Published online Jul 5, 2021. doi: 10.4292/wjgpt.v12.i4.79
Peer-review started: February 2, 2021
First decision: February 24, 2021
Revised: March 22, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 5, 2021
Processing time: 148 Days and 13.6 Hours
Preparation for colon capsule endoscopy (CCE) requires a large liquid laxative volume for capsule excretion, which compromises the procedure's tolerability.
To assess the safety and utility of castor oil-boosted bowel preparation.
This prospective cohort study including 20 patients (age range, 16-80 years; six men and 14 women) suspected of having colorectal disease was conducted at Kindai University Hospital from September 2017 to August 2019. All patients underwent CCE because of the following inclusion criteria: previous incomplete colonoscopy in other facility (n = 20), history of abdominal surgery (n = 7), or organ abnormalities such as multiple diverticulum (n = 4) and adhesion after surgery (n = 6). The exclusion criteria were as follows: Dysphagia, history of allergic reactions to the drugs used in this study (magnesium citrate, polyethylene glycol, metoclopramide, and castor oil), possibility of pregnancy, possibility of bowel obstruction or stenosis based on symptoms, or scheduled magnetic resonance imaging within 2 wk after CCE. The primary outcome was the capsule excretion rate within the battery life, as evaluated by the total large bowel observation rate, large bowel transit time, and bowel creasing level using a five-grade scale in different colorectal segments. The secondary outcomes were complications, colorectal lesion detection rates, and patients’ tolerability.
The castor oil-based regimen was implemented in 17 patients. Three patients cancelled CCE because they could tolerate castor oil, but not liquid laxatives. The capsule excretion rate within the battery life was 88% (15/17). The mean large bowel transit time was 236 min. Approximately 70% of patients had satisfactory colon cleansing levels. CCE detected colon polyps (14/17, 82%) and colonic diverticulum (4/12, 33%). The sensitivity, specificity, and diagnostic accuracy rates for detecting colorectal polyps (size ≥ 6 mm) were 76.9%, 75.0%, and 76.4%, respectively. The sensitivity, specificity, and diagnostic accuracy rates for detection of diverticulum were 100% each. Twelve patients (71%) rated CCE as more than “good”, confirming the new regimen’s tolerability. No serious adverse events occurred during this study.
The castor oil-based regimen could reduce bowel preparation dose and improve CCE tolerability.
Core Tip: Castor oil, a vegetable oil collected from castor oil plant seeds, is hydrolyzed into glycerin and retinoic acid in the small intestine, stimulating bowel movement in the small intestine. Among patients treated with castor oil as a booster, the rate of capsule excretion within battery life was 88%, whereas 70% of them had a more than “good” bowel cleansing level. The questionnaire of tolerability compared with previous colonoscopy showed that 71% of patients were satisfied with the new colon capsule endoscopy procedure. Sensitivity, specificity, and diagnostic accuracy of detecting colorectal polyps (size ≥ 6 mm) were 76.9%, 75.0%, and 76.4%, respectively.
- Citation: Takashima K, Komeda Y, Sakurai T, Masaki S, Nagai T, Matsui S, Hagiwara S, Takenaka M, Nishida N, Kashida H, Nakaji K, Watanabe T, Kudo M. Castor oil as booster for colon capsule endoscopy preparation reduction: A prospective pilot study and patient questionnaire. World J Gastrointest Pharmacol Ther 2021; 12(4): 79-89
- URL: https://www.wjgnet.com/2150-5349/full/v12/i4/79.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v12.i4.79
Colonoscopy is a well-established examination for detecting various types of colorectal diseases, including inflammatory bowel disease and colorectal tumors[1-3]. In addition to disease detection, colonoscopy plays an indispensable role in colorectal cancer (CRC) prevention through detection and removal of precancerous adenomatous polyps and early CRC. Although colonoscopy is usually performed under conscious sedation, a significant number of patients undergoing colonoscopy complain of pain and discomfort even under sedation. Thus, pain associated with colonoscopy is a major obstacle that prevents patients from undergoing this procedure for colorectal disease detection.
Such painful nature of colonoscopy is considered to result in a lower examination attendance rate than that of other types of cancer screening methods[4,5]. Colon capsule endoscopy (CCE), a recently developed technique for the detection of colorectal diseases, was approved for reimbursement under the national health insurance system of Japan in 2014. CCE is recognized as a noninvasive imaging modality that can be performed in patients complaining of colonoscopy-associated pain and discomfort. In fact, the usefulness of CCE as an alternative screening method for CRC prevention has been reported by several groups[6-8]. However, a major weakness of CCE lies on the fact that bowel preparations for CCE require a larger volume of laxative than that used in conventional colonoscopy because of the need for completion of capsule excretion[9-13]. For smooth capsule excretion, > 4.5 L of polyethylene glycol (PEG) is usually required for CCE[9-13]. Thus, patients need to take more than twice the volume of PEG for the observation of the entire colon by CCE than that used for conventional colonoscopy. Such a high liquid laxative volume may reduce patients’ tolerability and compliance.
Therefore, the development of a new bowel preparation method with comparable liquid volume to colonoscopy is necessary to increase patient tolerability of CCE. One way to achieve clean preparation and volume reduction of liquid laxative is to use a booster to accelerate capsule excretion though the colon. Castor oil, a type of vegetable oil collected from the castor oil plant seeds, is hydrolyzed into glycerin and retinoic acid by lipase in the small intestine, which stimulates the bowel movement in the small intestine[14-16]. Castor oil is widely used as a laxative in traditional medicine in western countries[14-16]. Indeed, several regimens consisting of PEG and castor oil, the latter of which functions as a booster, were proposed for the reduction of laxative liquid volumes. Such a booster effect by castor oil has the potential to accelerate capsule excretion through the colon and reduce the volume of the liquid laxative. Thus, the use of castor oil as a booster may help us develop tolerable bowel preparation methods for patients receiving CCE.
In this study, we aimed to determine the feasibility of a new bowel preparation regimen consisting of a low volume of PEG (2 L) combined with castor oil as a booster and provide evidence that it can achieve both effective capsule excretion and sufficient colon cleansing in CCE.
In total, 20 patients who were suspected of having colorectal diseases were enrolled in this study. This prospective pilot cohort study was performed at Kindai University Hospital from September 2017 to August 2019. This study was approved by the Institutional Review Board of Kindai University Hospital (29-087) and the procedures were in accordance with the Declaration of Helsinki. All study participants, or their legal guardian, provided written consent prior to study enrollment. The clinical trial is registered with University Hospital Medical Information Network, using identifier UMIN000028694. Details can be found at https://upload.umin.ac.jp/cgi-openbin/ ctr_e/ctr_view.cgi?recptno=R000032809.
Patients aged between 16 and 80 years suspected of having colorectal disease were included. All 20 patients (age range, 16-80 years; sex, six men and 14 women) underwent CCE because of previous incomplete colonoscopy in other facilities (n = 20), history of abdominal surgery (n = 7), or organ abnormalities, such as multiple diverticulum (n = 4) and adhesion after surgery (n = 6).
The exclusion criteria for this study were dysphagia, history of allergic reactions to the drugs used in this study (magnesium citrate, PEG, metoclopramide, and castor oil), possibility of pregnancy, possibility of bowel obstruction or stenosis based on symptoms, or scheduled magnetic resonance imaging within 2 wk after CCE.
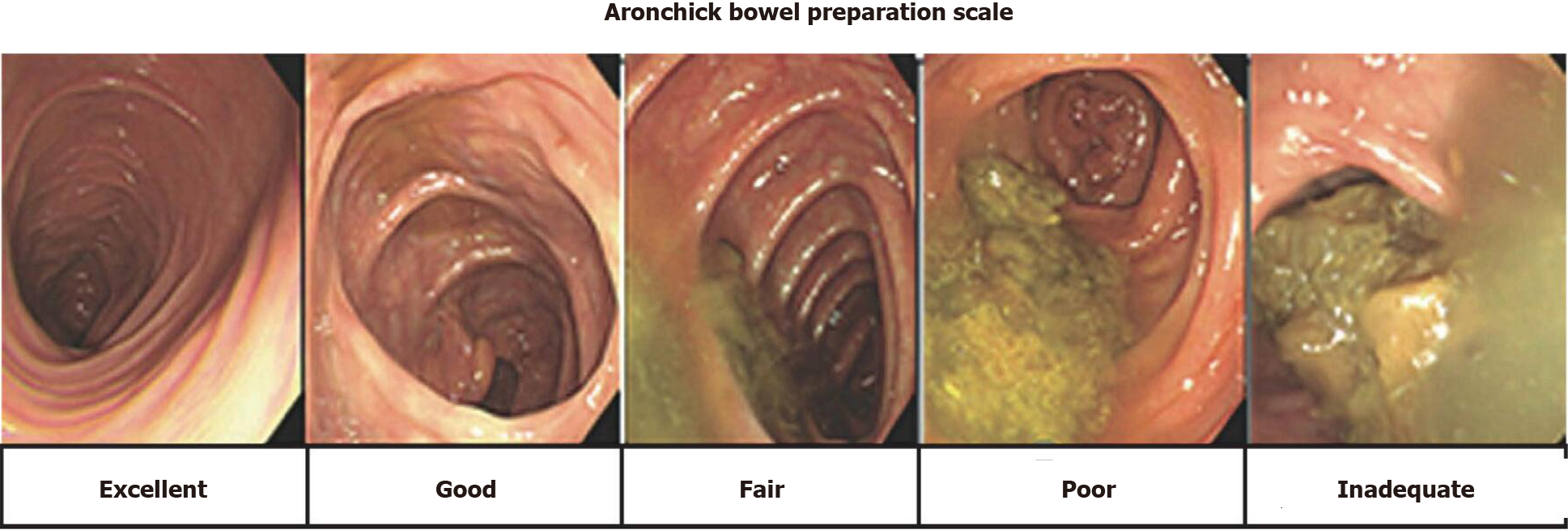
The primary outcome was the capsule excretion rate within battery life as evaluated by total large bowel observation rate, large bowel transit time, and bowel cleansing level using a five-point scale (excellent, good, fair, poor, and inadequate), as described in the Aronchick Global Assessment Scale[17] (Figure 1). The degree of colon cleansing level was rated in different segments of the colorectum (cecum, ascending colon, transverse colon, descending-sigmoid colon, and rectum).
The secondary outcomes were complications, diagnostic accuracy of colorectal lesion, and patients’ tolerability evaluated using the CCE questionnaire. The diagnosis of colorectal disease obtained by CCE was verified by subsequent colonoscopy in our university hospital (Kindai University Hospital), which is a high-volume center and, thus, it is fully equipped with endoscopy devices and many experienced colonoscopists. Therefore, our facility can perform total colonoscopy even in patients who have undergone incomplete colonoscopy at other facilities.
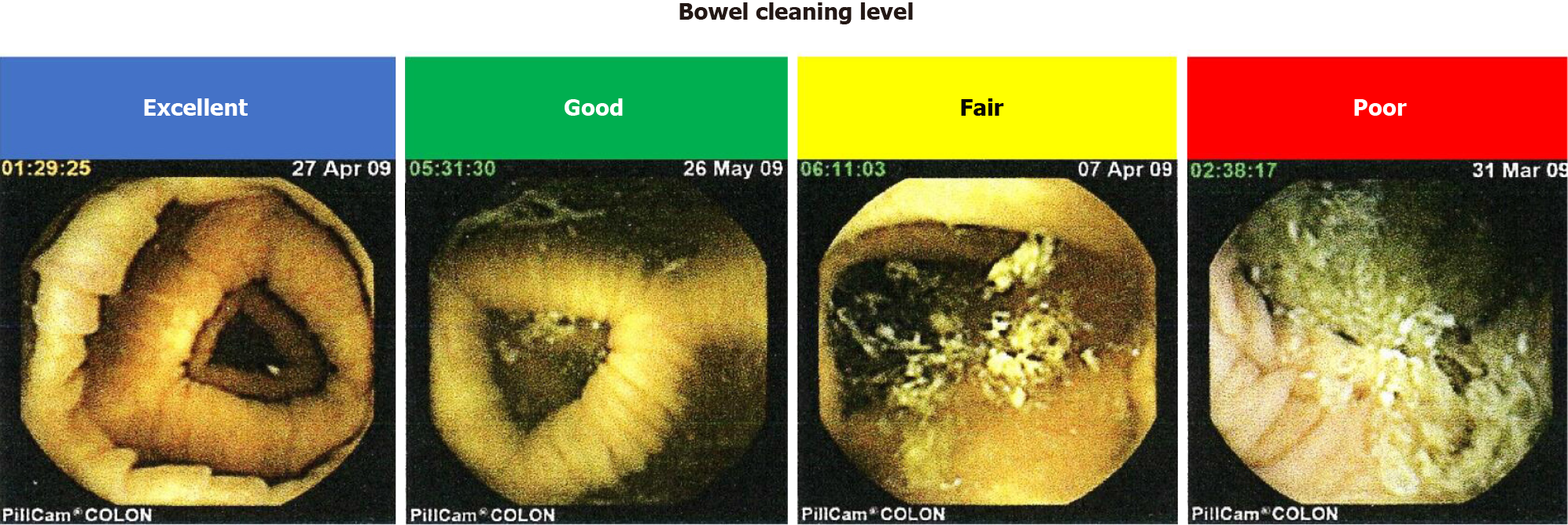
To assess patient-reported outcome, the patients were asked to complete a questionnaire regarding the tolerability of CCE. The questionnaire included CCE bowel preparation, taste of castor oil as a booster, total period of CCE procedure, and overall evaluation of CCE examination. The satisfaction level was rated on a five-point scale (excellent, very good, good, fair, and poor) in each point.
CCE was performed using PillCam COLON Capsule (Medtronic, Minneapolis, MN, USA). The details of our modified CCE regimen using castor oil, as a booster, are shown in Table 1.
| Modified colon capsule endoscopy regimen | ||
| Day before endoscopy | 21:00 | Magnesium citrate P 50 g + water 180 mL |
| Day of endoscopy | 06:00 | PEG 1000 mL + water 500 mL |
| 09:00 | Patient comes to the hospital | |
| 09:30 | Capsule ingestion, intravenous metoclopramide 10 mg | |
| 10:00 | Patient goes home after the capsule reaches the duodenum | |
| When patient reaches home | Castor oil 20 mL, PEG 500 mL + water 250 mL | |
| 1 h later | PEG 500 mL + water 250 mL | |
| 2 h later | Magnesium citrate 50 g + water 180 mL | |
| 2 h later | Castor oil 20 mL | |
Our regimen used 50 g of magnesium citrate (Magcorol P; Horii Pharmaceutical Industry, Ltd, Osaka, Japan) dissolved in 180 mL of water on the day before examination. On the examination day, patients took 1 L of PEG solution plus ascorbic acid (MoviPrep; EA Pharma Co., Ltd, Tokyo, Japan) together with 500 mL of water at 6:00 AM. Patients visited the hospital at 9:00 AM and took the capsule 30 min later. Then, metoclopramide (10 mg) intravenously administered. Patients went home after the capsule reached the duodenum. When they got home, they took 20 mL of castor oil (Himashi Oil; Yoshida Pharmaceutical, Tokyo, Japan) as a booster, together with 500 mL Moviprep and 250 mL water. After 1 h, they took 500 mL of Moviprep and 250 of mL water. When the capsule was not excreted at 2 h after receiving the castor oil, they took additional Magcorol P (50 g) dissolved in 180 mL water together with 20 mL of castor oil. Our regimen required 2 L of PEG, which is approximately 50% of the volume of the bowel preparation without castor oil.
The degree of the colon cleansing level was rated in different segments of the colorectum (cecum, ascending colon, transverse colon, descending-sigmoid colon, and rectum), as described in the Aronchick Global Assessment Scale[17], and the overall cleansing level in the entire colon was determined.
The sensitivity, specificity, positive predictive value, negative predictive value, accuracy of adenoma detection ≥ 6 mm, accuracy of adenoma detection ≤ 5 mm, and diverticula detection were calculated for each classification category. The statistical methods of this study were reviewed by biomedical statistician Satoru Hagiwara from Kindai University Hospital.
Seventeen patients (85%) successfully followed our modified CCE regimen using castor oil. Three patients cancelled CCE because they could tolerate only castor oil, but not liquid laxatives. Seven patients experienced incomplete colonoscopy because of an abdominal surgery history (Table 2). The reasons for taking CCE were abdominal pain, diarrhea or constipation, elevation of tumor markers, anemia, bloody stool, and follow-up examination results after colon polypectomy or after colon cancer operation.
| Characteristics | |
| Age (yr) | |
| mean ± SD | 59.5 ± 16.8 |
| Range | 37-80 |
| Sex, n (%) | |
| Male | 3 (18) |
| Female | 14 (82) |
| History of abdominal surgery, n (%) | 7 (41) |
| Reason for colon capsule endoscopy, n (%) | |
| Abdominal pain | 5 (29) |
| Constipation | 5 (29) |
| Elevation of tumor marker | 2 (12) |
| Follow-up examination after polypectomy | 2 (12) |
| Anemia | 1 (6) |
| Bloody stool | 1 (6) |
| Follow-up after colon cancer surgery | 1 (6) |
As shown in Table 3, the capsule excretion rate within the battery life was 88% (15/17) among patients treated with castor oil as a booster. Two patients did not expel the capsule within the battery life duration. Thus, the combination of PEG with castor oil for bowel preparation promoted capsule excretion in CCE.
| Variables | n (%) or mean (range) |
| Capsule excretion rate within battery life | 15 (88) |
| Large bowel transit time (min) | 236 (16-725) |
| Cases within 60 min | 5 (29) |
| Cleansing level (overall) | 12 (70) |
| Excellent | 6 |
| Good | 6 |
| Fair | 5 |
| Poor | 0 |
| Polyp detection rate | 14 (82) |
The median time of large bowel transition was 236 min. The overall cleansing level of the colon was “excellent”, “good”, “fair”, and “poor” in seven, five, four, and one cases, respectively. No cases were judged as having “inadequate” cleansing (Tables 3 and 4). Thus, > 70% (12/17) of patients treated with PEG in combination with castor oil as a booster exhibited enough level of colon cleansing for the detection of colorectal diseases by CCE. More detailed information regarding the colon cleansing levels at five different sites is shown in Figure 2.
| Disorder | No disorder | |
| Detection of adenoma ≥ 6 mm | ||
| Positive test result | True positive: 10 | False positive: 1 |
| Negative test result | False negative: 3 | True negative: 3 |
| Sensitivity, 76.9%; Specificity, 75.0%; Diagnostic accuracy, 76.5% | ||
| Detection of adenoma ≤ 5 mm | ||
| Positive test result | True positive: 2 | False positive: 1 |
| Negative test result | False negative: 3 | True negative: 3 |
| Sensitivity, 50.0%; Specificity, 66.7%; Diagnostic accuracy, 55.6% | ||
| Detection of diverticulums | ||
| Positive test result | True positive: 4 | False positive: 0 |
| Negative test result | False negative: 0 | True negative: 13 |
| Sensitivity, 100%; Specificity, 100%; Diagnostic accuracy, 100% | ||
The percentage of those who had a more than “good” bowel cleansing level was 70% (12/17). The percentages of patients exhibiting a cleansing level of “excellent” or “very good” were higher in the proximal than in the descending-sigmoid colon. Thus, the distal sites of the colon tended to show poor cleansing compared with the proximal sites. Such lower cleansing levels at the descending-sigmoid colon can be partially explained by the presence of diverticulum at this site. In fact, the diverticulum was detected in four (80%) out of five patients with a “fair” cleansing level at the descending-sigmoid colon. Although floating of oil originating from the castor oil degradation was sometimes observed, the presence of oil in the colonic lumen did not affect the detection of colorectal disease.
CCE detected colon polyps (14/17, 82%) and colonic diverticulum (4/12, 33%). No patient had CRCs or inflammatory bowel disease (Table 3). These colorectal diseases diagnosed by CCE were verified by subsequent colonoscopy.
The sensitivity, specificity, and diagnostic accuracy rates in adenoma detection of ≥ 6 mm were 76.9%, 75.0%, and 76.4%, respectively (Table 4). Most cases of inconsistent diagnosis between CCE and colonoscopy were those bearing colon polyps ≤ 5 mm because the diagnostic accuracy for small polyps ≤ 5 mm was low. Indeed, the sensitivity, specificity, and diagnostic accuracy rates in detecting adenoma ≤ 5 mm were 50.0%, 66.7%, and 55.6%, respectively (Table 4). Regarding the detection of diverticulum, the sensitivity, specificity, and diagnostic accuracy rates were all 100% (Table 4).
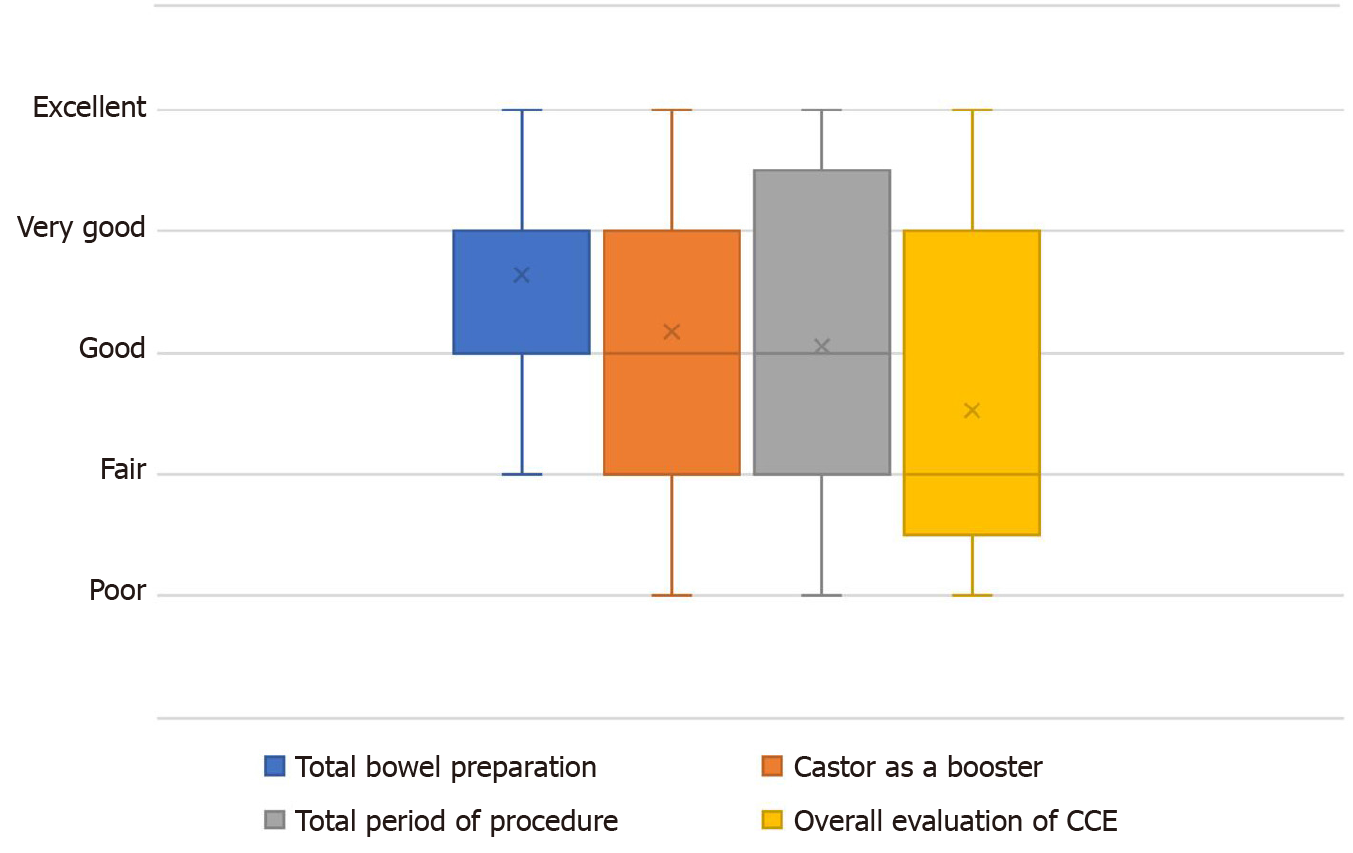
All participants completed the questionnaire regarding the tolerability of the bowel preparation method using castor oil as a booster for CCE. The results of the questionnaire evaluating the tolerability of our new CCE procedure from four different aspects are shown in Figure 3. Concerning total preparation of CCE, castor oil as a booster, total procedure time, and overall evaluation of CCE, 41% (7/17), 53% (9/17), 59% (10/17), and 71% (12/17) of the participants, respectively, graded each component of our new procedure as more than “good”.
None of the patients experienced adverse events associated with the use of castor oil as a booster, such as bleeding, perforation, abdominal pain, vomiting, aspiration pneumonia, or allergic reaction.
In this study, we assessed the safety and utility of castor oil-boosted bowel preparation for CCE and found that this method can achieve capsule excretion and colon cleansing in CCE. This new bowel preparation method enabled us to reduce the volume of liquid laxatives to 3.5 L after using castor oil as a booster, as evidenced by the fact that 17 patients (85%) successfully completed CCE using our castor oil-boosted bowel preparation without causing severe adverse events. As aforementioned, conventional laxative regimen for CCE requires large amounts of liquid laxatives (4.5-6.0 L) to obtain a sufficient capsule excretion rate (70%-95%)[9,11,12,18,19]. Nakaji et al[20] showed that in their historical control group, in which patients did not receive castor oil (total liquid laxatives, 4.1 L; n = 82), the capsule excretion rate (total large intestine observation) was 83%, the average colon transit time was 259 min, the bowel cleaning level (excellent/good) was 82% and the colorectal polyp detection rate was 49%. Interestingly, no adverse events were observed.
Our castor oil-boosted regimen achieved a significant reduction in the liquid laxative volume and a high capsule excretion rate (15/17, 88%). Such a reduction in liquid laxative volume achieved tolerability of CCE, as > 70% of patients were satisfied with the CCE in the overall assessment in the questionnaire scores. In addition to tolerability, the colon cleansing levels by castor oil-based bowel preparation methods were comparable to those of conventional preparation methods. Collectively, these data suggested that the castor oil-boosted bowel preparation method is useful and safe for CCE.
Although the castor oil-boosted colon preparation regimen used in this study was tolerable in most patients undergoing CCE, there is still room for improvement in our regimen. In the questionnaire, half (54%) of the patients pointed out the unique taste and sticky texture of castor oil. Therefore, it is desirable to encapsulate or add flavor to the oil to resolve this issue. Nevertheless, > 70% of the patients rated the castor oil-boosted CCE in the present form as “good” or “better”. Moreover, 76% of the patients indicated that they are willing to undergo CCE in the next examination.
Aside from castor oil, other boosters are used for the reduction of liquid laxative volume. Togashi et al[21] used gastrografin and reported a high capsule excretion rate within battery life (97%). However, gastrografin cannot be taken by individuals allergic to iodine. Thus, it is not widely used as a booster for CCE.
In line with our data, Ohmiya et al[22] recently reported the safety and feasibility of castor oil-boosted bowel preparation methods for CCE in a multicenter retrospective study. In their study, the capsule excretion rate within its battery life with castor oil was 97%, which is comparable to that of gastrografin-boosted preparation. Given the possibility of allergic reactions to gastrografin, castor oil appears to be superior as a booster despite the almost equal capsule excretion rates and the comparable volumes of liquid laxatives.
In our study, the mean large bowel transit time was 236 min (range, 16–725 min), which was longer than that reported by Ohmiya et al[22]. The longer colon transit time in our regimen as compared with that reported in the previous study[22] may be explained by the presence or absence of sodium picosulfate, sennoside, or mosapride. They administered sodium picosulfate or sennoside the day before CCE and mosapride on the day of CCE. In contrast, the patients enrolled in this study did not take any of these drugs. Therefore, additional laxatives and mosapride may further enhance the efficacy of castor oil-boosted bowel preparation for CCE. Whether the addition of sodium picosulfate, sennoside, or mosapride is absolutely required for CCE with castor oil-boosted awaits the performance of further prospective studies. Interestingly, a significant number of patients (5/17, 29%) exhibited very fast colon transit times (within 60 min) in our regimen, and the findings were consistent in 80% of them. In contrast, four cases were found in the group with a very slow transit time of ≥ 300 min, and the findings were consistent in 75% of them. However, we did not obtain significantly different results because of the limited data.
Concerning the colorectal cleansing levels, > 70% (12/17) of the patients who used our castor oil-boosted regimen achieved more than “good” bowel preparation. These data regarding the cleansing level were comparable to those of previous reports[10,13,23-25]. Therefore, the addition of sodium picosulfate, sennoside, or mosapride to our regimen can affect the colon transit time rather than the cleansing level. Despite a relatively small number of cases and poor detection rates of adenomas ≤ 5 mm and no sampling the tissue for capsule endoscopy as inherent limitation, we evaluated the diagnostic performance of colorectal polyps. The sensitivity, specificity, and diagnostic accuracy rates in detecting colorectal polyps with a maximum size ≥ 6 mm were approximately 75%. Comparable to our present data, the sensitivity and specificity rates of second-generation CCE with at least one polyp with a size ≥ 6 and ≤ 5 mm were reported to range between 84% and 94% and between 64% and 94%, respectively[7,24-27].
Reduction of liquid laxative volume and sufficient capsule rate can be achieved using our castor oil-boosted bowel preparation method for CCE. This study provides evidence regarding the safety and feasibility of this new bowel preparation method despite its limitation in the study design (i.e., the small cohort size in a single university hospital). Nevertheless, it should be emphasized that the castor oil-boosted bowel preparation may help us perform tolerable and safe CCE, and this needs to be confirmed in future prospective multicenter studies.
Colon capsule endoscopy (CCE) is a usefulness imaging modality because it can be performed non-invasively. However, there is one major limitation of CCE, as bowel preparations for CCE require a larger volume of laxative than that used in conventional colonoscopy because of the need for capsule excretion completion. Therefore, the development of a new bowel preparation method with comparable liquid volume to colonoscopy is necessary to increase patients’ CCE tolerability.
Castor oil could have the potential to accelerate the capsule excretion through the colon and reduce the volume of the liquid laxative.
In this study, we attempted to clarify the effectiveness and tolerability of our modified regimen, which uses castor oil as a booster.
Twenty patients suspected of colorectal diseases were enrolled in this prospective cohort study. We used modified CCE regimen using castor oil as a booster. The capsule excretion rate within the battery life, bowel cleansing level in different segments of the colorectum, and detection rates of colorectal lesions were evaluated. In this study, we asked the patients to complete a questionnaire to assess the CCE tolerability.
Seventeen patients (85%) successfully followed our castor oil–based regimen, whereas three patients (15%) were unable to ingest castor oil because of its taste and failed to expel the capsule within the duration of battery life. The mean large bowel transit time was 236 min. The percentage of patients with satisfactory colon cleansing levels was 70%. The sensitivity, specificity, and diagnostic accuracy rates in detecting colorectal polyps with a size ≥ 6 mm were 76.9%, 75.0%, and 76.4%, respectively. Twelve patients (71%) evaluated the CCE procedure as more than “good” in the questionnaire, thus confirming the tolerability of our new regimen.
This study shows the safety and utility of modified bowel preparation for CCE, which uses castor oil, and found that that it can achieve capsule excretion, colon cleansing, high tolerability of CCE preparation, and reduction of liquid laxative volume.
A prospective multicenter trial is required to assess the safety and utility of castor oil–boosted bowel preparation for CCE.
We would like to thank Professor Tsuji N, Dr. Honjo H, Dr. Kono M, Dr. Okamoto K, and Dr. Yamada M for the endoscopic examinations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta N, Schwabl P S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1504] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3128] [Article Influence: 97.8] [Reference Citation Analysis (1)] |
| 3. | Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, Ederle A, Fantin A, Ferrari A, Fracchia M, Ferrero F, Gasperoni S, Recchia S, Risio M, Rubeca T, Saracco G, Zappa M; SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132:2304-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Lisi D, Hassan C, Crespi M; AMOD Study Group. Participation in colorectal cancer screening with FOBT and colonoscopy: an Italian, multicentre, randomized population study. Dig Liver Dis. 2010;42:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Holleran G, Leen R, O'Morain C, McNamara D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy. 2014;46:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Rondonotti E, Borghi C, Mandelli G, Radaelli F, Paggi S, Amato A, Imperiali G, Terreni N, Lenoci N, Terruzzi V, Baccarin A, Martegani A, Spinzi G. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol. 2014;12:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Rex DK, Adler SN, Aisenberg J, Burch WC Jr, Carretero C, Chowers Y, Fein SA, Fern SE, Fernandez-Urien Sainz I, Fich A, Gal E, Horlander JC Sr, Isaacs KL, Kariv R, Lahat A, Leung WK, Malik PR, Morgan D, Papageorgiou N, Romeo DP, Shah SS, Waterman M. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015; 148: 948-957. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Sieg A, Friedrich K, Sieg U. Is PillCam COLON capsule endoscopy ready for colorectal cancer screening? Am J Gastroenterol. 2009;104:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, Costamagna G, Riccioni ME, Spada C, Petruzziello L, Fraser C, Postgate A, Fitzpatrick A, Hagenmuller F, Keuchel M, Schoofs N, Devière J. Capsule endoscopy vs colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Gay G, Delvaux M, Frederic M, Fassler I. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy? Am J Gastroenterol. 2010;105:1076-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Watson WC, Gordon RS Jr. Studies on the digestion, absorption and metabolism of castor oil. Biochem Pharmacol. 1962;11:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 15. | Gaginella TS, Phillips SF. Ricinoleic acid: current view of an ancient oil. Am J Dig Dis. 1975;20:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci USA. 2012;109:9179-9184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, Carretero C, Charton JP, Van Gossum A, Wientjes CA, Sacher-Huvelin S, Delvaux M, Nemeth A, Petruzziello L, de Frias CP, Mayershofer R, Amininejad L, Dekker E, Galmiche JP, Frederic M, Johansson GW, Cesaro P, Costamagna G. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc 2011; 74: 581-589. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Argüelles-Arias F, San-Juan-Acosta M, Belda A, García-Montes JM, Pellicer F, Polo J, Caunedo-Álvarez Á, Herrerías-Gutiérrez JM. Preparations for colon capsule endoscopy. Prospective and randomized comparative study between two preparations for colon capsule endoscopy: PEG 2 Liters + ascorbic acid vs PEG 4 Liters. Rev Esp Enferm Dig. 2014;106:312-317. [PubMed] |
| 20. | Nakaji K, Matsumoto H, Shiotani A, Oka S, Kunihara S, Tanaka S, Ohda Y, Miwa H, Hamamoto T, Kawano S, Igawa A, Okada H, Kobayashi M, Takahashi S, Higaki S, Nakae Y. Prospective and multicenter study of bowel preparation consisting of polyethylene glycol that contained ascorbic acid and glycerin enema for colon capsule endoscopy. Gastroenterol Endosc. 2019;61:2590-2596. [DOI] [Full Text] |
| 21. | Togashi K, Fujita T, Utano K, Waga E, Katsuki S, Isohata N, Endo S, Lefor AK. Gastrografin as an alternative booster to sodium phosphate in colon capsule endoscopy: safety and efficacy pilot study. Endosc Int Open. 2015;3:E659-E661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ohmiya N, Hotta N, Mitsufuji S, Nakamura M, Omori T, Maeda K, Okuda K, Yatsuya H, Tajiri H. Multicenter feasibility study of bowel preparation with castor oil for colon capsule endoscopy. Dig Endosc. 2019;31:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, Kopelman Y, Adler SN. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Kakugawa Y, Saito Y, Saito S, Watanabe K, Ohmiya N, Murano M, Oka S, Arakawa T, Goto H, Higuchi K, Tanaka S, Ishikawa H, Tajiri H. New reduced volume preparation regimen in colon capsule endoscopy. World J Gastroenterol. 2012;18:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Hartmann D, Keuchel M, Philipper M, Gralnek IM, Jakobs R, Hagenmüller F, Neuhaus H, Riemann JF. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012;44:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Minelli Grazioli L, Senore C, Brizi G, Costamagna I, Alvaro G, Iannitti M, Salsano M, Ciolina M, Laghi A, Bonomo L, Costamagna G. Colon capsule vs CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015;64:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Saito Y, Saito S, Oka S, Kakugawa Y, Matsumoto M, Aihara H, Watari I, Aoyama T, Nouda S, Kuramoto T, Watanabe K, Ohmiya N, Higuchi K, Goto H, Arakawa T, Tanaka S, Tajiri H. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. Gastrointest Endosc. 2015;82:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |