Copyright
©The Author(s) 2023.
World J Clin Oncol. Dec 24, 2023; 14(12): 549-569
Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.549
Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.549
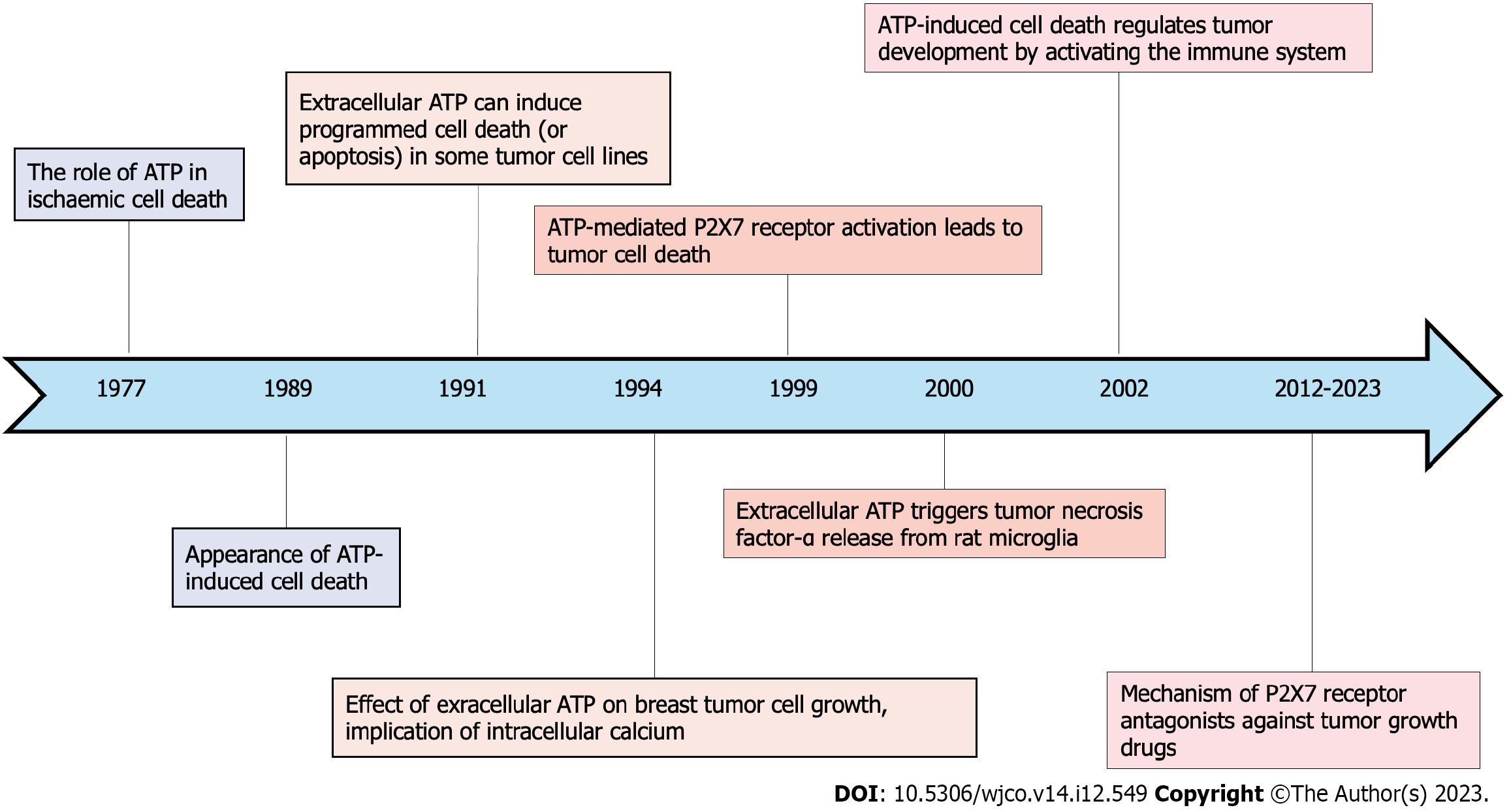
Figure 1 Chronological depiction of key milestones in the exploration of adenosine triphosphate induced cell death.
ATP: Adenosine triphosphate.
Figure 2 The process of adenosine triphosphate production necessitates the sequential progression through a series of reactions encompassing glycolysis, pyruvate decarboxylation, the krebs cycle, and the respiratory chain.
Cellular entities harness carbon sources to generate adenosine triphosphate (ATP) via glycolysis and the respiratory chain. Engineered cellular systems, when designed along specific pathways to facilitate targeted product synthesis, incur heightened ATP consumption for processes such as sugar uptake, cellular proliferation, biosynthesis, product efflux, and the acquisition of tolerance to cytotoxic agents. Furthermore, the equilibrium of ATP is influenced by a range of factors, including pH levels and oxygen availability. Perturbations in these dynamics can result in the overproduction of intracellular ATP, leading to its efflux through membrane-associated signaling channels or extracellular vesicles. Subsequent activation of cell membrane-associated P2 receptors by extracellular ATP triggers the influx of intracellular calcium ions, culminating in apoptotic cell demise. ATP: Adenosine triphosphate.
Figure 3 Illustration of the mechanism of adenosine triphosphate induced cell death, which involves several interconnected pathways.
Upon binding to the purinergic receptor P2X7 (P2X7R), extracellular adenosine triphosphate (ATP) induces a surge in intracellular calcium levels, leading to caspase activation and subsequent cell death. Additionally, ATP activates the NOD-like receptor family pyrin domain containing 3 inflammasome by releasing High Mobility Group Box 1/Toll-Like Receptor 4, triggering caspase-1 activation and promoting cell apoptosis. The interaction between ATP and P2X7Rs also activates the Nuclear Factor-kappa B and Phosphatidylinositol 3-kinase-protein kinase B/hypoxia-inducible factor pathways, resulting in DNA damage and cell death. Simultaneously, the continuous accumulation of intracellular Ca2+ stimulates the opening of the mitochondrial permeability transition pore, leading to DNA damage and ultimately cell necrosis. Ca2+ induces mitochondria to release cytochrome c, further contributing to the apoptotic process. Moreover, ATP-triggered cellular demise instigates a transformative shift within the extracellular microenvironment, concurrently unleashing a plethora of cytokines. Lastly, apart from elucidating the fundamental underpinnings of ATP induced cell death, this Figure also encapsulates a synthesized appraisal of the plausible mechanisms governing microenvironmental equilibrium, as extrapolated from relevant literature. ATP: Adenosine triphosphate; NF-κB: Nuclear Factor-kappa B; NLRP3: NOD-like receptor family pyrin domain containing 3; PI3K-AKT: Phosphatidylinositol 3-kinase-protein kinase B; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; ASC: Apoptosis-related speckle-like protein; STAT: Signal transducer and activator of transcription.
- Citation: Zhang HL, Sandai D, Zhang ZW, Song ZJ, Babu D, Tabana Y, Dahham SS, Adam Ahmed Adam M, Wang Y, Wang W, Zhang HL, Zhao R, Barakat K, Harun MSR, Shapudin SNM, Lok B. Adenosine triphosphate induced cell death: Mechanisms and implications in cancer biology and therapy. World J Clin Oncol 2023; 14(12): 549-569
- URL: https://www.wjgnet.com/2218-4333/full/v14/i12/549.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i12.549