Published online Aug 6, 2014. doi: 10.4292/wjgpt.v5.i3.122
Revised: June 20, 2014
Accepted: July 17, 2014
Published online: August 6, 2014
Processing time: 113 Days and 6.9 Hours
Functional gastrointestinal disorders (FGID) are common clinical syndromes diagnosed in the absence of biochemical, structural, or metabolic abnormalities. They account for significant morbidity and health care expenditures and are identifiable across variable age, geography, and culture. Etiology of abdominal pain associated FGIDs, including functional dyspepsia (FD), remains incompletely understood, but growing evidence implicates the importance of visceral hypersensitivity and electromechanical dysfunction. This manuscript explores data supporting the role of visceral hypersensitivity and electromechanical dysfunction in FD, with focus on pediatric data when available, and provides a summary of potential therapeutic targets.
Core tip: Functional dyspepsia (FD) is a common disorder of upper gastrointestinal symptoms in adults and children. Etiology and mechanisms of FD are complex, and improved understanding could help direct therapy. Visceral sensitivity and intestinal electromechanical function both are demonstrated to be altered in some FD patients and are potential targets for treatment. Limited studies in pediatric FD are available, but available evidence supports adult data that targeting visceral hypersensitivity and electromechanical dysfunction is warranted, particularly in the context of the biopsychosocial model. Future studies in pediatrics are needed to determine optimal therapy and appropriate patient application.
- Citation: Rosen JM, Cocjin JT, Schurman JV, Colombo JM, Friesen CA. Visceral hypersensitivity and electromechanical dysfunction as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther 2014; 5(3): 122-138
- URL: https://www.wjgnet.com/2150-5349/full/v5/i3/122.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v5.i3.122
Functional gastrointestinal disorders (FGIDs) account for more than 80% of chronic abdominal pain complaints in children. Although additional studies are needed, pediatric FGID prevalence and impact are described broadly in North America[1,2] and Europe[3,4], and with increasing recognition in other parts of the world[5-7]. The impact of pediatric FGIDs on patients and health-care systems cannot be overstated. In one epidemiologic study, 38% of school-aged children in the United States reported abdominal pain weekly and 24% reported abdominal pain persisting for more than 8 wk[8]. Further, FGIDs frequently are associated with somatic symptoms[9], decreased quality of life[10,11], psychological comorbidities[12], and school absenteeism[8]. Consequently, the burden on public health care[13] and associated financial costs are enormous[14,15].
In the late 1950s, Apley and Naish described an entity of recurrent abdominal pain (RAP)[16]. RAP was defined by 3 or more bouts of pain severe enough to interfere with activities and occurring over at least a 3 mo period. Children with a wide variety of clinical presentations and etiologies were included under the single entity of RAP. This entity was rendered inadequate for clinical practice due to broad inclusivity. Over the past decade there was an effort to reclassify RAP into discrete groups that are known as FGIDs. FGIDs are defined by symptom-based clinical criteria set forth by an expert panel generally referred to as the Rome Committee. The committee met for the third time in 2006 (Rome III) to update the criteria[17]. Rome III defines abdominal pain associated FGIDs in children as pain occurring at least weekly for longer than 2 mo and without identifiable biochemical, structural, or metabolic abnormalities to explain symptoms. However, abdominal pain associated FGIDs are diagnosed even in the absence of laboratory, radiologic, and endoscopic testing, or in the presence of mild chronic inflammation of the intestinal mucosa[18,19]. Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are among the most common pediatric FGIDs[20]. FD is diagnosed in children by: (1) upper abdominal pain or discomfort several times a week or more often; (2) upper abdominal pain or discomfort longer than 2 mo duration; (3) pain “sometimes” or less relieved by defecation; and (4) pain “once in a while” or less associated with a change in stool form or frequency. FD is differentiated from IBS in that IBS pain can be upper or lower abdomen, is more often relieved with defecation, and is often associated with change in stool form or frequency. Although distinctions are made within the criteria, it is debatable whether the two disorders are truly distinct in etiology or mechanism and ultimately may be symptom-defined diagnoses sharing a common underlying pathophysiology[21,22].
In adults, FD is further delineated by two subtypes: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS). PDS is defined by the presence of upper abdominal fullness or early satiety after normal size meals, whereas EPS is defined by predominance of epigastric pain or burning. PDS and EPS are not included within the pediatric FD symptom definition due to lack of supportive evidence in children. However, subsequent to Rome III, evidence emerged that adult subtypes also may be relevant in the pediatric population. For example, children with PDS-type symptoms have been found to have increased anxiety[23,24], a phenotype demonstrated in adults with PDS[25].
FD, as true of other FGIDS, is considered to be etiologically multi-factorial. The biopsychosocial model proposes contributions from and interactions between biologic, psychologic, and social systems. Factors within any of these systems may initiate, exacerbate or alter the course of the pain syndrome. In addition, adverse events early or later in life may lead to brain-gut axis changes, including long-term alterations in visceral electromechanical function, sensitivity, immunity, and brain-gut stress response. Examples of early adverse events span the biopsychosocial spectrum to include infection[26], inflammation, surgery[27,28], abuse[29], and wartime exposure[30].
We previously reviewed the role of inflammation (specifically eosinophils and mast cells) in pediatric FD[31]. In this companion review, we explore the role of visceral hypersensitivity and gastrointestinal electromechanical dysfunction in generation and maintenance of FD symptoms or subtypes, as well as their potential as therapeutic targets. Although they will generally be treated as separate entities in this discussion, visceral sensation, motor function and inflammation interrelate and should be considered as such when pursuing patient diagnosis and treatment.
Visceral sensory output from organs (e.g., intestine, bladder) to the central nervous system occurs continuously. Signals result from stimuli including hollow organ distension, inflammation, traction on the mesentery, and ischemia. Normal physiologic function of the visceral organs, including gastrointestinal distension and contraction, is typically nonpainful. However, the subjective interpretation may change due to increased frequency or amplitude of the visceral stimulus, or increased sensitivity to a typically painful (hyperalgesia) or nonpainful (allodynia) stimulus. Visceral hypersensitivity may result from alterations in the peripheral or central nervous system and has complex but increasingly understood etiology[32]. Human and animal studies have identified numerous contributing factors to this alteration, with visceral hypersensitivity now considered one of the central mechanisms of FGIDs.
Visceral hypersensitivity in FD may result in early satiety, abdominal pain, and nausea. Results from pediatric and adult investigations strongly suggest that sensory thresholds in FD patients are different than in subjects with other intestinal disorders and healthy controls. Visceral hypersensitivity was studied in 11 FD, 8 IBS and 11 FD-IBS overlap adults utilizing gastric and rectal barostats[33]. FD patients had predominant gastric (91% of subjects) over rectal (18%) hypersensitivity, IBS patients had only rectal (75%) hypersensitivity, and overlap patients had hypersensitivity to both (82% gastric, 91% rectal). Findings from this study suggest that hypersensitivity in FD may be localized to the stomach. However, other studies have failed to demonstrate these location-specific findings[34,35]. Differences in findings across studies may be related, at least in part, to heterogeneity in patient selection and/or in hypersensitivity definition.
Visceral sensitivity of the intestine is measured using a variety of methods in clinical studies. Patients undergo specific interventions, then either subjective pain reports or objective clinical data (e.g., biometrics, functional brain imaging) are collected and analyzed. Tests utilized include water load, balloon distension, and inflammatory/nociceptive challenge. In many studies, tests of visceral sensitivity are conducted in a multimodal design, both to determine correlation and to validate outcomes. Water load testing requires subjects to drink a maximal amount of water in a brief discrete time period (typically 5 min). Outcomes include subjective symptoms and quantity of water ingested. Balloon distension of hollow organs, including gastric barostat, measures distension thresholds and corresponding signs and symptoms. Of note, balloon distension also is used in animal models of visceral pain, with electromyographic recording included as an additional objective outcome. Inflammatory/nociceptive challenges directly stimulate intestinal mucosal sensory nerves by application of a chemical (e.g., acid or lipid) and measuring subjective pain thresholds. Both water load and balloon distension tests are affected by gastric accommodation and emptying, further demonstrating that separating sensation from function is a practical but artificial distinction.
Water load test: The water load test is advocated as a means of identifying patients with visceral hypersensitivity. Although the water load test may not be useful for identification of pediatric FD due to suboptimal sensitivity, children diagnosed with FD often have abnormal test results[36]. In a controlled study by Schurman et al[36], 68 pediatric patients with FGIDs and 26 healthy children completed the Behavioral Assessment Scale for Children-Self-Report Form (BASC-SR) and underwent a rapid water load test (maximal tolerable volume within 3 min). Children with FD, with or without corresponding IBS, had lower water consumption than healthy controls. This was not true of children with IBS only. Using the 10th percentile for water volume consumption in the control group as a lower limit of normal, the water load test had 28% sensitivity and 100% specificity in identifying patients with the diagnosis of FD as determined by the clinician. Consistent with the biopsychosocial model, self-reported anxiety was negatively correlated with volume of water intake; however, it accounted for only 6% of the variance.
A variation on the water load test measuring satiety was evaluated in 28 pediatric patients diagnosed with FD using Rome III criteria[37]. Participants drank a liquid meal at a constant rate and repeatedly scored satiety until reaching maximal possible score or 5 min time. Total intake volume was decreased in dyspeptic patients compared to healthy controls. Another study of 15 adolescents with FD who consumed a liquid meal at a constant rate to maximal tolerable volume found no statistical difference in total ingested volume or time to satiation compared to controls[38]. However, total volume was over 10% less and time to satiation over 20% sooner in FD subjects. Additionally, postprandial nausea and bloating were greater in dyspeptics, with 7/15 subjects reporting postprandial pain scores > 99th percentile of scores for healthy adolescents. Of note, in a study of 101 children with functional abdominal pain that utilized multiple validated questionnaires in addition to a water load test, children believing they could modify their own pain (high problem-focused pain efficacy) had decreased visceral sensitivity compared to those who perceived little control over pain[39]. Although the direct application to children with FD is unclear given different inclusion criteria, findings support consideration of visceral sensitivity to gastric distension as a possible pathophysiologic mechanism and, further, the potential beneficial role of CNS-mediated inhibition.
Measures of visceral sensitivity are studied more extensively in adult patients with FGIDs including FD. While water load testing in adults with FD has yielded similar results[40-44] to those reported above for pediatric studies, studies in adults contain expanded data investigating other upper GI conditions, demographic and psychosocial factors, and liquid composition. In one study of adults, patients with FD (n = 59), GERD (n = 101), and ulcer (n = 55) all demonstrated decreased maximal ingested volume of water over 5 min compared to 30 healthy controls[45]. Although this again supports visceral sensitivity mechanisms, it also raises concern regarding the specificity of the water load test as an assessment for FD. Strid et al[43] evaluated 35 FD adults and 56 controls. Depressed mood and poor overall health correlated with lower tolerated volumes in FD patients only, again reinforcing the brain-gut connection/biopsychosocial model and the useful but artificial construct of measuring visceral sensitivity in isolation. In contrast, Jones et al[44] found no correlation between psychological measures and specific water load test outcomes. Composition of the liquid also appears to affect the postprandial symptom profile in FD. Lee et al[46] compared 30 adults with FD to 12 healthy controls and found that symptoms of bloating and abdominal pain within 30 min following ingestion were greater in FD patients after a nutrient drink as compared to water, while there was no symptom difference between the two liquids in healthy controls[46]. Interpretation of liquid loading needs to take into consideration the psychologic state of the subject and the nutrient content of the ingested liquid.
Gastric barostat: Barostat testing is the traditional “gold standard” for evaluating mechanical hypersensitivity in adults. In FD, the evaluation utilizes balloon distension of the fundus and subjective scoring of discomfort. Hoffman et al[47] found that FD children had abdominal discomfort at lower gastric distension pressures compared to healthy young adults. This is consistent with a separate study utilizing barostat testing in which visceral hypersensitivity was identified at a higher frequency in children with RAP as compared to healthy controls[48]. The RAP group likely included children with FD as well as other abdominal pain disorders.
Gastric barostat studies in adult FD generally replicate, and also extend, pediatric findings. Evaluation of 8 dyspeptic adults found lower sensation threshold to gastric distension compared to controls, although maximal tolerated distension pressure and volume were similar[49]. These 8 patients had not previously consulted health care professionals regarding symptoms, suggesting that visceral hypersensitivity to balloon distension is independent of referral bias and certain psychosocial characteristics (such as high anxiety regarding symptoms). FD patient heterogeneity was demonstrated in two other studies, however, suggesting that sensitivity to balloon distension is not universal. Specifically, relative pressure (intraballoon pressure/intraabdominal pressure) to produce discomfort was abnormal in only 37% of 160 consecutive patients with FD when compared to 80 healthy controls and gastric hypersensitivity was found in only 44% of “pain-predominant” and 25% of “discomfort-predominant” FD adults[50]. Hypersensitivity to balloon distention is enhanced in the postprandial state in FD patients (but not controls) and correlates with preprandial sensitivity, impaired accommodation, and the severity of meal-related symptoms[51]. Taken together, studies suggest that mechanical hypersensitivity may be associated with an increased prevalence of postprandial pain.
Duodenal infusion: Although chemosensitivity has not been evaluated in children with FD, adults with FD have demonstrated increased symptoms to both duodenal[52] and gastric[53] acid infusion. Duodenal acid infusion has most often been associated with nausea but also bloating and pain[52,54-56]. Duodenal acid infusion decreases antral motility and alters response to balloon distention[46,55]. In a study of adults with FD, Feinle et al[57] showed that duodenal lipid exposure affects gastric sensitivity to balloon distension supporting the effect of lipids and cholecystykinin on visceral sensitivity. Lipid infusion, but not glucose infusion, enhances perception to gastric distention and lipid infusion is associated with nausea[58]. In addition to mechanical sensitivity, chemosensitivity represents another potential therapeutic target.
Visceral hypersensitivity is a complex process which may occur both within the CNS and at the level of the peripheral nervous system. Mechanisms of increased visceral sensitivity to balloon distension have been studied extensively in animal models[59,60] and in several cohorts of adults with FD, but have not been reproduced in dyspeptic children. Neuroimaging studies conducted in adults with FD support the presence of abnormal CNS processing of pain signals as compared to controls and in FD patients with hypersensitivity as compared to FD patients with normal sensation[61,62]. Vandenberghe et al[63] postulated that intense stimulation of low threshold multimodal afferent pathways, as opposed to sensitization of nociceptive pathways, occurs in hypersensitive FD adults. Their conclusion is based on studying 48 FD adults (hypersensitive, n = 20) in whom non-pain symptoms were induced at similar distending pressures that resulted in pain. At a peripheral level, hypersensitivity may be induced by a number of factors, including alterations in mediator release (e.g., serotonin) or receptors (e.g., 5-HT or TRPV1), inflammation, or the stress response.
Serotonin (5-HT) is abundant throughout the intestine and is an important neurotransmitter within the brain and the GI tract where it plays a key role in the regulation of motility and sensation. The effects of serotonin are modified by 5-HT receptors and its reuptake controlled by SERT. In adults with FD, plasma levels of 5-HT are decreased in the basal and postprandial states[64]. This has not been studied directly in children with FD; however, gastric 5-HT content and SERT mRNA do not differ between children with FD and controls[65]. Due to its important role in sensation, serotonin (broadly or specific serotonin receptors) represents a potentially important treatment target.
Transient receptor potential (TRP) channels survey the gastrointestinal contents for chemicals ingested, produced within the gastrointestinal tract (including those produced by the microbiome), and/or generated by inflammatory responses[66]. TRP vanilloid type 1 (TRPV1) is a polymodal nociceptor on GI afferent neurons and is the specific sensor for capsaicin. Based on oral capsaicin capsule titration, the majority of adults with FD demonstrate visceral chemosensitivity involving TRPV1 pathways[67-69]. Repeated ingestion of capsaicin in healthy volunteers initially increases symptoms, but after 4 wk decreases symptoms through desensitization of both chemo- and mechanoreceptors[70]. The effects on sensitivity appear to be dependent on length of exposure. In healthy volunteers with 7 d exposure, chemoreceptors remain sensitized while threshold of mechanoreceptors to distention decreases[71]. TRPV1 potentially plays a key role in chemosensation and possibly mechanosensitivity; as such, TRPV1 may represent another therapeutic target.
Inflammation and stress have been implicated in the pathophysiology of visceral hypersensitivity in FD. Consistent with the biopsychosocial model, electromechanical dysfunction may also be influenced by anxiety and the stress response. Anxiety is the most highly implicated psychological contributor to the development and maintenance of FGIDs including FD. Approximately 50% of children and adolescents with FD demonstrate elevated anxiety scores[72]. Anxiety can trigger the stress response which is mediated primarily through the release of corticotrophin releasing hormone (CRH) from the hypothalamus. The stress response results in physiologic effects relevant to FGIDs including inflammation (particularly mast cell activation), sympathetic nervous system activation, altered gastric accommodation, gastric dysmotility, and visceral hypersensitivity. CRH also alters central processing of nociceptive messages. The effects of CRH on hypersensitivity and electromechanical dysfunction may be direct and mediated via CRH1 and CRH2 receptors. Downstream effects of CRH-induced mast cell activation and mediator release can stimulate afferent nerves signaling pain, sensitize afferent nerves resulting in visceral hypersensitivity, and alter electromechanical function. In adults with FD, hypersensitivity is associated with mast cell degranulation after balloon distention of the proximal stomach[73].
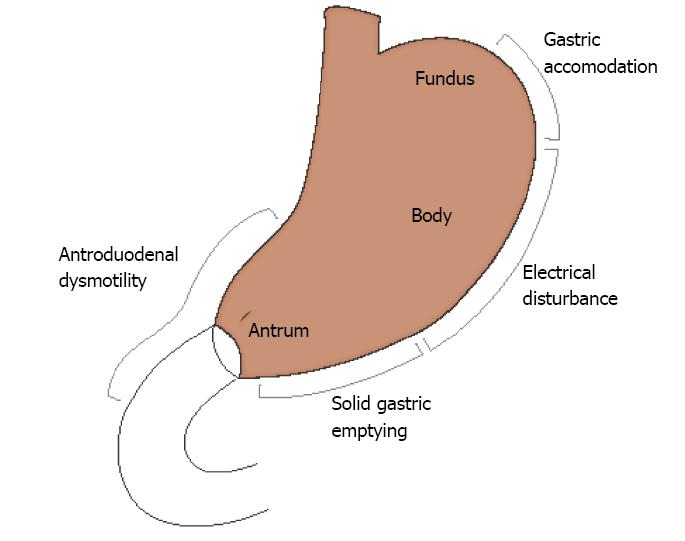
Visceral hypersensitivity undoubtedly has a role in dyspeptic symptoms, but it is identified in only a fraction of patients diagnosed clinically with FD. In contrast, disordered accommodation, delayed gastric emptying, gastric electrical rhythm disturbances, and altered antroduodenal motility are all physiologically relevant and common in FD (Figure 1). As reviewed by Azpiroz et al[74], gastric motor function is interdependent on visceral sensation and is a complex function affected by both tonic and induced stimuli. Understanding physiologic abnormalities in specific disorders such as FD can guide effective therapy.
Motor function of the stomach and duodenum is a coordinated activity meant to prepare food for digestion and initiate passage through the small intestine. The stomach serves as a reservoir for ingested food and functions to grind food and then provide passage to the intestine at a rate appropriate for effective nutrient absorption. In the interdigestive period, gastroduodenal motility is modulated by the migrating motor complex (MMC) which is a multiphase action propagated from the gastric antrum into the small intestine controlled by the enteric nervous system, central nervous system, and intestinal regulatory hormones. Gastroduodenal motility depends on prandial state, food composition, presence and type of inflammation, distal intestinal motor function, and both motor and autonomic neural input. Symptoms related to altered gastroduodenal motor function may include abdominal pain, nausea, vomiting, and early satiety and can occur due to rapid[75] or delayed gastric emptying, or altered proximal stomach accommodation with normal gastric emptying. Gastroduodenal mechanical function can be measured with a variety of tools including scintigraphic or breath gastric emptying study (GES), gastric barostat, antroduodenal manometry (ADM), and electrogastrography (EGG) as well as newer studies including single-photon emission computed tomography (SPECT), and the wireless motility capsule (WMC). Each test measures related but different aspects of physiology including compliance, accommodation, contractility, coordination, and propagation as highlighted below.
Pediatric studies have identified abnormal gastric emptying in FD. In a study of 15 FD adolescents using the 13C-s platensis breath test, gastric emptying of solids was significantly delayed[38]. Solid-phase delays were similarly identified in 26% of dyspeptic children when evaluated with the 13C-octanoic breath test[37]. Emptying function has also been evaluated in pediatric dypeptics with scintigraphy using 99mTc-sulfur colloid and a standard meal[76]. Although a majority of the 57 patients had normal gastric emptying at 2- and 4-h post meal, abnormalities of rapid (20%) and slow (20%) gastric emptying were observed. Symptoms did not correlate with emptying rates in these children. Another study utilizing scintigraphy demonstrated delayed solid emptying in 47% of patients, but again there was no relationship between emptying and symptom severity[77]. In contrast, Devanarayana et al[78] recently used antral ultrasound to correlate gastric emptying after a liquid meal with symptoms in pediatric dyspeptics. Forty-one FD patients had delay in both gastric emptying rate (% change in antral cross sectional area from 1 to 15 min post ingestion) and antral motility index (product of contractile amplitude and frequency) compared to healthy controls. Severity of symptoms correlated negatively with gastric emptying rate (r = -0.35), but not with other measures of motility. Gastric emptying appears to have no relationship to satiety in children[37]. Delays in gastric emptying may also be affected by concurrence of constipation in pediatric dyspepsia[79]. FD patients with constipation had longer gastric emptying times than FD patients without constipation, and treatment with lactulose over 3 mo resolved the difference.
Abnormal gastric emptying by scintigraphic evaluation has been demonstrated in a significant proportion of adults with FD[80-82] although findings may be affected by the modality of measurement as well as meal volume and contents[83]. In adults, there have been no reproducible relationships between impaired emptying and specific symptoms. Some studies have revealed no or only weak associations with symptoms[84-86]. Other studies have reported variable and highly inconsistent associations with nausea, vomiting, postprandial fullness, and bloating with both positive and negative relationships with regard to pain[87-91]. Postprandial fullness and nausea, and severe early satiety have been reported with delayed liquid emptying[88,90].
Gastric accommodation, the ability of the proximal stomach to relax and serve as a reservoir for food, is implicated as a motor abnormality responsible for symptoms in some dyspeptic patients[92]. Impaired accommodation has been associated with early satiety in some but not all studies[84,91,93]. Assessment of accommodation can be conducted with gastric barostat, ultrasound, MRI, and SPECT. Gastric emptying and water-load capacity are certainly affected by accommodation, but neither is a specific measure of fundic relaxation. Impaired accommodation was demonstrated in pediatric RAP patients assessed by 2-dimensional ultrasound[94]. Participants, most of whom had dyspeptic symptoms, had decreased proximal stomach saggital area and increased rate of proximal stomach emptying after a liquid meal when compared to healthy controls. A similar assessment of RAP patients utilized 3-dimensional ultrasound to assess antral relaxation and gastric distribution of ingested liquids[95]. Participants demonstrated decreased postprandial proximal filling (accommodation) and altered liquid distribution favoring the distal stomach despite no difference in gastric emptying rate. Ultrasound evaluation of children with FD also showed increased antral distension after a mixed solid-liquid meal, but without specific evaluation of the proximal stomach[96]. Adolescents with FD also have a lower postprandial gastric volume change than healthy adults when assessed by SPECT[38]. No MRI studies of gastric function in pediatric FD patients have been published.
In adults with FD, decreased gastric accommodation and abnormal gastric volumes are widely demonstrated using barostat[90,97,98], ultrasound[99,100], SPECT[80,101,102], and MRI[83]. Accommodation defects have been reported in 40% of adults with FD as assessed by barostat and in 47% as assessed by SPECT[93,101]. It is less clear whether symptoms are associated with abnormal accommodation or gastric volumes[91] and whether newer imaging modalities such as MRI will consistently support these findings[103].
Electrogastrography (EGG) is a noninvasive method to evaluate gastric myoelectrical activity. It can assess rhythmic gastric slow waves associated with frequency and propagation of contractions, as well as superimposed activity (spike/second) indicative of antral contractility. Cutaneous abdominal electrodes are utilized to obtain raw data, then computer analysis is performed to determine targeted values for comparison. Normative data are considered similar in children, adolescents, and adults[104], but not in neonates or toddlers[105].
Children with FD have abnormal EGG compared to healthy children, indicating underlying myoelectrical dysfunction. Chen et al[106] assessed 15 pediatric patients with FD compared to 17 healthy controls using surface electrodes. Children with FD had a lower percentage of slow waves and more time with no rhythmic activity in fasting and fed states. In the postprandial state, frequency of gastric slow waves also increased less in subjects than controls although measures of contractility (power) were similar. In an independent study of 30 children with FD, EGGs were abnormal in 50% and correlated with symptom severity[77].
Electrogastrogram abnormalities in adults with FD are similar to those described in children[42,107]. Patients with abnormal EGG also had higher postprandial pain scores, and patients with a history of vomiting had more frequent fasting bradygastria and fewer normal slow waves. This symptom correlation suggests clinical relevance of EGG abnormalities and is consistent with other data correlating EGG and symptoms in pediatric FD[106,108]. However, the role of EGG abnormalities as a therapeutic target remains to be established.
Antroduodenal manometry also demonstrates abnormal motility in children with FD[109]. A study of 34 children and 35 adults with FD found a majority with abnormal motility with a neuropathic pattern observed most commonly[110]. Several studies of antroduodenal motility also demonstrate abnormalities in adults with FD[111,112], but symptoms, intestinal dysmotility, and gastric emptying delays are not clearly correlated[112]. The relationship between motility studies is made even less clear in that a study of 31 adults with FD showed abnormal EGG was not associated with concurrent abnormalities in antroduodenal manometry[113], and available pediatric data supports this concept[114]. The clinical significance of altered antroduodenal motility, particularly as a therapeutic target, is not established.
The WMC shows promise as a relatively noninvasive, clinically relevant measure of gastrointestinal motility[115]. It is used to study prokinetic medication efficacy[116] and to describe an adult irritable bowel syndrome cohort[117]. Data is not yet available in adult or pediatric dyspeptics, but an initial study suggests the WMC is a sensitive detector of motor abnormalities in pediatric patients with upper gastrointestinal symptoms[118].
The specific cause of electromechanical dysfunction in FD is unclear, but may be related to immune activation[119]. Inflammation is implicated as a contributor in dyspepsia-associated dysmotility[31,120]. However, this effect appears to require specific inflammatory pathways. For example, EGG abnormalities in children and adolescents with FD are independent of chronic gastritis, but associated with antral mast cell and eosinophil density[121,122]. Likewise, in children with FD, increased antral mast cell density is associated with slower gastric emptying[121].
As alluded to previously, the stress response also has effects on electromechanical function. Experimentally induced stress has been shown to increase symptoms and inhibit normal postprandial EGG responses in some, but not all studies[123,124]. Stress is shown to impair accommodation and to decrease gastric emptying[125,126]. The effect on gastric emptying appears to be mediated primarily via CRH receptors.
Proper identification of functional dyspepsia using symptom based criteria (Rome III) is the first step in treatment. Diagnostic and screening tests to evaluate for diseases with similar symptoms are sometimes important, but not necessary for FD diagnosis. Providing a named diagnosis (i.e., FD) and the expectation of treatment success potentially increases the treatment response rate. Importantly, the placebo effect may be particularly strong in children with FGIDs and should be considered when interpreting efficacy of studied interventions[127].
Reassurance and education regarding FGIDs is imperative. Validating that subjective symptoms are real and putting them in the context of the biopsychosocial model aids in directing effective treatment and provides hope for patients and families. Visceral hypersensitivity and electromechanical dysfunction represent potential targets, but patients may be more effectively managed if underlying factors (such as inflammation, anxiety, etc.) are considered in the treatment plan. Treating FD, like other FGIDs, in the conceptual framework of the biopsychosocial model necessitates inclusion of both medical and psychological interventions. Effective medical therapy targeted to the specific pathophysiologic mechanism is preferred, but symptom-based therapy may also be useful. Although we will discuss medications in the context of their most likely target, it should be noted that visceral sensation, motor function, and inflammation do not exist in a vacuum; many medications exert an effect on more than one domain of sensation and mechanical function.
Treatment of visceral sensitivity related to distension in FD has focused largely on antidepressant therapy, including tricyclic antidepressants (TCAs), selective serotonin uptake inhibitors (SSRIs), and related medications. Antidepressants may have primary effects on comorbid anxiety/depression that secondarily alter symptom perception, coping skills, arousal thresholds, and/or sleep quality. Alternately, they may affect functional gastrointestinal pain through central nervous system analgesia or a direct effect on gastrointestinal tract sensitivity. Serotonergic neurons have a role in gastrointestinal pain as discussed above, but antinociceptive effects of these medications cannot always be dissociated from their influence on motility and, in some cases, may be integral to effective treatment[128]. For example, TCAs slow gastric emptying and small bowel transit in healthy patients[129,130], but do not affect SPECT-determined gastric accomodation or outcomes of the nutrient drink test, except for post-satiation nausea[129]. Similarly, SSRIs shorten small bowel transit time in healthy patients, but do not clearly decrease gastric sensitivity or compliance[131,132]. Treatment with TCAs, SSRIs, and related medications must be carefully weighed against potential adverse effects, including cardiac dysrhythmias, suicidality, and anticholinergic effects, and monitored to minimize these relatively rare, but potentially life-threatening issues.
Several studies have investigated whether TCAs, SSRIs, and related medications alter visceral sensitivity and overall symptoms in adult FD. Data in healthy adult volunteers demonstrate no change in tolerated gastric volume in the nutrient drink test after a short treatment course with desipramine (TCA) or escitalopram (SSRI)[133]. Although total symptom scores induced by the nutrient drink test were influenced, treatment effects were nullified in multivariate analysis considering age, gender, BMI, and baseline scores. Fluoxetine (SSRI) improved symptom scores in depressed adults with FD[134], but non-depressed subjects had no change in symptom scores and EGG measures were similar across all groups. Sertraline (SSRI) similarly failed to alter global symptoms or quality of life in adults with FD[135]. Finally, a randomized clinical trial (RCT) of venlafaxine, a medication with combined SSRI and selective norepinephrine reuptake inhibition (SNRI), demonstrated significant patient dropout due to medication adverse effects and no differences in symptom scores, health-related quality of life, anxiety, or depression[136]. Taken together, current evidence does not support a strong direct effect of SSRIs or TCAs on visceral sensitivity in adults. The potential role of these medications in treatment of visceral hypersensitivity, as well as gastroduodenal motility, may be further clarified by an international multicenter placebo-controlled RCT currently underway to compare escitalopram to amitriptyline in adults with FD. This trial has completed enrollment and data collection for the primary outcome of global symptom score, and also is assessing solid gastric emptying, liquid nutrient drink test, and SPECT (http://clinicaltrials.gov, NCT00248651).
Limited data exists regarding treatment of pediatric FD with TCAs and SSRIs, and studies typically include a mixed cohort of FGIDs. A double-blind placebo-controlled RCT of amitriptyline (TCA) in 33 pediatric patients with IBS treated for 8 wk demonstrated improvement in QOL and some IBS-associated symptoms[137]. Symptom improvement was limited to very specific symptoms (i.e., right lower quadrant pain) and the reason for such specificity is not clear. Amitriptyline also was studied in 90 pediatric FGID patients in a multicenter double-blinded placebo-controlled RCT[138]. Few patients were diagnosed with FD (8% placebo, 13% amitriptyline), but primary outcome of symptom relief was not different when analyzed by diagnosis. No difference in symptom relief, depression, or functional disability was noted, although anxiety was decreased in subjects receiving treatment. Notably, at least “fair” improvement in pain relief was seen in greater than 2/3 of subjects receiving placebo. A retrospective study of 98 pediatric FGID patients (n = 16 with FD) treated with TCAs found greater than 75% symptom response rate in all FGID subtypes, but limitations include lack of validated outcome measures, blinding, and control subjects[139]. A 12-wk open label study of citalopram (SSRI) in 25 pediatric patients with RAP identified improvement in global symptoms, somatic symptoms, anxiety, and functional impairment[140]. There are no published placebo-controlled RCTs of SSRIs for treatment of pediatric FGIDs in general or FD in particular. Given the questionable efficacy in adults with FD, SSRIs should not be viewed as first-line therapy, if at all, in pediatric FD.
In addition to mechanosensitivity, visceral chemosensitivity may represent a valid therapeutic target. Lipid sensitivity may be addressed through diet modification, but there have not been any studies demonstrating long-term benefit from low fat diets. Acid sensitivity may be addressed more directly through acid reducing medications. Acid-suppressive therapy with histamine-2 receptor antagonists (H2RA) and proton-pump inhibitors (PPI) improve pain in adults with FD[141-143]. PPI therapy may be more effective than H2RA[144], but studies typically have a mixed cohort without control for presence of H. pylori infection, gastroesophageal reflux disease (GERD), or both. A randomized, controlled trial in adults found that PPI therapy improved symptoms only in FD patients with concurrent heartburn[145]. Whether the therapeutic benefit is related to acid hypersensitivity is not clear as these medications may be treating a component of acid mucosal injury or co-morbid GERD, or may also improve dyspeptic symptoms related to delayed gastric emptying[146]. Still, acid reduction therapy remains the most common treatment prescribed empirically by pediatric gastroenterologists for FD in children[147]. In children with abdominal pain, famotidine has demonstrated superiority to placebo in global improvement, and additional benefit is noted in children with FD[148]. In a large pediatric cohort, omeprazole had no benefit over ranitidine or famotidine in the relief of pain, nausea, or vomiting[149]. Although acid suppression appears promising, the specific mechanism of action in FD remains unclear.
Treatment of visceral chemosenstivity in FD also has targeted specific nociceptors including TRPV1. As described earlier, healthy adults ingesting capsaicin achieve desensitization following initial increase in symptoms, and FD adults may have increased chemosensitivity to TRPV1 agonists. A double-blind, placebo-controlled trial of red pepper powder in 30 FD adults demonstrated efficacy in decreasing overall symptoms, epigastric pain, and epigastric fullness within 3 wk[150]. Although some initial discomfort occurred in treatment group patients, only two discontinued the study due to severe pain or burning. Capsaicin or other TRPV1 agents have promise in FD patients with demonstrated chemosensitivity.
Therapies for electromechanical dysfunction in FD can be broken down into those targeting gastric motility/emptying and those targeting gastric accommodation. Therapies to increase gastrointestinal motility and emptying have met with mixed results for FD. A meta-analysis of 1844 adult patients with FD and 1599 controls found that prokinetics were effective in decreasing symptoms[151]. The authors importantly note that most studies of prokinetics assess short-term efficacy only. Interestingly, a separate analysis of studies including measures of symptom improvement and gastric emptying found no correlation between the two, suggesting that alternate effects of prokinetics are responsible for symptom improvement[152].
Prokinetics evaluated in adults include agents primarily targeting 5-HT (5-HT3 antagonists and 5-HT4 agonists), dopamine, and motilin receptors. Cisapride, a 5-HT4 receptor agonist, demonstrated symptom reduction in adults with FD in one meta-analysis, but potential bias and inclusion of specific FD-subtypes may affect applicability of findings[153]. In pediatric patients with dyspepsia it may normalize gastric myoelectric activity[154], but data on clinical effects is not available. Cisapride and newer 5-HT4 receptor agonists regulate intestinal motility through effects on enteric cholinergic neurons, enhancing gastric emptying and accomodation, as well as potentially modulating visceral sensitivity[155,156]. Although cisapride was withdrawn from the United States and European markets due to concern for potentially fatal cardiac arrythmias, it is not clear that these effects are common in otherwise healthy children[157] and the medication can still be used in limited capacity with close supervision. Another serotonergic/anti-dopaminergic compound, levosulpiride, has demonstrated noninferiority to cisapride[158] with safety and efficacy confirmed in an open-label trial of 279 adults with FD[159]. A selective 5-HT4 agonist and 5-HT3 antagonist (mosapride) has shown mixed results in FD symptom improvement[160-162]. Cinitapride, a relatively new 5-HT4 receptor agonist/dopamine-2 receptor antagonist, was demonstrated to relieve symptoms and reduce symptom severity as well as domperidone in a double-blind phase III RCT[163]. There is a lack of pediatric data regarding agents targeting 5-HT receptors.
Metoclopramide is a dopamine antagonist with a long history of use in FD as an effective promotility agent that reduces dyspeptic symptoms[164,165], but adverse effects may include irreversible extrapyramidal symptoms. There is evidence that metoclopramide liquid formulation may actually be more effective than the tablet[166]. Domperidone, a dopamine-2 receptor antagonist that does not cross the blood-brain barrier, is shown to improve symptoms in adults with FD[167] though it may be less effective when compared to cisapride[168,169]. Domperidone is currently available for pediatric patients only as an investigational new drug for compassionate use. Itopride, which is anti-dopaminergic and inhibits acetylcholinesterase, did not have promising results in a phase III trial in adults[170], but a meta-analysis that included a heterogenous patient population with potential comorbid disease (i.e., H. pylori) suggests that it may be effective in symptom reduction[171]. A lack of proven efficacy and significant potential side effects should limit the long-term use of metoclopramide in pediatric FD.
Erythromycin activates antral and small intestinal motilin receptors, and may have differential physiologic effects in children with underlying gastrointestinal disorders, including FD[172]. Erythromycin in adults with FD improved bloating and gastric emptying of liquids and solids, but did not affect meal related symptom severity[173]. The motilin agonist ABT 229 provided no symptom improvement in adults with FD[174]. Another motilin agonist, mitemcinal, showed promise in relieving gastroparesis-associated symptoms in adult diabetics[175]. Efficacy in a subset of those patients with lower body mass index and hemoglobin A1C suggests a role in nondiabetics with upper gastrointestinal symptoms[176]. Motilin receptor agonists are known to decrease gastric accommodation and compliance[177,178] and are susceptible to tachyphylaxis, both factors that may contribute to limited efficacy in FD.
Actiomide is a novel agent that has minimal interaction with serotonin and dopamine receptors. It affects gastrointestinal motility in adult FD, including improving accommodation and gastric emptying[179], through muscarinic receptor inhibition. This, in turn, increases acetylcholine release and inhibits its degradation. Elimination of meal-related symptoms, and improvement in symptom subgroups and quality of life was demonstrated in a phase III clinical trial in Japan[180] . Phase III trials are currently in preparation in the US and Europe.
Gastric accommodation represents another potential therapeutic target within the broad category of electromechanical dysfunction. Buspirone, a 5HT1a receptor agonist, increased accomodation and decreased symptom severity, postprandial symptoms, and liquid gastric emptying rate, but did not specifically affect gastric sensitivity to distension by barostat in adults with FD[181]. Tandospirone, a partial 5HT1a agonist similar to buspirone, also improved symptom scores in FD adults, but had no effect on early satiety implicating central anxiolytic effects rather than altered gastric accommodation[182]. Sumatriptan is another 5HT1 receptor agonist that alters gastric size in dyspeptics, but specific mechanical effect and association with symptom improvement remains unclear[93,183]. A subset of FD patients also showed improvement in nausea and accommodation when treated with ondansetron, a 5HT3 antagonist, but mechanical and clinical effects were disassociated[184]. Tegaserod, a partial 5HT4 receptor agonist, is shown to enhance gastric accommodation and two large randomized trials showed significant symptom relief compared to placebo[185]. Paroxetine, an SSRI, has been shown to enhance gastric accomodation in healthy volunteers but has not been studied in FD[186].
Cyproheptadine is efficacious in improving symptoms in children with FD[187]. As an antagonist of serotonin, histamine H1, and muscarinic receptors, it is possible that physiologic effects are due to increased gastric accommodation or decreased gastric hypersensitivity to distension. In a retrospective open-label study of 80 children, Rodriguez et al[187] showed FD-symptoms significantly improved in 33 (41%) and resolved in 11 (14%) with very good medication tolerance even in nonresponders. It was previously found to be effective in a RCT of children with functional abdominal pain[188].
Complementary therapies such as ginger[189,190], peppermint oil[191], and iberogast[192], may also have a role in the treatment of FD. Ginger enhances gastric emptying in healthy volunteers and adults with FD but had no impact on FD symptoms[193,194]. In healthy volunteers, peppermint oil enhances gastric emptying without effects on sensitivity or accomodation[195,196]. It was effective for irritable bowel syndrome in children, but has not been specifically studied in FD[191]. Iberogast (STW 5), an herbal preparation, improves symptoms in FD, but there is not clear data determining whether effects are directly mediated by acceleration of gastric emptying or an alternate mechanism[192,197].
Gastrointestinal motility can also be influenced by mechanical devices including the gastric electrical stimulator. The device utilizes electrodes implanted into the antrum to deliver high frequency, low amplitude stimulation. Adult studies show the device decreases symptom severity and improves quality of life[198] and findings were recently replicated in pediatric trials[10,199]. The study of 24 pediatric FD patients included those who did not improve with conventional medical therapy and most underwent temporary endoscopic gastric pacemaker placement to assess for symptom improvement prior to implantation of the permanent device[10]. Most patients showed significant gastrointestinal symptom improvement, as well as improved quality of life and global health scores.
Given the interaction between the stress response, visceral hypersensitivity, and electromechanical dysfunction, non-medication treatment of stress and anxiety likely have a role in the management of these patients.. Psychological and relaxation interventions studied in children with FGIDs include cognitive behavioral therapy, gut-directed hypnotherapy[200,201], yoga[202], and biofeedback-assisted relaxation therapy (BART)[203]. Children receiving a standardized course of targeted medication plus BART demonstrated better outcomes including decrease in pain intensity, decrease in pain episode duration, and global pain improvement as compared to children receiving only the medication component.
This gives rise to the hope that treatments addressing multiple, complementary targets within the biopsychosocial model can improve outcomes for children with FD, although further research needs to be done with multiple-component treatments to determine optimal combinations for individual children.
FGIDs, including functional dyspepsia, are incompletely understood despite high prevalence and significant impact on patient quality of life and healthcare costs. FGIDs are best approached utilizing a biopsychosocial model in which all relevant factors (biologic, psychologic and social) are identified and targeted in treatment. As mechanisms of disease are further investigated, both in laboratory and clinical models, opportunities arise to target therapies. In addition to inflammation (addressed elsewhere), visceral hypersensitivity and gastrointestinal dysmotility are pathophysiolgic alterations that may respond to directed treatment. Despite limited evidence in children, the role of pharmacologic agents within a broader biopsychosocial treatment context remains promising.
There remains a need for placebo-controlled trials of therapy targeting visceral hypersensitivity and electromechanical dysfunction in children with FD. Likewise, there is a need to better understand the diagnostic and prognostic utility of various tests of upper intestinal sensory and mechanical function including visceral sensitivity, accommodation, and gastric emptying. Application of knowledge from placebo-controlled trials and specific tests of function may improve directed medical therapy for children with FD.
P- Reviewer: Akiho H, Hoff DAL, Schmidt PT, Pehl C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220-226. [PubMed] |
| 2. | Saps M, Adams P, Bonilla S, Chogle A, Nichols-Vinueza D. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J Pediatr Gastroenterol Nutr. 2012;55:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Vila M, Kramer T, Obiols JE, Garralda ME. Abdominal pain in British young people: associations, impairment and health care use. J Psychosom Res. 2012;73:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Spee LA, Lisman-Van Leeuwen Y, Benninga MA, Bierma-Zeinstra SM, Berger MY. Prevalence, characteristics, and management of childhood functional abdominal pain in general practice. Scand J Prim Health Care. 2013;31:197-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Dong L, Dingguo L, Xiaoxing X, Hanming L. An epidemiologic study of irritable bowel syndrome in adolescents and children in China: a school-based study. Pediatrics. 2005;116:e393-e396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Rajindrajith S, Devanarayana NM. Subtypes and Symptomatology of Irritable Bowel Syndrome in Children and Adolescents: A School-based Survey Using Rome III Criteria. J Neurogastroenterol Motil. 2012;18:298-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 7. | Saps M, Nichols-Vinueza DX, Rosen JM, Velasco-Benítez CA. Prevalence of functional gastrointestinal disorders in Colombian school children. J Pediatr. 2014;164:542-545.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Dengler-Crish CM, Horst SN, Walker LS. Somatic complaints in childhood functional abdominal pain are associated with functional gastrointestinal disorders in adolescence and adulthood. J Pediatr Gastroenterol Nutr. 2011;52:162-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Lu PL, Teich S, Di Lorenzo C, Skaggs B, Alhajj M, Mousa HM. Improvement of quality of life and symptoms after gastric electrical stimulation in children with functional dyspepsia. Neurogastroenterol Motil. 2013;25:567-e456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Demyttenaere K, Tack J. Risk factors for impaired health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther. 2011;33:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20 Suppl 1:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;51:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:671-682. [PubMed] |
| 16. | APLEY J, NAISH N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958;33:165-170. [PubMed] |
| 17. | Walker LS C-DA, Rasquin-Weber , A . Questionnaire on Pediatric Gastrointestinal Symptoms, Rome III Version (QPGS-RIII). Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, Virginia: Degnon Associates, Inc 2006; 963-990. |
| 18. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1075] [Article Influence: 56.6] [Reference Citation Analysis (6)] |
| 19. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1191] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 20. | Schurman JV, Friesen CA, Danda CE, Andre L, Welchert E, Lavenbarg T, Cocjin JT, Hyman PE. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41:291-295. [PubMed] |
| 21. | Hyams JS, Davis P, Sylvester FA, Zeiter DK, Justinich CJ, Lerer T. Dyspepsia in children and adolescents: a prospective study. J Pediatr Gastroenterol Nutr. 2000;30:413-418. [PubMed] |
| 22. | Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Schurman JV, Singh M, Singh V, Neilan N, Friesen CA. Symptoms and subtypes in pediatric functional dyspepsia: relation to mucosal inflammation and psychological functioning. J Pediatr Gastroenterol Nutr. 2010;51:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Rippel SW, Acra S, Correa H, Vaezi M, Di Lorenzo C, Walker LS. Pediatric patients with dyspepsia have chronic symptoms, anxiety, and lower quality of life as adolescents and adults. Gastroenterology. 2012;142:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, Ronkainen J. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Ford AC, Thabane M, Collins SM, Moayyedi P, Garg AX, Clark WF, Marshall JK. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: a cohort study. Gastroenterology. 2010;138:1727-1736; quiz e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Rosen JM, Adams PN, Saps M. Umbilical hernia repair increases the rate of functional gastrointestinal disorders in children. J Pediatr. 2013;163:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Saps M, Bonilla S. Early life events: infants with pyloric stenosis have a higher risk of developing chronic abdominal pain in childhood. J Pediatr. 2011;159:551-554.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Geeraerts B, Van Oudenhove L, Fischler B, Vandenberghe J, Caenepeel P, Janssens J, Tack J. Influence of abuse history on gastric sensorimotor function in functional dyspepsia. Neurogastroenterol Motil. 2009;21:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Klooker TK, Braak B, Painter RC, de Rooij SR, van Elburg RM, van den Wijngaard RM, Roseboom TJ, Boeckxstaens GE. Exposure to severe wartime conditions in early life is associated with an increased risk of irritable bowel syndrome: a population-based cohort study. Am J Gastroenterol. 2009;104:2250-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Friesen CA, Schurman JV, Colombo JM, Abdel-Rahman SM. Eosinophils and mast cells as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther. 2013;4:86-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085-G1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 33. | Bouin M, Lupien F, Riberdy M, Boivin M, Plourde V, Poitras P. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil. 2004;16:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Moriarty KJ, Dawson AM. Functional abdominal pain: further evidence that whole gut is affected. Br Med J (Clin Res Ed). 1982;284:1670-1672. [PubMed] |
| 35. | Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci. 1995;40:1607-1613. [PubMed] |
| 36. | Schurman JV, Friesen CA, Andre L, Welchert E, Lavenbarg T, Danda CE, Cocjin JT, Hyman PE. Diagnostic utility of the water load test in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Hoffman I, Tack J. Assessment of gastric motor function in childhood functional dyspepsia and obesity. Neurogastroenterol Motil. 2012;24:108-112, e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Chitkara DK, Camilleri M, Zinsmeister AR, Burton D, El-Youssef M, Freese D, Walker L, Stephens D. Gastric sensory and motor dysfunction in adolescents with functional dyspepsia. J Pediatr. 2005;146:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Anderson JL, Acra S, Bruehl S, Walker LS. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:309-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Boeckxstaens GE, Hirsch DP, van den Elzen BD, Heisterkamp SH, Tytgat GN. Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology. 2001;121:1054-1063. [PubMed] |
| 41. | Jones MP, Hoffman S, Shah D, Patel K, Ebert CC. The water load test: observations from healthy controls and patients with functional dyspepsia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G896-G904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Koch KL, Hong SP, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J Clin Gastroenterol. 2000;31:125-129. [PubMed] |
| 43. | Strid H, Norström M, Sjöberg J, Simrén M, Svedlund J, Abrahamsson H, Björnsson ES. Impact of sex and psychological factors on the water loading test in functional dyspepsia. Scand J Gastroenterol. 2001;36:725-730. [PubMed] |
| 44. | Jones MP, Roth LM, Crowell MD. Symptom reporting by functional dyspeptics during the water load test. Am J Gastroenterol. 2005;100:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Chen CL, Hu CT, Lin HH, Yi CH. Clinical utility of electrogastrography and the water load test in patients with upper gastrointestinal symptoms. J Smooth Muscle Res. 2006;42:149-157. [PubMed] |
| 46. | Lee KJ, Kim JH, Cho SW. Dietary influence on electro-gastrography and association of alterations in gastric myoelectrical activity with symptoms in patients with functional dyspepsia. J Gastroenterol Hepatol. 2006;21:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Hoffman I, Vos R, Tack J. Assessment of gastric sensorimotor function in paediatric patients with unexplained dyspeptic symptoms and poor weight gain. Neurogastroenterol Motil. 2007;19:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Di Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Holtmann G, Gschossmann J, Neufang-Hüber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332-336. [PubMed] |
| 50. | Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526-535. [PubMed] |
| 51. | Farré R, Vanheel H, Vanuytsel T, Masaoka T, Törnblom H, Simrén M, Van Oudenhove L, Tack JF. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. 2013;145:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515-520. [PubMed] |
| 53. | Miwa H, Nakajima K, Yamaguchi K, Fujimoto K, Veldhuyzen VAN Zanten SJ, Kinoshita Y, Adachi K, Kusunoki H, Haruma K. Generation of dyspeptic symptoms by direct acid infusion into the stomach of healthy Japanese subjects. Aliment Pharmacol Ther. 2007;26:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Schwartz MP, Samsom M, Smout AJ. Human duodenal motor activity in response to acid and different nutrients. Dig Dis Sci. 2001;46:1472-1481. [PubMed] |
| 55. | Ishii M, Manabe N, Kusunoki H, Kamada T, Sato M, Imamura H, Shiotani A, Hata J, Haruma K. Real-time evaluation of dyspeptic symptoms and gastric motility induced by duodenal acidification using noninvasive transnasal endoscopy. J Gastroenterol. 2008;43:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | di Stefano M, Vos R, Vanuytsel T, Janssens J, Tack J. Prolonged duodenal acid perfusion and dyspeptic symptom occurrence in healthy volunteers. Neurogastroenterol Motil. 2009;21:712-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Feinle C, Meier O, Otto B, D’Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347-355. [PubMed] |
| 58. | Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51 Suppl 1:i54-i57. [PubMed] |
| 59. | Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc. 2007;2:2624-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Miranda A, Mickle A, Medda B, Zhang Z, Phillips RJ, Tipnis N, Powley TL, Shaker R, Sengupta JN. Altered mechanosensitive properties of vagal afferent fibers innervating the stomach following gastric surgery in rats. Neuroscience. 2009;162:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Zeng F, Qin W, Liang F, Liu J, Tang Y, Liu X, Yuan K, Yu S, Song W, Liu M. Abnormal resting brain activity in patients with functional dyspepsia is related to symptom severity. Gastroenterology. 2011;141:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Van Oudenhove L, Vandenberghe J, Dupont P, Geeraerts B, Vos R, Dirix S, Bormans G, Vanderghinste D, Van Laere K, Demyttenaere K. Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study. Am J Gastroenterol. 2010;105:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Vandenberghe J, Vos R, Persoons P, Demyttenaere K, Janssens J, Tack J. Dyspeptic patients with visceral hypersensitivity: sensitisation of pain specific or multimodal pathways? Gut. 2005;54:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Cheung CK, Lee YY, Chan Y, Cheong PK, Law WT, Lee SF, Sung JJ, Chan FK, Wu JC. Decreased Basal and postprandial plasma serotonin levels in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2013;11:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131:142-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 67. | Li X, Cao Y, Wong RK, Ho KY, Wilder-Smith CH. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol Motil. 2013;25:246-253, e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Führer M, Vogelsang H, Hammer J. A placebo-controlled trial of an oral capsaicin load in patients with functional dyspepsia. Neurogastroenterol Motil. 2011;23:918-e397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Hammer J, Führer M, Pipal L, Matiasek J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol Motil. 2008;20:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Führer M, Hammer J. Effect of repeated, long term capsaicin ingestion on intestinal chemo- and mechanosensation in healthy volunteers. Neurogastroenterol Motil. 2009;21:521-527, e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Hammer J. Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation. Aliment Pharmacol Ther. 2006;24:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Schurman JV, Danda CE, Friesen CA, Hyman PE, Simon SD, Cocjin JT. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings. 2008;15:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Hou XH, Zhu LR, Li QX, Chen JDZ. Alterations in mast cells and 5-HT positive cells in gastric mucosa in functional dyspepsia patients with hypersensitivity. Neurogastroenterol Motil. 2001;13:398-399. |
| 74. | Azpiroz F, Feinle-Bisset C, Grundy D, Tack J. Gastric sensitivity and reflexes: basic mechanisms underlying clinical problems. J Gastroenterol. 2014;49:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Kusano M, Zai H, Shimoyama Y, Hosaka H, Kuribayashi S, Kawamura O, Mori M. Rapid gastric emptying, rather than delayed gastric emptying, might provoke functional dyspepsia. J Gastroenterol Hepatol. 2011;26 Suppl 3:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Chitkara DK, Delgado-Aros S, Bredenoord AJ, Cremonini F, El-Youssef M, Freese D, Camilleri M. Functional dyspepsia, upper gastrointestinal symptoms, and transit in children. J Pediatr. 2003;143:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Friesen CA, Lin Z, Hyman PE, Andre L, Welchert E, Schurman JV, Cocjin JT, Burchell N, Pulliam S, Moore A. Electrogastrography in pediatric functional dyspepsia: relationship to gastric emptying and symptom severity. J Pediatr Gastroenterol Nutr. 2006;42:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Devanarayana NM, Rajindrajith S, Perera MS, Nishanthanie SW, Benninga MA. Gastric emptying and antral motility parameters in children with functional dyspepsia: association with symptom severity. J Gastroenterol Hepatol. 2013;28:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Boccia G, Buonavolontà R, Coccorullo P, Manguso F, Fuiano L, Staiano A. Dyspeptic symptoms in children: the result of a constipation-induced cologastric brake? Clin Gastroenterol Hepatol. 2008;6:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685-1694. [PubMed] |
| 81. | Troncon LE, Herculano JR, Savoldelli RD, Moraes ER, Secaf M, Oliveira RB. Relationships between intragastric food maldistribution, disturbances of antral contractility, and symptoms in functional dyspepsia. Dig Dis Sci. 2006;51:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Waldron B, Cullen PT, Kumar R, Smith D, Jankowski J, Hopwood D, Sutton D, Kennedy N, Campbell FC. Evidence for hypomotility in non-ulcer dyspepsia: a prospective multifactorial study. Gut. 1991;32:246-251. [PubMed] |
| 83. | Fruehauf H, Steingoetter A, Fox MR, Kwiatek MA, Boesiger P, Schwizer W, Fried M, Thumshirn M, Goetze O. Characterization of gastric volume responses and liquid emptying in functional dyspepsia and health by MRI or barostat and simultaneous C-acetate breath test. Neurogastroenterol Motil. 2009;21:697-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | van Lelyveld N, Schipper M, Samsom M. Lack of relationship between chronic upper abdominal symptoms and gastric function in functional dyspepsia. Dig Dis Sci. 2008;53:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Talley NJ, Locke GR, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, Rojavin MA, Tack J. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 86. | Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Pallotta N, Pezzotti P, Calabrese E, Baccini F, Corazziari E. Relationship between gastrointestinal and extra-gastrointestinal symptoms and delayed gastric emptying in functional dyspeptic patients. World J Gastroenterol. 2005;11:4375-4381. [PubMed] |
| 88. | Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 89. | Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036-1042. [PubMed] |
| 90. | Bisschops R, Karamanolis G, Arts J, Caenepeel P, Verbeke K, Janssens J, Tack J. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. 2008;57:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 91. | Kindt S, Dubois D, Van Oudenhove L, Caenepeel P, Arts J, Bisschops R, Tack J. Relationship between symptom pattern, assessed by the PAGI-SYM questionnaire, and gastric sensorimotor dysfunction in functional dyspepsia. Neurogastroenterol Motil. 2009;21:1183-e105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Bisschops R, Tack J. Dysaccommodation of the stomach: therapeutic nirvana? Neurogastroenterol Motil. 2007;19:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [PubMed] |
| 94. | Olafsdottir E, Gilja OH, Aslaksen A, Berstad A, Fluge G. Impaired accommodation of the proximal stomach in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2000;30:157-163. [PubMed] |
| 95. | Olafsdottir E, Gilja OH, Tefera S, Fluge G, Berstad A. Intragastric maldistribution of a liquid meal in children with recurrent abdominal pain assessed by three-dimensional ultrasonography. Scand J Gastroenterol. 2003;38:819-825. [PubMed] |
| 96. | Cucchiara S, Minella R, Iorio R, Emiliano M, Az-Zeqeh N, Vallone G, Bali MA, Alfieri E, Scoppa A. Real-time ultrasound reveals gastric motor abnormalities in children investigated for dyspeptic symptoms. J Pediatr Gastroenterol Nutr. 1995;21:446-453. [PubMed] |
| 97. | Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006;130:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Lunding JA, Tefera S, Bayati A, Gilja OH, Mattsson H, Hausken T, Berstad A. Pressure-induced gastric accommodation studied with a new distension paradigm. Abnormally low accommodation rate in patients with functional dyspepsia. Scand J Gastroenterol. 2006;41:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Gilja OH, Hausken T, Wilhelmsen I, Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689-696. [PubMed] |
| 100. | Hata T, Kato M, Kudo T, Nishida M, Nishida U, Imai A, Yoshida T, Hirota J, Kamada G, Ono S. Comparison of gastric relaxation and sensory functions between functional dyspepsia and healthy subjects using novel drinking-ultrasonography test. Digestion. 2013;87:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264-272. [PubMed] |
| 102. | van den Elzen BD, Bennink RJ, Holman R, Tytgat GN, Boeckxstaens GE. Impaired drinking capacity in patients with functional dyspepsia: intragastric distribution and distal stomach volume. Neurogastroenterol Motil. 2007;19:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Bharucha AE, Manduca A, Lake DS, Fidler J, Edwards P, Grimm RC, Zinsmeister AR, Riederer SJ. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil. 2011;23:617-e252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Friesen CA, Lin Z, Schurman JV, Andre L, McCallum RW. An evaluation of adult electrogastrography criteria in healthy children. Dig Dis Sci. 2006;51:1824-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Chen JD, Co E, Liang J, Pan J, Sutphen J, Torres-Pinedo RB, Orr WC. Patterns of gastric myoelectrical activity in human subjects of different ages. Am J Physiol. 1997;272:G1022-G1027. [PubMed] |
| 106. | Chen JD, Lin X, Zhang M, Torres-Pinedo RB, Orr WC. Gastric myoelectrical activity in healthy children and children with functional dyspepsia. Dig Dis Sci. 1998;43:2384-2391. [PubMed] |
| 107. | Sha W, Pasricha PJ, Chen JD. Rhythmic and spatial abnormalities of gastric slow waves in patients with functional dyspepsia. J Clin Gastroenterol. 2009;43:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 108. | Riezzo G, Chiloiro M, Guerra V, Borrelli O, Salvia G, Cucchiara S. Comparison of gastric electrical activity and gastric emptying in healthy and dyspeptic children. Dig Dis Sci. 2000;45:517-524. [PubMed] |
| 109. | Cucchiara S, Bortolotti M, Colombo C, Boccieri A, De Stefano M, Vitiello G, Pagano A, Ronchi A, Auricchio S. Abnormalities of gastrointestinal motility in children with nonulcer dyspepsia and in children with gastroesophageal reflux disease. Dig Dis Sci. 1991;36:1066-1073. [PubMed] |
| 110. | Di Lorenzo C, Hyman PE, Flores AF, Kashyap P, Tomomasa T, Lo S, Snape WJ. Antroduodenal manometry in children and adults with severe non-ulcer dyspepsia. Scand J Gastroenterol. 1994;29:799-806. [PubMed] |
| 111. | Jebbink HJ, vanBerge-Henegouwen GP, Akkermans LM, Smout AJ. Small intestinal motor abnormalities in patients with functional dyspepsia demonstrated by ambulatory manometry. Gut. 1996;38:694-700. [PubMed] |
| 112. | Wilmer A, Van Cutsem E, Andrioli A, Tack J, Coremans G, Janssens J. Ambulatory gastrojejunal manometry in severe motility-like dyspepsia: lack of correlation between dysmotility, symptoms, and gastric emptying. Gut. 1998;42:235-242. [PubMed] |
| 113. | Sha W, Pasricha PJ, Chen JD. Correlations among electrogastrogram, gastric dysmotility, and duodenal dysmotility in patients with functional dyspepsia. J Clin Gastroenterol. 2009;43:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Di Lorenzo C, Reddy SN, Flores AF, Hyman PE. Is electrogastrography a substitute for manometric studies in children with functional gastrointestinal disorders? Dig Dis Sci. 1997;42:2310-2316. [PubMed] |
| 115. | Rao SS, Mysore K, Attaluri A, Valestin J. Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol. 2011;45:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Rozov-Ung I, Mreyoud A, Moore J, Wilding GE, Khawam E, Lackner JM, Semler JR, Sitrin MD. Detection of drug effects on gastric emptying and contractility using a wireless motility capsule. BMC Gastroenterol. 2014;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | DuPont AW, Jiang ZD, Harold SA, Snyder N, Galler GW, Garcia-Torres F, DuPont HL. Motility abnormalities in irritable bowel syndrome. Digestion. 2014;89:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Green AD, Belkind-Gerson J, Surjanhata BC, Mousa H, Kuo B, Di Lorenzo C. Wireless motility capsule test in children with upper gastrointestinal symptoms. J Pediatr. 2013;162:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Liebregts T, Adam B, Bredack C, Gururatsakul M, Pilkington KR, Brierley SM, Blackshaw LA, Gerken G, Talley NJ, Holtmann G. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 120. | Schäppi MG, Borrelli O, Knafelz D, Williams S, Smith VV, Milla PJ, Lindley KJ. Mast cell-nerve interactions in children with functional dyspepsia. J Pediatr Gastroenterol Nutr. 2008;47:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Friesen CA, Lin Z, Singh M, Singh V, Schurman JV, Burchell N, Cocjin JT, McCallum RW. Antral inflammatory cells, gastric emptying, and electrogastrography in pediatric functional dyspepsia. Dig Dis Sci. 2008;53:2634-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Friesen CA, Lin Z, Garola R, Andre L, Burchell N, Moore A, Roberts CC, McCallum RW. Chronic gastritis is not associated with gastric dysrhythmia or delayed solid emptying in children with dyspepsia. Dig Dis Sci. 2005;50:1012-1018. [PubMed] |
| 123. | Yin J, Levanon D, Chen JD. Inhibitory effects of stress on postprandial gastric myoelectrical activity and vagal tone in healthy subjects. Neurogastroenterol Motil. 2004;16:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | De Giorgi F, Sarnelli G, Cirillo C, Savino IG, Turco F, Nardone G, Rocco A, Cuomo R. Increased severity of dyspeptic symptoms related to mental stress is associated with sympathetic hyperactivity and enhanced endocrine response in patients with postprandial distress syndrome. Neurogastroenterol Motil. 2013;25:31-8.e2-31-8.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | van den Elzen BD, Boeckxstaens GE. Review article: a critical view on impaired accommodation as therapeutic target for functional dyspepsia. Aliment Pharmacol Ther. 2006;23:1499-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Lee HS, An YS, Kang J, Yoo JH, Lee KJ. Effect of acute auditory stress on gastric motor responses to a meal in healthy volunteers. J Gastroenterol Hepatol. 2013;28:1699-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Benninga MA, Mayer EA. The power of placebo in pediatric functional gastrointestinal disease. Gastroenterology. 2009;137:1207-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Grover M, Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J Gastroenterol. 2013;48:177-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 129. | Bouras EP, Talley NJ, Camilleri M, Burton DD, Heckman MG, Crook JE, Richelson E. Effects of amitriptyline on gastric sensorimotor function and postprandial symptoms in healthy individuals: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2008;103:2043-2050. [PubMed] |
| 130. | Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 1995;40:86-95. [PubMed] |
| 131. | Gorard DA, Libby GW, Farthing MJ. 5-Hydroxytryptamine and human small intestinal motility: effect of inhibiting 5-hydroxytryptamine reuptake. Gut. 1994;35:496-500. [PubMed] |
| 132. | Ladabaum U, Glidden D. Effect of the selective serotonin reuptake inhibitor sertraline on gastric sensitivity and compliance in healthy humans. Neurogastroenterol Motil. 2002;14:395-402. [PubMed] |
| 133. | Talley NJ, Camilleri M, Chitkara DK, Bouras E, Locke GR, Burton D, Rucker MJ, Thapa P, Zinsmeister AR. Effects of desipramine and escitalopram on postprandial symptoms induced by the nutrient drink test in healthy volunteers: a randomized, double-blind, placebo-controlled study. Digestion. 2005;72:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Wu CY, Chou LT, Chen HP, Chang CS, Wong PG, Chen GH. Effect of fluoxetine on symptoms and gastric dysrhythmia in patients with functional dyspepsia. Hepatogastroenterology. 2003;50:278-283. [PubMed] |
| 135. | Tan VP, Cheung TK, Wong WM, Pang R, Wong BC. Treatment of functional dyspepsia with sertraline: a double-blind randomized placebo-controlled pilot study. World J Gastroenterol. 2012;18:6127-6133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 136. | van Kerkhoven LA, Laheij RJ, Aparicio N, De Boer WA, Van den Hazel S, Tan AC, Witteman BJ, Jansen JB. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2008;6:746-752; quiz 718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 137. | Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, Di Lorenzo C. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. 2009;137:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Teitelbaum JE, Arora R. Long-term efficacy of low-dose tricyclic antidepressants for children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2011;53:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Campo JV, Perel J, Lucas A, Bridge J, Ehmann M, Kalas C, Monk K, Axelson D, Birmaher B, Ryan N. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: an exploratory study. J Am Acad Child Adolesc Psychiatry. 2004;43:1234-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Redstone HA, Barrowman N, Veldhuyzen Van Zanten SJ. H2-receptor antagonists in the treatment of functional (nonulcer) dyspepsia: a meta-analysis of randomized controlled clinical trials. Aliment Pharmacol Ther. 2001;15:1291-1299. [PubMed] |
| 142. | Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337. [PubMed] |
| 143. | Meineche-Schmidt V, Christensen E, Bytzer P. Randomised clinical trial: identification of responders to short-term treatment with esomeprazole for dyspepsia in primary care - a randomised, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 144. | Jones RH, Baxter G. Lansoprazole 30 mg daily versus ranitidine 150 mg b.d. in the treatment of acid-related dyspepsia in general practice. Aliment Pharmacol Ther. 1997;11:541-546. [PubMed] |
| 145. | Peura DA, Kovacs TO, Metz DC, Siepman N, Pilmer BL, Talley NJ. Lansoprazole in the treatment of functional dyspepsia: two double-blind, randomized, placebo-controlled trials. Am J Med. 2004;116:740-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 146. | Futagami S, Shimpuku M, Song JM, Kodaka Y, Yamawaki H, Nagoya H, Shindo T, Kawagoe T, Horie A, Gudis K. Nizatidine improves clinical symptoms and gastric emptying in patients with functional dyspepsia accompanied by impaired gastric emptying. Digestion. 2012;86:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. J Pediatr Gastroenterol Nutr. 2010;50:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 148. | See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci. 2001;46:985-992. [PubMed] |
| 149. | Dehghani SM, Imanieh MH, Oboodi R, Haghighat M. The comparative study of the effectiveness of cimetidine, ranitidine, famotidine, and omeprazole in treatment of children with dyspepsia. ISRN Pediatr. 2011;2011:219287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075-1082. [PubMed] |
| 151. | Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Nishi N, Chayama K, Haruma K. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 152. | Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom H, Van Oudenhove L, Simrén M, Tack J. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 153. | Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;CD001960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 154. | Riezzo G, Cucchiara S, Chiloiro M, Minella R, Guerra V, Giorgio I. Gastric emptying and myoelectrical activity in children with nonulcer dyspepsia. Effect of cisapride. Dig Dis Sci. 1995;40:1428-1434. [PubMed] |
| 155. | Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000;47:667-674. [PubMed] |
| 156. | Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354-360. [PubMed] |
| 157. | Levy J, Hayes C, Kern J, Harris J, Flores A, Hyams J, Murray R, Tolia V. Does cisapride influence cardiac rhythm? Results of a United States multicenter, double-blind, placebo-controlled pediatric study. J Pediatr Gastroenterol Nutr. 2001;32:458-463. [PubMed] |
| 158. | Mearin F, Rodrigo L, Pérez-Mota A, Balboa A, Jiménez I, Sebastián JJ, Patón C. Levosulpiride and cisapride in the treatment of dysmotility-like functional dyspepsia: a randomized, double-masked trial. Clin Gastroenterol Hepatol. 2004;2:301-308. [PubMed] |
| 159. | Lozano R, Concha MP, Montealegre A, de Leon L, Villalba JO, Esteban HL, Cromeyer M, García JR, Brossa A, Lluberes G. Effectiveness and safety of levosulpiride in the treatment of dysmotility-like functional dyspepsia. Ther Clin Risk Manag. 2007;3:149-155. [PubMed] |
| 160. | Hallerbäck BI, Bommelaer G, Bredberg E, Campbell M, Hellblom M, Lauritsen K, Wienbeck M, Holmgren LL. Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. 2002;16:959-967. [PubMed] |
| 161. | Kinoshita Y, Hashimoto T, Kawamura A, Yuki M, Amano K, Sato H, Adachi K, Sato S, Oshima N, Takashima T. Effects of famotidine, mosapride and tandospirone for treatment of functional dyspepsia. Aliment Pharmacol Ther. 2005;21 Suppl 2:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Otaka M, Jin M, Odashima M, Matsuhashi T, Wada I, Horikawa Y, Komatsu K, Ohba R, Oyake J, Hatakeyama N. New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment Pharmacol Ther. 2005;21 Suppl 2:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 163. | Du Y, Su T, Song X, Gao J, Zou D, Zuo C, Xie W, Wang B, Zhang Z, Xu J. Efficacy and safety of cinitapride in the treatment of mild to moderate postprandial distress syndrome-predominant functional dyspepsia. J Clin Gastroenterol. 2014;48:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 164. | Albibi R, McCallum RW. Metoclopramide: pharmacology and clinical application. Ann Intern Med. 1983;98:86-95. [PubMed] |
| 165. | Fumagalli I, Hammer B. Cisapride versus metoclopramide in the treatment of functional dyspepsia. A double-blind comparative trial. Scand J Gastroenterol. 1994;29:33-37. [PubMed] |
| 166. | Banani SJ, Lankarani KB, Taghavi A, Bagheri MH, Sefidbakht S, Geramizadeh B. Comparison of metoclopramide oral tablets and solution in treatment of dysmotility-like dyspepsia. Am J Health Syst Pharm. 2008;65:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 167. | Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 168. | Halter F, Staub P, Hammer B, Guyot J, Miazza BM. Study with two prokinetics in functional dyspepsia and GORD: domperidone vs. cisapride. J Physiol Pharmacol. 1997;48:185-192. [PubMed] |
| 169. | Van Outryve M, De Nutte N, Van Eeghem P, Gooris JP. Efficacy of cisapride in functional dyspepsia resistant to domperidone or metoclopramide: a double-blind, placebo-controlled study. Scand J Gastroenterol Suppl. 1993;195:47-52; discussion 52-53. [PubMed] |
| 170. | Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 171. | Huang X, Lv B, Zhang S, Fan YH, Meng LN. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol. 2012;18:7371-7377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 172. | Cucchiara S, Minella R, Scoppa A, Emiliano M, Calabrese F, Az-Zeqeh N, Rea B, Salvia G. Antroduodenal motor effects of intravenous erythromycin in children with abnormalities of gastrointestinal motility. J Pediatr Gastroenterol Nutr. 1997;24:411-418. [PubMed] |
| 173. | Arts J, Caenepeel P, Verbeke K, Tack J. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut. 2005;54:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 174. | Talley NJ, Verlinden M, Snape W, Beker JA, Ducrotte P, Dettmer A, Brinkhoff H, Eaker E, Ohning G, Miner PB. Failure of a motilin receptor agonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2000;14:1653-1661. [PubMed] |
| 175. | McCallum RW, Cynshi O. Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment Pharmacol Ther. 2007;26:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 176. | Takanashi H, Cynshi O. Motilides: a long and winding road: lessons from mitemcinal (GM-611) on diabetic gastroparesis. Regul Pept. 2009;155:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 177. | Kamerling IM, Van Haarst AD, Burggraaf J, Schoemaker RC, Biemond I, Heinzerling H, Jones R, Cohen AF, Masclee AA. Motilin effects on the proximal stomach in patients with functional dyspepsia and healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2003;284:G776-G781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Cuomo R, Vandaele P, Coulie B, Peeters T, Depoortere I, Janssens J, Tack J. Influence of motilin on gastric fundus tone and on meal-induced satiety in man: role of cholinergic pathways. Am J Gastroenterol. 2006;101:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 179. | Kusunoki H, Haruma K, Manabe N, Imamura H, Kamada T, Shiotani A, Hata J, Sugioka H, Saito Y, Kato H. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24:540-545, 540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 180. | Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 181. | Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 182. | Miwa H, Nagahara A, Tominaga K, Yokoyama T, Sawada Y, Inoue K, Ashida K, Fukuchi T, Hojo M, Yamashita H. Efficacy of the 5-HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. Am J Gastroenterol. 2009;104:2779-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 183. | Malatesta MG, Fascetti E, Ciccaglione AF, Cappello G, Grossi L, Ferri A, Marzio L. 5-HT1-receptor agonist sumatriptan modifies gastric size after 500 ml of water in dyspeptic patients and normal subjects. Dig Dis Sci. 2002;47:2591-2595. [PubMed] |
| 184. | Marzio L, Cappello G, Grossi L, Manzoli L. Effect of the 5-HT3 receptor antagonist, ondansetron, on gastric size in dyspeptic patients with impaired gastric accommodation. Dig Liver Dis. 2008;40:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 185. | Vakil N, Laine L, Talley NJ, Zakko SF, Tack J, Chey WD, Kralstein J, Earnest DL, Ligozio G, Cohard-Radice M. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am J Gastroenterol. 2008;103:1906-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 186. | Tack J, Broekaert D, Coulie B, Fischler B, Janssens J. Influence of the selective serotonin re-uptake inhibitor, paroxetine, on gastric sensorimotor function in humans. Aliment Pharmacol Ther. 2003;17:603-608. [PubMed] |
| 187. | Rodriguez L, Diaz J, Nurko S. Safety and efficacy of cyproheptadine for treating dyspeptic symptoms in children. J Pediatr. 2013;163:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 188. | Sadeghian M, Farahmand F, Fallahi GH, Abbasi A. Cyproheptadine for the treatment of functional abdominal pain in childhood: a double-blinded randomized placebo-controlled trial. Minerva Pediatr. 2008;60:1367-1374. [PubMed] |
| 189. | Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 190. | Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2013;53:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 191. | Kline RM, Kline JJ, Di Palma J GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138:125-128. [PubMed] |
| 192. | Braden B, Caspary W, Börner N, Vinson B, Schneider AR. Clinical effects of STW 5 (Iberogast) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil. 2009;21:632-638, e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 193. | Wu KL, Rayner CK, Chuah SK, Changchien CS, Lu SN, Chiu YC, Chiu KW, Lee CM. Effects of ginger on gastric emptying and motility in healthy humans. Eur J Gastroenterol Hepatol. 2008;20:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 194. | Hu ML, Rayner CK, Wu KL, Chuah SK, Tai WC, Chou YP, Chiu YC, Chiu KW, Hu TH. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J Gastroenterol. 2011;17:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 195. | Inamori M, Akiyama T, Akimoto K, Fujita K, Takahashi H, Yoneda M, Abe Y, Kubota K, Saito S, Ueno N. Early effects of peppermint oil on gastric emptying: a crossover study using a continuous real-time 13C breath test (BreathID system). J Gastroenterol. 2007;42:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 196. | Papathanasopoulos A, Rotondo A, Janssen P, Boesmans W, Farré R, Vanden Berghe P, Tack J. Effect of acute peppermint oil administration on gastric sensorimotor function and nutrient tolerance in health. Neurogastroenterol Motil. 2013;25:e263-e271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 197. | von Arnim U, Peitz U, Vinson B, Gundermann KJ, Malfertheiner P. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 198. | O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009;33:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 199. | Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 200. | Vlieger AM, Rutten JM, Govers AM, Frankenhuis C, Benninga MA. Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol. 2012;107:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 201. | van Tilburg MA, Chitkara DK, Palsson OS, Turner M, Blois-Martin N, Ulshen M, Whitehead WE. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics. 2009;124:e890-e897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 202. | Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11:217-223. [PubMed] |
| 203. | Schurman JV, Wu YP, Grayson P, Friesen CA. A pilot study to assess the efficacy of biofeedback-assisted relaxation training as an adjunct treatment for pediatric functional dyspepsia associated with duodenal eosinophilia. J Pediatr Psychol. 2010;35:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |