics of tumor cells, such as lacunar, adenoid, vascular, or cystic structure, intraluminal mucus, anaplastic and mitotic features, lined with flat or cubic cells. Immunohistochemical staining of adenomatous tumors shows strong expression of CK and calretinin. To ensure the best clinical management, it is important that they are differentiated from angioleiomyoma, lymphangioma, leiomyolipoma, adenocarcinoma, and signet ring cell carcinoma[13].
In this patient, the adenomatous tumor occurred in the oviduct with a typical morphology. The immunohistochemical staining for CK and calretinin showed strong positive expression, which supported the diagnosis of an adenomatoid tumor (Figure 4). This patient underwent a tubal ligation in 2004. Whether this procedure potentially induced the development of the adenomatoid tumor needs to be further investigated. An adenomatoid tumor and endometrial clear cell carcinoma coexisted in this case (Figure 5). There was a clear boundary between the adenomatoid tumor and endometrial clear cell carcinoma. There was no clear cell carcinoma component within the adenomatoid tumor, indicating that it was more difficult to invade than normal uterine smooth muscle tissue. The expression of CK and calretinin in cancer tissues suggested that the two tumors had different origins and were not the same tumor, however further investigation is needed to confirm this.
In summary, we have shown that endometrial clear cell carcinoma, a tumor that can invade the oviduct, can cooccur with oviduct adenomatoid tumors. Endometrial clear cell carcinoma is Napsin A and P16 positive, and ER and PR negative. Furthermore, the presence of endometrial clear cell carcinoma does not affect the expression of CK and calretinin in an adenomatoid tumor. In pathological diagnosis, when investigating invasion of the oviduct, one should pay close attention to distinguishing whether the endometrial clear cell carcinoma invades the oviduct or whether it is accompanied by an oviduct adenomatoid tumor. The significance of this investigation is to determine whether the endometrial adenocarcinoma has tubal metastasis. This is critical for the confirmation of T3a in tumor-node-metastasis staging.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Viswanath Y S-Editor: Huang P L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Creasman WT, Ali S, Mutch DG, Zaino RJ, Powell MA, Mannel RS, Backes FJ, DiSilvestro PA, Argenta PA, Pearl ML, Lele SB, Guntupalli SR, Waggoner S, Spirtos N, Boggess JF, Edwards RP, Filiaci VL, Miller DS. Surgical-pathological findings in type 1 and 2 endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol. 2017;145:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Schwartz EJ, Longacre TA. Adenomatoid tumors of the female and male genital tracts express WT1. Int J Gynecol Pathol. 2004;23:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Nieto K, Adams W, Pham N, Block AM, Grover S, Small W Jr, Harkenrider MM. Adjuvant therapy in patients with clear cell endometrial carcinoma: An analysis of the National Cancer Database. Gynecol Oncol. 2018;148:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Alldredge JK, Eskander RN. EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers. Gynecol Oncol Res Pract. 2017;4:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Gu Y, Hao YJ, Qian N, Yan PP, Wu WF, Tang YF. Uterine clear cell carcinoma: a clinicopathologic analysis of 33 cases. Linchuang Yu Shiyan Binglixue Zazhi. 2018;34:1331-1334. [DOI] [Full Text] |
| 6. | Lim D, Ip PP, Cheung AN, Kiyokawa T, Oliva E. Immunohistochemical Comparison of Ovarian and Uterine Endometrioid Carcinoma, Endometrioid Carcinoma With Clear Cell Change, and Clear Cell Carcinoma. Am J Surg Pathol. 2015;39:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Ping Q, Gao BL. The expression and significance of NapsinA, TTF-1, PAX8 and CA125 in ovarian and uterine clear cell carcinoma and endometrioid carcinoma. Linchuang Yu Shiyan Binglixue Zazhi. 2017;2:192-194. [DOI] [Full Text] |
| 8. | Li C, Xu Y, Zhang WM, Tang JL, Zhang ZH, Fan QH, Wang C. Primary uterine serous carcinoma: a clinicopathologic analysis of 17 cases. Linchuang Yu Shiyan Bingli Xue Zazhi. 2017;11:1278-1281. [DOI] [Full Text] |
| 9. | Yasuda M. Immunohistochemical characterization of endometrial carcinomas: endometrioid, serous and clear cell adenocarcinomas in association with genetic analysis. J Obstet Gynaecol Res. 2014;40:2167-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ranjan R, Singh L, Nath D, Sable MN, Malhotra N, Bhatla N, Kumar S, Datta Gupta S. Uterine Adenomatoid Tumors: A Study of Five Cases Including Three Cases of the Rare Leiomyoadenomatoid Variant. J Obstet Gynaecol India. 2015;65:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4th edition. Lyon: IARC Press. 2014; 151-154. |
| 12. | Deng J, Wang JW, Zhou WX. Leiomyo-matoid tumor of uterus: a clinicopathological analysis. Zhenduan Binglixue Zazhi. 2011;18:371-373. [DOI] [Full Text] |
| 13. | Zhu L, Li B. [Clinical pathological analysis of adenomatoid tumor in uterus and ovaries]. Zhonghua Bing Li Xue Za Zhi. 2001;30:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |