Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.193
Peer-review started: April 12, 2015
First decision: July 10, 2015
Revised: July 22, 2015
Accepted: September 10, 2015
Article in press: September 16, 2015
Published online: November 15, 2015
Processing time: 220 Days and 20.8 Hours
Chronic pancreatitis (CP) is a chronic inflammatory disease of the pancreas. The main symptom of patients with CP is chronic and severe abdominal pain. However, the pathophysiology of pain in CP remains obscure. Traditionally, researchers believed that the pain was caused by anatomical changes in pancreatic structure. However, treatment outcomes based on such beliefs are considered unsatisfactory. The emerging explanations of pain in CP are trending toward neurobiological theories. This article aims to review current evidence regarding the neuropathophysiology of pain in CP and its potential implications for the development of new treatments for pain in CP.
Core tip: Abdominal pain is the main symptom of patients with chronic pancreatitis (CP), yet the underlying mechanisms are not well understood. The emerging explanations of pain in CP are trending toward neurobiological theories. This article reviews these emerging concepts and their potential implications for the development of new treatments for pain in CP. Three major concepts attempting to explain the pathogenesis of CP pain: Pancreatic nociception and sensitization-induced pain, neuropathic remodeling, and central mechanism of pancreatitis pain are summarized, along with the specific molecules involved in each and potential therapeutic targets.
- Citation: Atsawarungruangkit A, Pongprasobchai S. Current understanding of the neuropathophysiology of pain in chronic pancreatitis. World J Gastrointest Pathophysiol 2015; 6(4): 193-202
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/193.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.193
Chronic pancreatitis (CP) is a persistent and chronic inflammatory disease of the pancreas. Approximately, 80%-90% of patients with CP typically suffer from pancreatic pain[1], which is commonly described as a constant, severe, and dull pain in the mid-epigastrium that radiates to the back and worsens by with high-fat meals. Unsurprisingly, the pancreatic pain can have substantial psychological and economic impact on patients. In addition, a recent study confirmed that the life quality of patients with CP is significantly worsened by pain severity and disease-related complications[2].
The pathogenesis of pancreatic pain is still not fully understood. Thus, management of this pain cannot be specific, leading to unnecessarily high treatment costs and ineffective outcomes. Many theories have been proposed to explain the pain mechanism based on anatomical changes including high pressure within the pancreatic duct, high pressure in the pancreatic parenchyma, and complications of pancreatic and extra-pancreatic structures (i.e., pseudocysts, duodenal and bile duct obstruction, and peptic ulcer). These anatomical changes are believed to be noxious stimuli that activate pancreatic pain via nociceptive pathways. However, a number of human studies of CP have demonstrated evidence against the above theories, finding, for example, no relationship between pain and pancreatic duct pressure reduction[3,4], no relationship between pain and increase of parenchymal pressure[5], no pancreatic duct dilation in some patients with severe pancreatic pain[6], and no relationship between pain and severity of CP-related structural changes on imaging[7]. Therefore, the pain of CP patients cannot be explained by mechanical stimulation of nociceptive pathways alone.
Since the late 1990s, investigators have been trending toward neurobiological theories to explain pain in CP[8]. Therefore, the main objective of this paper was to review the current neurobiological theories and emerging concepts that might lead to the development of new treatment regimens for alleviating pain in CP patients.
The pancreas is innervated by a complex structure of two groups of afferent fibers. The first group consists of branches of the abdominal vagus nerve, and the second fibers that run through the celiac plexus and reach the lower thoracic segments of the spinal cord via the splanchnic nerves[9]. The latter group is best known for stimulating visceral pain.
The nociceptive pathway in the pancreas begins with nociceptors located at the ends of the primary afferent neurons and function as afferent nerve endings[10]. Unlike those in other visceral organs, these primary afferent neurons convey only pain stimuli. One special subset of theses nociceptors contains a group called “silent nociceptors”, which are only activated during inflammatory processes[11]. Furthermore, the pancreatic nociceptors can be activated by various noxious stimuli through mechanosensitive and chemosensitive mechanisms[12]. The former mechanism is located on blood vessels that supply the pancreas and pancreatic parenchyma and can be stimulated by stretching, ischemia, and necrosis. The latter mechanism can be stimulated by inflammatory mediators, but the exact location of this mechanism is not completely known.
The pathogenesis of CP is strongly related to prolonged exposure to noxious stimuli, which causes chronic inflammation. Noxious stimuli not only stimulate nociceptors, but can also damage pancreatic tissues and nerves surrounding the pancreas[13]. The injured tissues can release pro-inflammatory mediators such as prostanoid, bradykinin, tachykinin, serotonin, and growth factors[14]. Induced by the above mediators, primary sensory neurons then become more sensitive to further stimulation by either noxious (hyperalgesia) or non-noxious (allodynia) stimuli. This process is called peripheral sensitization[15], which indicates that the noxious stimuli can evoke nociceptor plasticity. Moreover, there is another mechanism by which pain can be exacerbated via peripheral sensitization, which begins with the activation of silent nociceptors by peripheral inflammation, and the silent nociceptors consequently facilitate and increase afferent activities in the spinal cord.
Once stimulated by pro-inflammatory mediators, the nociceptors will transform the stimuli into action potentials by unbalancing the Na and K currents on the neuronal membrane. The action potentials travel along both unmyelinated C-fibers and small myelinated Aδ fibers of primary sensory neurons[11,12]. These neurons traverse paravertebral and prevertebral ganglia to synapse with secondary sensory neurons at laminae I, II, V, and X of the dorsal horn of the spinal cord at the T5-L2 level. Based on an animal study, the secondary sensory neurons related to the pancreas are primarily located at the T10-T11 level[12]. Consequently, the primary sensory axons release glutamate, substance P, and calcitonin gene-related peptide (CGRP). Glutamate activates both α-amino-3-hydroxy-5-methyl-4-isoxazole propionate and N-methyl-D-aspartate (NMDA) receptors, while substance P activates NK1 receptors[16,17]. These three receptors are located on secondary sensory neurons within the dorsal horn. At this level of stimulation, prolonged stimulation from peripheral sensitization can facilitate excitation of dorsal horn neurons, which can increase spontaneous activities, decrease the firing threshold, and expand the receptive field of the dorsal horn neurons. This process is called central sensitization and can result in hyperalgesia and allodynia[11].
After the activation of secondary sensory neurons, action potentials are generated and transmitted to the thalamus via the spinothalamic tract to activate tertiary sensory neurons. These tertiary sensory neurons then transmit the signal to the somatosensory cortex for cognitive integration of pain and the limbic system and hypothalamus for autonomic/affective integration of the pain[18].
Furthermore, the central nervous system (CNS) can modulate pain signaling at the spinal cord level via either facilitation, increasing the spinal transmission of pain impulses, or inhibition, decreasing the spinal transmission of pain impulses. The combination of facilitation and inhibition generates the signal that will determine the pain perception in the brain.
After the primary sensory neurons are activated, neurotransmitters (glutamate, substance P, and CGRP) are not only released to the dorsal horn of the spinal cord, but also to primary nerve endings located on the pancreas, where they act as inflammatory mediators that create pancreatic inflammation characterized by vasodilation, edema, and neutrophil infiltration. This process is also known as neurogenic inflammation[19-21]. Additionally, this neurogenic inflammation can facilitate the activation of peripheral sensitization[10].
Chronic inflammation in the pancreas has been shown to spread to the pancreatic nerve[22,23]. Additionally, perineural inflammatory cells including eosinophils, CD4+ and CD8+ lymphocytes, macrophages, and mast cells are evidenced in patients with painful CP[24-27]. This finding is consistent with the increased percentage of eosinophils observed in perineural inflammatory cell infiltrates, which may be related to the release of a nociceptive substance[13]. In addition, numerous studies[28-34] have reported the increase of various perineural inflammatory mediators including histamine, serotonin, interleukin, bradykinin, substance P, CGRP, tumor necrosis factor-alpha, and several neurotrophins [i.e., growth-associated protein 43, brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF)]. Specifically, BDNF and NGF up-regulation has been shown in CP patients[24,26].
Such evidence has recently become the main focus of many studies attempting to explain the pathogenesis of pain based on three concepts: pancreatic nociception and sensitization-induced pain, neuropathic remodeling (neuropathic pain), and central mechanism of pancreatitis pain. Each of these aspects is complex and involves specific molecules that are described in the following sections.
There is much evidence to support that peripheral and central sensitization is largely associated with the pancreatic pain in CP. The evidence related to the molecules and receptors that have been found to be involved in the sensitization mechanisms will be discussed one-by-one in the following paragraphs.
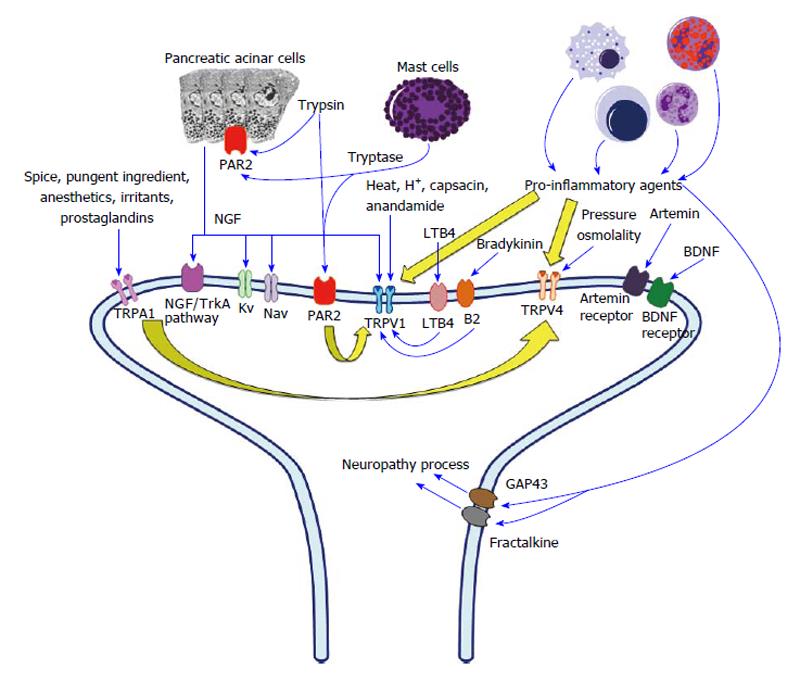
The transient receptor potential (TRP) family is a group of ion channels localized mainly to the plasma membrane of neurons. Three molecules strongly related to pain and inflammation in the TRP family are TRP vanilloid 1 (TRPV1), TRPV4, and TRP ankyrin 1 (TRPA1)[35]. These three TRP channels are also associated with pain in CP patients through the sensitization of pancreatic afferent neurons and development of neurogenic inflammation. The primary sensory nerve endings that supply the pancreas contain these three types of TRP, which can be stimulated by specific stimuli including inflammatory mediators. After the receptors are stimulated, primary sensory neurons then release substance P and CGRP at both the spinal cord and peripheral sites, thus causing pancreatic inflammation via neurogenic inflammation[36-40]. The mechanism of peripheral sensitization (Figure 1) is discussed below.
TRPV1 can be directly activated by many factors, including heat, extra-cellular proton and tissue acidosis, capsaicin, biologically active compounds (anandamide and hydrogen sulfide), and endogenous lipid metabolites from the arachidonic acid pathway[41,42]. Furthermore, TRPV1 can be indirectly activated by pro-inflammatory bradykinin and pro-inflammatory leukotriene[43]. By modulating TRPV1 activity, pro-inflammatory bradykinin can indirectly activate TRPV1 via B2 receptors residing on primary sensory neurons. By binding to their leukotriene B4 receptors, pro-inflammatory leukotriene B4 can activate TRPV1 via an intra-neural signaling pathway. Furthermore, pro-inflammatory agents can sensitize TRPV1 by reducing the threshold of thermal stimuli (hyperalgesia)[44].
In animal and human studies, TRPV1 plays an important role in explaining pain in CP. After TRPV1 receptor activation by capsaicin in rats with induced CP, peripheral sensitization is evidenced by the significant upregulation of TRPV1 at both mRNA and protein levels in the dorsal root ganglion (DRG) and pancreas-specific sensory neurons[45]. Moreover, the same study found significant reduction of pain behavior and hyperalgesia after administration of a systemic TRPV1 antagonist. Significant upregulation of TRPV1 is also seen in the pancreatic tissue of patients with painful CP; however, no relationship was found between the pain score level and the level of TRPV1 expression[46].
TRPA1 is responsive to various stimuli that can be categorized into five groups: The pungent ingredients of spices, environmental irritants, endogenous agonists of TRPA1[39], cyclopentenone prostaglandins, and general anesthetics[47]. The pungent ingredients of spices include mustard oil[48], garlic[48], and cinnamon[48,49], and environmental irritants include acrolein[48,50], formaldehyde[48,51], and cigarette smoke[36,48]. Cyclopentenone prostaglandins include PGA2, PGA1, and PGJ2[52,53]. Pro-inflammatory agents also sensitize TRPA1 leading to hyperalgesia[54-56].
TRPV4 responds to changes in tonicity[57,58], moderate heat (> 27 °C)[37], and mechanical pain[37]. Changes in tonicity can cause cell swelling and activate phospholipase A2; this process leads to the generation of arachidonic acid[59], which is an endogenous agonist of TRPV4. In addition, 4α-phorbol 12,13-didecanoate (4αPDD) is a synthetic TRPV4 agonist[60,61]. Similar to TRPV1 and TRPA1, pro-inflammatory agents can sensitize TRPV4 causing hyperalgesia to mechanical stimuli[62-64].
To the best of our knowledge, the first evidence that TRPA1 and TRPV4 contribute to pancreatitis pain was reported in rats with induced acute pancreatitis[48]. Another study also demonstrated that TRPA1 mediates CP pain in mice[54]. In a recent study using mice in which CP was induced through repetitive cerulein injections, TRPV1 and TRPA1 antagonists were important in alleviating neurogenic inflammation in pancreatitis, reducing pain-related behavior, and preventing the transition from acute to chronic inflammation[65]. Therefore, TRPV1, TRPA1, and TRPV4 are likely to be targets for therapeutic pain management in CP patients by reducing peripheral sensitization and neuropathic inflammation.
Proteinase-activated receptor 2 (PAR2) is one of the chief regulators of pancreatic exocrine secretion in pancreatic acinar cells and ductal epithelium. Notably, trypsin is recognized as the strongest activator of PAR2. There is also evidence supporting a relationship between PAR2 and pancreatic pain. PAR2 expression was detected in sensory neurons supplying the pancreas; in fact, primary sensory neurons could be activated and sensitized by administering PAR2-specific proteinase activating peptide and trypsin in an in vivo study[66,67]. Moreover, both PAR2-specific proteinase activating peptide and trypsin-induced behavioral pain response have been observed in awake rats[67]. Another study discovered that tryptase, a substance released from activated mast cells, can stimulate PAR2[27], which might explain the relationship between mast cells and pain in CP patients. In an experimental animal model of pancreatitis pain, the administration of two proteinase inhibitors (camostat mesylate and nafamostat mesylate) reduced sensitivity to abdominal pain[68]. Likewise, nafamostat was associated with a significant reduction of pain duration induced by acute pancreatitis[69].
Based on in vitro findings, PAR2 activation causes TRPV1 sensitization by enhancing capsaicin; consequently, this process leads to the significant release of CGRP[70]. Similarly, in in vivo studies, PAR2 activation resulted in pain-related behavior[55,70,71]. As additional supporting evidence that PAR2 is involved in the development of hyperalgesia, PAR2 was significantly upregulated in DRG neurons along with decreased thermal withdrawal latencies in a rat model of CP[72]. In short, PAR2 agonist peptides, trypsin and tryptase, are related to the pathogenesis of pain in CP via nociception and sensitization caused by the interaction between TRPV1 and PAR2.
NGF, a type of neurotrophin, is a protein important for the growth, maintenance, regulation of survival, and specialization of sensory neurons. Moreover, NGF is an essential mediator of peripheral sensitization[73]. Although the islets of pancreatocytes typically generate NGF, NGF was found to be upregulated and surprisingly expressed in pancreatic acinar cells and ductal epithelium in a rat model of pancreatitis[74]. However, the upregulation of NGF returned to normal after the pancreatic inflammation resolved[16]. Many studies have attempted to explain the mechanism of NGF-induced pancreatitis pain on sensitization via modulation of TRPV1 and excitability of K and Na currents[73,75-77]. Another hypothesized mechanism underlying pain in CP is activation of the NGF/trkA pathway[78,79]. In a study of rats with CP induced by trinitrobenzene sulfonic acid, both anti-NGF antibodies and trkA-immunoglobulin G substantially reduced hyperalgesia[80,81].
Artemin is a neurotrophin classified as a glial cell line-derived neurotrophic factor. Overexpression of artemin and its co-receptor GFR alpha 3 has been reported to strongly relate to the increased frequency and intensity of pain in rats with CP[82].
BDNF is also a member of the neurotrophin family found in the brain and periphery. An in vivo study reported that BDNF is upregulated in primary sensory neurons in rats with CP, and that BDNF antagonist treatment was associated with a reduction of pain-related behavior in these animals[83]. Another study of pancreatic tissue in patients with CP found that pain was positively related with BDNF levels and increased in CP patients compared to healthy control. These findings suggest that BDNF is essential to the nociceptive pathway of CP.
Studies have also reported associations between pain in CP and other substances that could be related to peripheral sensitization, for example, the over-expression of interleukin 1[84], interleukin 6[85], interleukin 8[86], and fractalkine[87].
Previous findings in patients with painful CP indicate overexpression of neurokinin 1[88], neurokinin 2[88], CGRP[16], and substance P[16,88]. Therefore, overexpression of these neurotransmitters may result from activation of nociceptive pathways and peripheral sensitization.
In clinico-pathological studies, the intra-pancreatic nerves in patients with painful CP demonstrate immune cell infiltration, indicating pancreatic neuritis[13,89], and characteristics of pancreatic neuropathy, which can be described as the increase of neural density, hypertrophy, and spouting[13,90-92]. Both pancreatic neuritis and pancreatic neuropathy are believed to relate with the inflammatory process, which is a key pathogenic factor in CP as indicated by the following evidence. The increase of fractalkine and its receptor is correlated with fibrosis, neuropathic changes, pain duration of CP and the degree of inflammatory cell infiltrate[87,91,92]. Moreover, the expression of growth-associated protein 43 (GAP43), which is a member of the neurotrophin family, is reported to have a relationship with pancreatic neuropathy, pancreatic neuritis, and pancreatic pain. Consequently, GAP43 may be considered a potential marker of neuronal plasticity during development and injury[87,89,91,92].
Patients with painful CP have been reported to demonstrate significant alterations in pancreatic innervation, with a marked decrease in sympathetic innervation but no statistically significant difference in cholinergic innervation[92]. In the same study, stronger expression of pain-related behavior was also noted in patients with painful CP, indicating neuronal regeneration after neuron injury.
In conclusion, the inflammatory process leaves pancreatic neurons damaged and characterized as showing either neuropathy or neuritis. Correspondingly, these neurons express GAP43, leading to the remodeling of pancreatic innervation. This process might explain pancreatic pain in CP patients. Such a process is similar to pancreatic nociception and sensitization-induced pain in the sense that both processes involve inflammatory mediators. However, the mechanism by which inflammatory mediators induce neuropathic pain is by destroying the neurons, leading to permanent neuronal lesions without involving noxious stimuli and the sensitization process.
As previously described, several factors can induce pain in CP by triggering the CNS, for instance, chronic stimulation of pain through nociceptive pathways, peripheral sensitization caused by inflammatory processes in the pancreas, and nerve damage. Consequently, prolonged peripheral sensitization can lead to central sensitization, which will be discussed next.
Using quantitative sensory testing in human experiments, researchers found that the brain activity of patients with CP demonstrated increased areas of referred pain and increased heterogeneity of referred pain location compared to the control group after electrical stimulation of the esophagus, stomach, and duodenum[93]. The sensitization caused by CP could decrease the pain threshold and increase the referred pain area[94,95].
By using electroencephalography (EEG) to measure brain activities, studies of pain in CP can be categorized as either resting-state EEG or evoked potential (EP) tests[84,96]. In resting-state EEG, alpha activities were found to demonstrate increased amplitude strength in CP patients compared to healthy volunteers[97], and pain duration was negatively correlated with the average peak alpha frequency[98]. Notably, the relationship between chronic pain and the change in alpha activity could be the result of thalamocortical dysrhythmia, which is activated by T-type calcium channels[99]. In EP tests, constant electrical stimulation of the upper gastrointestinal tract significantly decreased latencies of the early EP components in CP patients compared to healthy volunteers[93]. Moreover, hyperalgesia and prolonged latencies of early visceral EPs components in the frontal region of the cortex were seen following electrical stimulation in CP patients compared to healthy subjects[100].
As observed with functional magnetic resonance imaging, pain sensation is processed and localized in somatosensory cortex, insula, anterior cingulate cortex, prefrontal cortex, and thalamus. Recently reported evidence indicates that plasticity, i.e., functional or structural changes, in the CNS may be associated with pain in chronic syndromes. The structural reduction of cortical thickness[101] and microstructural changes in the insula and frontal cortex[102] also have been observed in magnetic resonance imaging studies.
The above findings support the hypothesis that the pain experienced by CP patients can be triggered by central sensitization, which is derived from sustained and increased peripheral nociceptive drivers. Moreover, recent studies have demonstrated that descending inhibitory modulators are significantly impaired in patients with CP compared to healthy controls[95,103]. Descending facilitation from the brainstem was also reported to be a critical factor in pancreatic pain in rats with CP[20].
Generally, drug discovery involves finding a new drug with the ability to increase or decrease the activities of selected targets or unrelated targets. The greater our understanding of the neuropathophysiology of pain in CP, the better our opportunity to identify potential treatment alternatives. Currently, there are two groups of potential treatment alternatives and their drug targets, which are summarized in Table 1. The first group of potential treatment alternatives is directed at attenuating the peripheral sensitization process by targeting related molecules and receptors, such as NGF, TRPV1, PAR2, trypsin, tryptase, interleukin 1, and interleukin 6. The second group of potential treatment alternatives focuses on attenuating the central sensitization process.
| Drug target | Potential treatment alternatives |
| NGF | Tanezumab |
| TRPV1 | TRPV1 antagonist |
| PAR2 | Trypsin inhibitors |
| Mast cell | Ketotifen |
| Interleukin 1 | Recombinant interleukin-1 receptor antagonist |
| Interleukin 6 | Interleukin-6 antagonist |
| Central sensitization | Ketamine, dextromethrophan, pregabalin, tricyclic antidepressants, and noradrenaline reuptake inhibitors |
Anti-NGF antibody demonstrated a significant effect on attenuating the changes in the excitation of pancreatic nociceptors in rats with CP[81]. Tanezumab, a humanized monoclonal antibody with specific binding to NGF, is able to relieve chronic pain in many conditions, for instance, chronic low back pain[8,104], interstitial cystitis[8,105,106], and osteoarthritis knee pain[8,107,108]. However, to the best of our knowledge, there has not been any human study to date using anti-NGF in CP.
A TRPV1 antagonist remarkably reduced both visceral pain behavior and referred somatic hyperalgesia in rats with CP[45]. Since not only TRPA1 but also TRPV4 are related to the peripheral sensitization of pain in CP, theoretically both TRPV1 and TRPV4 antagonists should be able to attenuate pain in CP. Nevertheless, we have not seen any study using a TRPV4 antagonist in CP.
Although PAR2 is the receptor that induces peripheral sensitization of pain in CP, direct PAR2 antagonists are very difficult to create[8]. As already mentioned, both trypsin and tryptase are agonists of the PAR2 receptor. Therefore, one researcher proposed that PAR2-sensitized pain can be inhibited indirectly by using trypsin inhibitors and a mast cell stabilizer (ketotifen)[8].
In the inflammatory process, interleukin 1 and interleukin 6 are associated with pain in CP. As a result, antagonists of both these interleukins may be able to attenuate pain. Researchers found that a recombinant interleukin-1 receptor antagonist[109] and interleukin-6 antagonist[85] can have an effect on attenuating pancreatitis-induced pain in rats with CP.
Central sensitization of pain in CP can be influenced by NMDA receptors, thalamocortical dysrhythmia, and impaired modulation pathways. Consequently, we can attenuate pain in CP by modifying the activities of these influencing factors. Several known drugs can reduce the effect of central sensitization, such as ketamine[8,110,111], dextromethrophan[8,112], pregabalin[113-115], tricyclic antidepressants[84], and noradrenaline reuptake inhibitors[84].
Chronic pain is an important issue that significantly lowers quality of life in patients with CP. The theories for underlying causes of pancreatic pain in CP have been shifting away from anatomical changes of pancreatic structure to changes in neurobiological structure, which include peripheral sensitization-induced pain, neuropathic remodeling, and central sensitization of pancreatic pain. Furthermore, researchers have identified numerous molecules related to pancreatic pain in CP, for example, TRPV1, TRPA1, TRPV4, PAR2, NGF, artemin, BDBF, GAP43, and fractalkine. As a result, the neuropathophysiological mechanisms of pain in CP show strong potential as targets for drug discovery to relieve the pain and improve quality of life in this patient population.
P- Reviewer: Drewes AM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Andrén-Sandberg A, Hoem D, Gislason H. Pain management in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14:957-970. [PubMed] |
| 2. | Olesen SS, Juel J, Nielsen AK, Frøkjær JB, Wilder-Smith OH, Drewes AM. Pain severity reduces life quality in chronic pancreatitis: Implications for design of future outcome trials. Pancreatology. 2014;14:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Okazaki K, Yamamoto Y, Ito K. Endoscopic measurement of papillary sphincter zone and pancreatic main ductal pressure in patients with chronic pancreatitis. Gastroenterology. 1986;91:409-418. [PubMed] |
| 4. | Renou C, Grandval P, Ville E, Laugier R. Endoscopic treatment of the main pancreatic duct: correlations among morphology, manometry, and clinical follow-up. Int J Pancreatol. 2000;27:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Manes G, Büchler M, Pieramico O, Di Sebastiano P, Malfertheiner P. Is increased pancreatic pressure related to pain in chronic pancreatitis? Int J Pancreatol. 1994;15:113-117. [PubMed] |
| 6. | Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut. 1992;33:1566-1571. [PubMed] |
| 7. | Bahuva R, Walsh RM, Kapural L, Stevens T. Morphologic abnormalities are poorly predictive of visceral pain in chronic pancreatitis. Pancreas. 2013;42:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Pasricha PJ. Unraveling the mystery of pain in chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2012;9:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95-138. [PubMed] |
| 10. | Barreto SG, Saccone GT. Pancreatic nociception--revisiting the physiology and pathophysiology. Pancreatology. 2012;12:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Olesen SS, Krarup AL, Brock C, Drewes AM. Gastrointestinal sensations and pain: a review on basic, experimental and clinical findings. Minerva Gastroenterol Dietol. 2009;55:301-313. [PubMed] |
| 12. | Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009;141:191-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Keith RG, Keshavjee SH, Kerenyi NR. Neuropathology of chronic pancreatitis in humans. Can J Surg. 1985;28:207-211. [PubMed] |
| 14. | Vardanyan M, Rilo HL. Pathogenesis of chronic pancreatitis-induced pain. Discov Med. 2010;9:304-310. [PubMed] |
| 15. | Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707-758. [PubMed] |
| 16. | Winston JH, He ZJ, Shenoy M, Xiao SY, Pasricha PJ. Molecular and behavioral changes in nociception in a novel rat model of chronic pancreatitis for the study of pain. Pain. 2005;117:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Büchler M, Weihe E, Friess H, Malfertheiner P, Bockman E, Müller S, Nohr D, Beger HG. Changes in peptidergic innervation in chronic pancreatitis. Pancreas. 1992;7:183-192. [PubMed] |
| 18. | Lieb JG, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:706-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551-559; discussion 559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Vera-Portocarrero L, Westlund KN. Role of neurogenic inflammation in pancreatitis and pancreatic pain. Neurosignals. 2005;14:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep. 2008;10:101-106. [PubMed] |
| 22. | Di Sebastiano P, di Mola FF, Buchler MW, Friess H. Pathogenesis of pain in chronic pancreatitis. Dig Dis. 2004;22:267-272. [PubMed] |
| 23. | Bornman PC, Marks IN, Girdwood AW, Berberat PO, Gulbinas A, Büchler MW. Pathogenesis of pain in chronic pancreatitis: ongoing enigma. World J Surg. 2003;27:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Shrikhande SV, Martignoni ME, Shrikhande M, Kappeler A, Ramesh H, Zimmermann A, Büchler MW, Friess H. Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg. 2003;90:1565-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Goecke H, Forssmann U, Uguccioni M, Friess H, Conejo-Garcia JR, Zimmermann A, Baggiolini M, Büchler MW. Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery. 2000;128:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hunger RE, Mueller C, Z‘graggen K, Friess H, Büchler MW. Cytotoxic cells are activated in cellular infiltrates of alcoholic chronic pancreatitis. Gastroenterology. 1997;112:1656-1663. [PubMed] |
| 27. | Hoogerwerf WA, Gondesen K, Xiao SY, Winston JH, Willis WD, Pasricha PJ. The role of mast cells in the pathogenesis of pain in chronic pancreatitis. BMC Gastroenterol. 2005;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol. 2006;19:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Di Sebastiano P. The quality of life in chronic pancreatitis: the role of surgery. JOP. 2006;7:120-121. [PubMed] |
| 30. | Talukdar R, Saikia N, Singal DK, Tandon R. Chronic pancreatitis: evolving paradigms. Pancreatology. 2006;6:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Madro A, Celiński K, Słomka M. The role of pancreatic stellate cells and cytokines in the development of chronic pancreatitis. Med Sci Monit. 2004;10:RA166-RA170. [PubMed] |
| 32. | Zhu ZW, Friess H, Wang L, Zimmermann A, Büchler MW. Brain-derived neurotrophic factor (BDNF) is upregulated and associated with pain in chronic pancreatitis. Dig Dis Sci. 2001;46:1633-1639. [PubMed] |
| 33. | DiMagno EP. Toward understanding (and management) of painful chronic pancreatitis. Gastroenterology. 1999;116:1252-1257. [PubMed] |
| 34. | Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Büchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230:615-624. [PubMed] |
| 35. | Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1064] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 36. | Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574-2582. [PubMed] |
| 37. | Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408-6414. [PubMed] |
| 38. | Su HC, Bishop AE, Power RF, Hamada Y, Polak JM. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci. 1987;7:2674-2687. [PubMed] |
| 39. | Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519-13524. [PubMed] |
| 40. | Vergnolle N, Cenac N, Altier C, Cellars L, Chapman K, Zamponi GW, Materazzi S, Nassini R, Liedtke W, Cattaruzza F. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol. 2010;159:1161-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155-6160. [PubMed] |
| 42. | McVey DC, Vigna SR. The role of leukotriene B4 in Clostridium difficile toxin A-induced ileitis in rats. Gastroenterology. 2005;128:1306-1316. [PubMed] |
| 43. | Geppetti P, Bertrand C, Ricciardolo FL, Nadel JA. New aspects on the role of kinins in neurogenic inflammation. Can J Physiol Pharmacol. 1995;73:843-847. [PubMed] |
| 44. | Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435-15439. [PubMed] |
| 45. | Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282-1292. [PubMed] |
| 46. | Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Büchler MW. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA. 2008;105:8784-8789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, Vaksman N, Liedtke W, Cohen DM, Grady EF. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G556-G571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260-265. [PubMed] |
| 50. | Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1475] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 51. | McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525-13530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 994] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 52. | Materazzi S, Nassini R, Andrè E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2008;105:12045-12050. [PubMed] |
| 53. | Taylor-Clark TE, Undem BJ, Macglashan DW, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol. 2008;73:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 54. | Cattaruzza F, Johnson C, Leggit A, Grady E, Schenk AK, Cevikbas F, Cedron W, Bondada S, Kirkwood R, Malone B. Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1002-G1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 56. | Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525-535. [PubMed] |
| 58. | Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100:13698-13703. [PubMed] |
| 59. | Pedersen S, Lambert IH, Thoroed SM, Hoffmann EK. Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur J Biochem. 2000;267:5531-5539. [PubMed] |
| 60. | Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569-13577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 472] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 61. | Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434-438. [PubMed] |
| 62. | Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497-511. [PubMed] |
| 63. | Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 64. | Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288-G1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 65. | Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33:5603-5611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci. 2001;21:9036-9042. [PubMed] |
| 67. | Hoogerwerf WA, Shenoy M, Winston JH, Xiao SY, He Z, Pasricha PJ. Trypsin mediates nociception via the proteinase-activated receptor 2: a potentially novel role in pancreatic pain. Gastroenterology. 2004;127:883-891. [PubMed] |
| 68. | Kawabata A, Matsunami M, Tsutsumi M, Ishiki T, Fukushima O, Sekiguchi F, Kawao N, Minami T, Kanke T, Saito N. Suppression of pancreatitis-related allodynia/hyperalgesia by proteinase-activated receptor-2 in mice. Br J Pharmacol. 2006;148:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. Am J Surg. 1996;171:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 107] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300-4312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 71. | Nishimura S, Ishikura H, Matsunami M, Shinozaki Y, Sekiguchi F, Naruse M, Kitamura T, Akashi R, Matsumura K, Kawabata A. The proteinase/proteinase-activated receptor-2/transient receptor potential vanilloid-1 cascade impacts pancreatic pain in mice. Life Sci. 2010;87:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Zhang W, Gao J, Zhao T, Wei L, Wu W, Bai Y, Zou D, Li Z. Proteinase-activated receptor 2 mediates thermal hyperalgesia and is upregulated in a rat model of chronic pancreatitis. Pancreas. 2011;40:300-307. [PubMed] |
| 73. | Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327-331. [PubMed] |
| 74. | Toma H, Winston J, Micci MA, Shenoy M, Pasricha PJ. Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology. 2000;119:1373-1381. [PubMed] |
| 75. | Djouhri L, Dawbarn D, Robertson A, Newton R, Lawson SN. Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci. 2001;21:8722-8733. [PubMed] |
| 76. | Lewin GR, Mendell LM. Maintenance of modality-specific connections in the spinal cord after neonatal nerve growth factor deprivation. Eur J Neurosci. 1996;8:1677-1684. [PubMed] |
| 77. | McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774-780. [PubMed] |
| 78. | Della Seta D, de Acetis L, Aloe L, Alleva E. NGF effects on hot plate behaviors in mice. Pharmacol Biochem Behav. 1994;49:701-705. [PubMed] |
| 79. | Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903-1912. [PubMed] |
| 80. | Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li C, Mehta K, Pasricha PJ. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology. 2011;141:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 81. | Zhu Y, Mehta K, Li C, Xu GY, Liu L, Colak T, Shenoy M, Pasricha PJ. Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G176-G181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Ceyhan GO, Bergmann F, Kadihasanoglu M, Erkan M, Park W, Hinz U, Giese T, Müller MW, Büchler MW, Giese NA. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Hughes MS, Shenoy M, Liu L, Colak T, Mehta K, Pasricha PJ. Brain-derived neurotrophic factor is upregulated in rats with chronic pancreatitis and mediates pain behavior. Pancreas. 2011;40:551-556. [PubMed] |
| 84. | Bouwense SA, de Vries M, Schreuder LT, Olesen SS, Frøkjær JB, Drewes AM, van Goor H, Wilder-Smith OH. Systematic mechanism-orientated approach to chronic pancreatitis pain. World J Gastroenterol. 2015;21:47-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 85. | Vardanyan M, Melemedjian OK, Price TJ, Ossipov MH, Lai J, Roberts E, Boos TL, Deschamps JR, Jacobson AE, Rice KC. Reversal of pancreatitis-induced pain by an orally available, small molecule interleukin-6 receptor antagonist. Pain. 2010;151:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Di Sebastiano P, di Mola FF, Di Febbo C, Baccante G, Porreca E, Innocenti P, Friess H, Büchler MW. Expression of interleukin 8 (IL-8) and substance P in human chronic pancreatitis. Gut. 2000;47:423-428. [PubMed] |
| 87. | Ceyhan GO, Deucker S, Demir IE, Erkan M, Schmelz M, Bergmann F, Müller MW, Giese T, Büchler MW, Giese NA. Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Lab Invest. 2009;89:347-361. [PubMed] |
| 88. | Michalski CW, Shi X, Reiser C, Fachinger P, Zimmermann A, Büchler MW, Di Sebastiano P, Friess H. Neurokinin-2 receptor levels correlate with intensity, frequency, and duration of pain in chronic pancreatitis. Ann Surg. 2007;246:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Di Sebastiano P, Fink T, Weihe E, Friess H, Innocenti P, Beger HG, Büchler MW. Immune cell infiltration and growth-associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology. 1997;112:1648-1655. [PubMed] |
| 90. | Friess H, Shrikhande S, Shrikhande M, Martignoni M, Kulli C, Zimmermann A, Kappeler A, Ramesh H, Büchler M. Neural alterations in surgical stage chronic pancreatitis are independent of the underlying aetiology. Gut. 2002;50:682-686. [PubMed] |
| 91. | Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Müller MW, Giese T, Büchler MW, Giese NA. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177-186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 92. | Ceyhan GO, Demir IE, Rauch U, Bergmann F, Müller MW, Büchler MW, Friess H, Schäfer KH. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104:2555-2565. [PubMed] |
| 93. | Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, Drewes AM. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology. 2007;132:1546-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Buscher HC, Wilder-Smith OH, van Goor H. Chronic pancreatitis patients show hyperalgesia of central origin: a pilot study. Eur J Pain. 2006;10:363-370. [PubMed] |
| 95. | Olesen SS, Brock C, Krarup AL, Funch-Jensen P, Arendt-Nielsen L, Wilder-Smith OH, Drewes AM. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724-730. [PubMed] |
| 96. | Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OH, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: evidence of abnormal cerebral pain processing? Eur J Gastroenterol Hepatol. 2011;23:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Olesen SS, Frøkjær JB, Lelic D, Valeriani M, Drewes AM. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology. 2010;10:742-751. [PubMed] |
| 98. | de Vries M, Wilder-Smith OH, Jongsma ML, van den Broeke EN, Arns M, van Goor H, van Rijn CM. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J Pain Res. 2013;6:815-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 99. | Frøkjær JB, Olesen SS, Graversen C, Andresen T, Lelic D, Drewes AM. Neuroimaging of the human visceral pain system-a methodoi: ogical review. Scand J Pain. 2011;2:95-104. |
| 100. | Drewes AM, Gratkowski M, Sami SA, Dimcevski G, Funch-Jensen P, Arendt-Nielsen L. Is the pain in chronic pancreatitis of neuropathic origin? Support from EEG studies during experimental pain. World J Gastroenterol. 2008;14:4020-4027. [PubMed] |
| 101. | Frøkjær JB, Bouwense SA, Olesen SS, Lundager FH, Eskildsen SF, van Goor H, Wilder-Smith OH, Drewes AM. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:434-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 102. | Frøkjær JB, Andersen LW, Brock C, Simrén M, Ljungberg M, Søfteland E, Dimcevski G, Yavarian Y, Gregersen H, Drewes AM. Altered brain microstructure assessed by diffusion tensor imaging in patients with diabetes and gastrointestinal symptoms. Diabetes Care. 2013;36:662-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Bouwense SA, Olesen SS, Drewes AM, Frøkjær JB, van Goor H, Wilder-Smith OH. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLoS One. 2013;8:e55460. [PubMed] |
| 104. | Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 105. | Te AE. A study to investigate tanezumab in patients with interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2011;12:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 107. | Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 515] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 108. | Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage. 2011;19:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Xu C, Shen J, Zhang J, Jia Z, He Z, Zhuang X, Xu T, Shi Y, Zhu S, Wu M. Recombinant interleukin-1 receptor antagonist attenuates the severity of chronic pancreatitis induced by TNBS in rats. Biochem Pharmacol. 2015;93:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Bouwense SA, Buscher HC, van Goor H, Wilder-Smith OH. S-ketamine modulates hyperalgesia in patients with chronic pancreatitis pain. Reg Anesth Pain Med. 2011;36:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Sprenger T, Valet M, Woltmann R, Zimmer C, Freynhagen R, Kochs EF, Tölle TR, Wagner KJ. Imaging pain modulation by subanesthetic S-(+)-ketamine. Anesth Analg. 2006;103:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Ilkjaer S, Dirks J, Brennum J, Wernberg M, Dahl JB. Effect of systemic N-methyl-D-aspartate receptor antagonist (dextromethorphan) on primary and secondary hyperalgesia in humans. Br J Anaesth. 1997;79:600-605. [PubMed] |
| 113. | Bouwense SA, Olesen SS, Drewes AM, Poley JW, van Goor H, Wilder-Smith OH. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One. 2012;7:e42096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | Olesen SS, Graversen C, Olesen AE, Frøkjaer JB, Wilder-Smith O, van Goor H, Valeriani M, Drewes AM. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment Pharmacol Ther. 2011;34:878-887. [PubMed] |
| 115. | Olesen SS, Bouwense SA, Wilder-Smith OH, van Goor H, Drewes AM. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |