Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.158
Revised: March 25, 2014
Accepted: May 29, 2014
Published online: August 15, 2014
Processing time: 217 Days and 14.1 Hours
Acute pancreatitis (AP) is a potentially life-threatening disease with a wide spectrum of severity. The overall mortality of AP is approximately 5%. According to the revised Atlanta classification system, AP can be classified as mild, moderate, or severe. Severe AP often takes a clinical course with two phases, an early and a late phase, which should both be considered separately. In this review article, we first discuss general aspects of AP, including incidence, pathophysiology, etiology, and grading of severity, then focus on the assessment of patients with suspected AP, including diagnosis and risk stratification, followed by the management of AP during the early phase, with special emphasis on fluid therapy, pain management, nutrition, and antibiotic prophylaxis.
Core tip: Acute pancreatitis is a frequent and potentially life-threatening disease. Therapy is currently mostly symptomatic with fluid resuscitation, pain management, and early oral feeding. Vigorous fluid resuscitation remains a cornerstone of early management of acute pancreatitis. Cross-sectional imaging during the early phase of evaluation has not been associated with improvement in outcome. There is no role for prophylactic antibiotics in the management of the early phase of acute pancreatitis (AP). Enteral nutrition in AP can reduce mortality, systemic infections, and multiorgan dysfunction compared to parenteral nutrition. Immediate endoscopic retrograde cholangiography is indicated only in patients with biliary pancreatitis with common bile duct obstruction and cholangitis.
- Citation: Phillip V, Steiner JM, Algül H. Early phase of acute pancreatitis: Assessment and management. World J Gastrointest Pathophysiol 2014; 5(3): 158-168
- URL: https://www.wjgnet.com/2150-5330/full/v5/i3/158.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i3.158
Acute pancreatitis (AP) is a potentially life-threatening disease with a wide spectrum of severity. The reported incidence of acute pancreatitis differs depending on geographic location and ranges from 14.7/100000 person years in the Netherlands to 45.1/100000 person years in Japan[1,2]. However, most studies show an incidence between 30 and 45/100000 person years[2-7]. Many studies report an increase in incidence over the last few decades[2,3,8], however, it is a matter of debate whether this represents a real increase in incidence due to increasing biliary AP in an increasingly obese population or whether this rise in incidence is due to improved diagnostic capabilities, a higher level of suspicion of this disease, or an overestimation of retrospective studies using administrative diagnostic codes[9-11]. In 2009, AP was the most common principal gastrointestinal diagnosis at discharge in the Unite States with estimated inpatient costs of $2.6 billion per year. Furthermore, it was the 14th most common cause of death with a crude rate of 1.0 per 100000 inhabitants[12]. The overall mortality of AP is about 5% and can reach up to 20%-30% in patients with severe AP and infected necrosis[13,14]. While there seems to be an increase in incidence, several studies have reported a decrease in mortality. Again, this could be a real decrease due to an earlier diagnosis and better therapeutic options or it may also be due to an improved sensitivity of diagnostic modalities, leading to an increase in the diagnosis of mild forms of pancreatitis[15].
In this review article, we first discuss general aspects of AP, including pathophysiology, etiology, and grading of severity, then focus on the assessment of patients with suspected AP, including diagnosis and risk stratification, followed by the management of AP during the early phase with special emphasis on fluid therapy, pain management, nutrition, and antibiotic prophylaxis.
The pathophysiology of AP with multi organ failure (MOF) is poorly understood. Researchers have long hypothesized that AP results from premature activation of digestive enzymes within the pancreas, a process referred to as autodigestion. Indeed, inherited mutations in genes encoding for digestive enzymes have been found in patients with a hereditary form of pancreatitis[16]. However, affected patients develop chronic, rather than acute pancreatitis. Therefore, in recent years, a novel concept has evolved, suggesting that systemic complications during AP result from uncontrolled activation of the inflammatory cascade. As indicated above, severe AP is associated with a significant mortality. Thus, early identification of severe forms of AP is crucial for outcome. In an attempt to identify surrogate parameters as predictors for severe AP, several association studies linking cytokines and chemokines with AP severity have been conducted[17]. Among these, serum levels of interleukin (IL)-6 and the IL-6-dependent acute phase protein, C-reactive protein (CRP) were identified as the most reliable predictors for severe AP[18,19]. Recent results from basic research have established that IL-6 or CRP are not only relevant markers to predict the severity of AP, but that the cytokine IL-6 also has a substantial pathophysiological impact on the course of the disease[19]. While excessive stimulation of the inflammatory cascade [hyper-inflammatory state, systemic inflammatory response syndrome (SIRS)] accounts for early systemic complications, paralysis of the inflammatory response, also termed compensatory anti-inflammatory response syndrome (CARS), contributes to local complications and sepsis associated with the late phase of the disease. Although these definitions are largely non-specific, they are undeniably useful in the clinical and research setting. Among the agents contributing to this anti-inflammatory response, IL-10 may be of importance. In fact, the protective role of IL-10 in experimental studies in animal models has been well documented[20]. Thus, the hypo-inflammatory status of CARS might facilitate superinfections that lead to extensive necrosis and/or septic complications. This interplay of these two contrasting phenomena requires an individualized therapeutic approach[20-22].
The identification of the etiology of AP is crucial for the management during the early phase of the disease and also for the prevention of recurrence of AP. Although there is no specific therapy for AP, the causing factor, e.g., choledocholithiasis in biliary AP, must be investigated and eliminated if identified. The most common causes of AP are gallstones and prolonged heavy use of alcohol, which together account for about 60%-80% of all cases. The incidence of biliary etiology differs considerably between different geographic regions. For example, there is a clear predominance for biliary AP over alcoholic AP in Greece (71.4% vs 6.0%) whereas the opposite is the case for Finland (6.3% vs 79.3%)[23,24]. The regional differences in frequency of biliary and alcoholic etiology are shown in Figure 1[6,7,23-31].
Other causes of AP include ERCP (0.4% to 11%[32,33]), idiosyncratic reactions to drugs (0.1% to 2%)[34], hypertriglyceridemia (1.1%-3.8%)[6,23,35], anatomic alterations[36], genetic predispositions[37], and other rare causes[38,39]. Despite a thorough clinical workup, 10%-25% of all cases remain idiopathic[6,11,23,33].
The severity of AP can be subclinical, mild without organ dysfunction, or can be severe. Patients with mild disease often improve spontaneously and heal within a few days. However, patients with severe disease may develop life-threatening local and/or systemic complications. According to the revised Atlanta classification system, AP can be classified as mild, moderate, or severe[40]. However, it is important to remember that AP is a rapidly evolving, dynamic condition in which the severity may change rapidly during the course of the disease[40]. Severe AP often takes a clinical course with two phases, an early and a late one, which should both be considered separately[40].
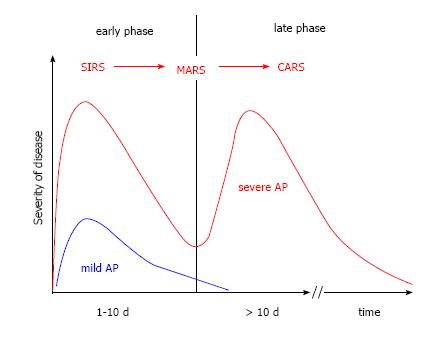
The early phase, which usually lasts for about one week, is characterized by a complex inflammatory reaction. The course of AP starts with a systemic proinflammatory phase systemic inflammatory response syndrome (SIRS), followed by a mixed inflammatory response syndrome mixed antagonist response syndrome (MARS), and finally leads to a phase with a suppressed inflammatory response compensatory anti-inflammatory response syndrome (CARS)[41-43]. In the phase of CARS, the immune system is downregulated and the chance of an infection of pancreatic and peripancreatic necrotic tissue rises. This is likely the reason why infections usually do not occur earlier than at the end of the first week[44]. During the stage of CARS pathogens can migrate unopposed from the intestinal lumen into necrotic tissue in and around the damaged pancreas. At that point, the clinical course of AP moves towards the second phase, including SIRS, sepsis, local and systemic complications, persistent organ failure, and possibly death. The model of the two-phase course is shown in Figure 2.
Efforts must be made to predict the severity of the disease as early as possible in order to know whether a patient diagnosed with AP can be treated as an outpatient, has to be admitted to a regular ward, to an intermediate care facility, or even to the intensive care unit. While it is generally recognized how important the prediction of severity of the disease is for the management of the individual patient, it is also recognized that such prediction is very difficult. Underestimation of the severity could be harmful for the patient, while overestimation could lead to unnecessary costs and a waste of resources. Therefore, the assessment and prediction of the severity is crucial for the management of the disease. A lot of research has been done over the last few decades trying to identify new tools to accurately predict the severity of pancreatitis, yet no gold standard for such prediction of the course of AP has been identified. An ideal predictor should be fast and easy to obtain, widely available, economical, and associated with a high sensitivity and specificity. Even though there are several clinical scores with a high sensitivity, specificity, positive, and/or negative predictive value, many of them are complicated to asses or can predict severity only after 48 h of admission to the hospital, which effectively means more than 72 h after the onset of disease[45]. This might be too late, as early aggressive fluid resuscitation is a cornerstone of AP therapy.
The diagnosis of AP can be made if ≥ 2 of the following three criteria are fulfilled: (1) abdominal pain characteristic of acute pancreatitis; (2) elevation of serum lipase or amylase activity > 3-fold of the upper limit of the reference interval; and (3) characteristic signs of pancreatitis on computed tomography (CT) imaging.
The first step in the diagnosis of AP should be a thorough clinical history. The pain caused by AP is typically dull, located in the epigastrium, may radiate into the back, and is usually severe, leading to hospital admission and often necessitating opioid therapy[45,46]. Furthermore, AP often causes nausea and vomiting. Known cholecystolithiasis and/or colics, alcohol excess within 48 h before the onset of pain, new medications, and the character of the pain should be evaluated. The second step of pancreatitis diagnosis is based on clinical chemistry. The measurement of serum lipase activity is generally thought to be more sensitive and specific than that of serum amylase activity and there is no additional value in simultaneous measurement of serum lipase and amylase activities[14,47]. Also, the degree of the elevation of serum pancreatic enzyme activities does not correlate with the severity of the disease, although, some studies would suggest such a correlation between serum enzyme activity and severity[6,45]. Only in patients with characteristic epigastric pain, but serum enzyme activities below 3-fold of the upper limit of the reference interval, a CT scan should be considered to rule out other differential diagnoses or to confirm AP. Apart from that, a CT in the early phase of AP is not recommended by current practice guidelines[14,48,49].
Risk factors: Obesity favors the development of local and systemic complications in patients with AP[50]. Since assessment for obesity is simple and free it should be assessed in every patient. The same applies for age, as patients 55 years or older are at increased risk for severe disease[14].
Scoring systems: Several single parameters and more or less complex scoring systems for the prediction of the severity of AP have been developed and clinically evaluated and all of them have been shown to be associated with advantages and disadvantages. The HAPScore (harmless acute pancreatitis score) was developed to identify patients with mild AP who can be treated as outpatients. Patients without rebound tenderness and/or guarding, a normal hematocrit, and a normal serum creatinine concentration have a high probability (positive predictive value: 98%-98.7%) to have a harmless course of the disease[51,52].
One of the oldest and probably best known and heavily used scores to predict a severe course of pancreatitis was developed in the early 70ties by John Ranson and colleagues[53]. The Ranson score is based on the presence or absence of simple parameters and is assessed differently at the time of admission (5 parameters; possible scores: 0-5) and 48 h later (6 parameters; possible scores: 0-6; Table 1).
| On admission | After 48 h |
| Age > 55 yr | Hematocrit fall > 10% |
| White blood cell count > 16000/mL | BUN increase > 1.8 mmol/L |
| Blood glucose concentration > 11.1 mmol/L | Serum calcium < 2 mmol/L |
| LDH > 350 IU/L | PaO2 < 60 mmHg |
| ASAT > 250 IU/L | Base deficit > 4 mmol/L |
| Fluid sequestration > 6 L |
Although a score ≥ 3 has a high sensitivity and specificity regarding a severe course of pancreatitis (83.9% and 78.0%, respectively) and a negative predictive value of 94.5%, the severity can be predicted no earlier than 48 h after admission[25,54]. A modification of the Ranson score by Clemens Imrie and colleagues (Imrie score or Glasgow score) was first reported in 1978 and is still widely used and has a similar accuracy as the Ranson score[25,55].
Currently, the score with the highest sensitivity regarding prediction of a severe course is the Acute Physiology And Chronic Health Evaluation (APACHE) II score[14,56]. Originally developed to predict mortality in intensive care patients, a value ≥ 8 of the APACHE II score predicts a severe course of AP with a sensitivity of 65%-83%, specificity of 77%-91%, positive predictive value (PPV) of 23%-69%, and negative predictive value (NPV) of 86%-99%[54,57]. However, the determination of an APACHE II score in a clinical patient is complex and time-consuming as it utilizes more than 15 parameters, which limits the clinical value of this score.
A score that was developed and validated more recently in almost 18000 patients, is the BISAP (Bedside Index of Severity in Acute Pancreatitis) score[58]. The main advantage of the BISAP score is its simplicity. One point each is given for blood urea nitrogen (BUN) > 8.9 mmol/L, impaired mental status (Glasgow Coma Scale < 15), presence of SIRS, age > 60 years, and pleural effusion (Table 2). A score ≥ 3 is predictive for a severe course (observed mortality of > 5%; Table 2) with a sensitivity of 83% and a PPV of 76.9%[58-60]. One disadvantage of the BISAP score is, that this score cannot easily distinguish patients with transient and persistent organ failure and therefore may overestimate severity and preclude differentiation between moderate and severe AP.
| BUN > 8.9 mmol/L | |
| Impaired mental status (Glasgow coma scale < 15) | |
| SIRS, defined by the presence of two or more | |
| Temperature | < 36 °C or > 38 °C (< 96.8 °F or > 100.4 °F) |
| Heart rate | > 90 per minute |
| Respiratory rate | > 20 per minute or PaCO2 < 32 mmHg |
| White blood cell count | < 4000/mL or > 12000/mL or > 10% immature neutrophils |
| Age > 60 yr | |
| Pleural effusion | |
| BISAP score | Mortality (%) |
| 0 | 0.1-0.2 |
| 1 | 0.5-0.7 |
| 2 | 1.9-2.1 |
| 3 | 5.3-8.3 |
| 4 | 12.7-19.3 |
| 5 | 22.5-26.7 |
In summary, there is currently no ideal predictor of severity of AP. All prognostic factors and scores show a good NPV, but suffer from a low PPV. Thus, the main value of severity assessment is to exclude a large number of patients with a low risk of mortality[57].
In addition to the laboratory/clinical scoring systems described above there are scoring systems based on imaging results to assess and predict the severity of AP. A CT scan for diagnostic purposes and severity assessment has been-and probably still is - standard practice in many centers[61]. The Balthazar score, developed in 1985, categorizes patients with AP into 5 groups (A-E) according to pancreatic and peripancreatic changes diagnosed by CT (Table 3)[62]. In 1990, Balthazar et al[63] modified this score, including assessment of the extent of pancreatic necrosis and named this score Computed Tomography Severity Index (CTSI) (Table 4). The CTSI is probably the most frequently used imaging score to assess severity in patients with AP and a score ≥ 4 has a negative predictive value of 94%-97% and a positive predictive value 53%-69% regarding the clinical severity of disease[61,64].
| Grade A | Normal pancreas |
| Grade B | Focal or diffuse enlargement of the pancreas |
| Grade C | Pancreatic changes associated with peripancreatic inflammation |
| Grade D | Single fluid collection |
| Grade E | Two or more fluid collections and/or presence of gas within the pancreas or within peripancreatic inflammation |
| Extent of necrosis | Points |
| Absence of necrosis | 0 |
| < 30% necrosis | 2 |
| 30%-50% necrosis | 4 |
| > 50% necrosis | 6 |
| Balthazar score | |
| A | 0 |
| B | 1 |
| C | 2 |
| D | 3 |
| E | 4 |
In addition to the Balthazar score and the CTSI, several other scores, e.g., pancreatic size index (PSI), mesenteric edema and peritoneal fluid (MOP) score, extrapancreatic (EP) score, extrapancreatic inflammation on CT (EPIC) score, modified CTSI (MCTSI), and MR severity index (MRSI) have been developed and evaluated[61,65]. However, none of these imaging scores were shown to be superior to clinical scoring systems. Thus, a CT on admission to predict severity of AP cannot be recommended at the current time[61].
In addition to laboratory/clinical and imaging scoring systems, single parameters have been evaluated to assess and predict severity.
A lot of research has been done evaluating hematocrit as an indicator for hemoconcentration. The first prospective cohort study showed a high NPV for a hematocrit ≥ 44 % (93% on admission and 97% 24 h later) but a poor PPV (26% and 27%, respectively) regarding organ failure in AP[66]. Similar results were obtained by several other studies focusing on the usefulness of hematocrit to predict a severe course of AP, organ failure, pancreatic necrosis, or death[67,68]. Due to its high negative predictive value, its low cost, and the ease of measurement, the hematocrit has value in predicting a non-severe course of AP.
The disruption of water balance can lead to hypoperfusion and a disturbance of pancreatic microcirculation[69], which in turn correlates with the severity of AP[70,71]. Understanding the water balance and the resulting changes in laboratory tests can help to predict severity and outcome of AP. In addition to hematocrit, other parameters, that mirror intravascular volume depletion, can also be helpful.
Serum creatinine has been identified as a predictor for pancreatic necrosis. Also, more recently, an estimated glomerular filtration rate (GFR) < 90 mL/min per 1.73 m2 on admission has been shown to predict pancreatic necrosis with a sensitivity, specificity, PPV, and NPV of 78.1%, 71%, 64%, and 83%, respectively[72,73]. While only one study has described GFR as a predictor of severity, BUN has been evaluated for many years and has been shown to be a good predictor for severity in AP in several large studies. A rise in BUN > 1.8 mmol/L after 48 h had already been included in the Ranson score 40 some years ago, is one of the 4 parameters used in the BISAP score, and has also been shown to have a high predictive value as a single parameter[74,75].
Besides parameters focusing on water balance and microcirculation, laboratory parameters suggesting the presence of an inflammatory process have been used as a predictor of severity. The most intensively studied parameter is CRP. In one study, a serum CRP concentration of 150 mg/L or greater predicted severe AP at 36 h after admission with a sensitivity, specificity, PPV, and NPV of 86%, 87%, 75%, and 93%, respectively[76]. However, the prediction of severity was only possible more than 24 h after admission, which, on average, is about 50 h after the onset of pain[45]. Also, several other studies showed a high predictive value of CRP during the course of AP in regards to severity, but a very low predictive value on admission[77,78].
Procalcitonin appears to be a valuable tool to discriminate between sterile and infected necrosis within the first days of AP[79,80]. However, data on the ability to predict the course of AP are not consistent. On one hand, a multicenter study from the United Kingdom found a significant difference of procalcitonin concentrations measured within 48 h of the onset of symptoms in patients with mild and severe AP and showed an accuracy of 94% in predicting death[81]. In a study from Slovakia, the PPV for predicting a fatal outcome reached 75% when a cut-off value of 5 ng/mL was used[82]. A third study evaluating procalcitonin showed an accuracy of 76% and a PPV of 75% for predicting a severe course of pancreatitis[83]. On the other hand, two studies reported that procalcitonin is not useful in predicting the severity of AP upon admission[79,84]. However, the time point for determination of procalcitonin concentrations, the assays used, and the cut-off values applied were different for all studies. Finally, measurement of procalcitonin is not widely available and is expensive.
A blood glucose concentration < 6.9 mmol/L on admission has a high negative predictive value (92%) for pancreatic necrosis and also can serve as a predictor for severity[85,86]. Blood glucose is easy, fast, and inexpensive to determine and widely available and therefore should be included in the risk stratification.
In summary, there is no single marker that can adequately predict the severity of AP, but there are several scoring systems that can be used to assess and predict the severity of AP. However, these scoring systems must be applied at the correct time, the correct place, and in the correct patient. Also, it is important to observe patients carefully and reassess severity frequently as the disease course can change rapidly at any given time.
Patients diagnosed with mild AP (according to the HAPScore) and no other risk factors can be treated as outpatients. In contrast, patients with any of the above-mentioned risk factors should be considered for admission to the hospital for close monitoring and timely reassessment of disease severity. In contrast, patients with a Ranson score ≥ 3, a BISAP score ≥ 3, an APACHE-II score ≥ 8, or patients with apparent organ failure should be transferred to an advanced medical care ward or facility.
Fluid therapy: Despite a lot of research, there is no pharmacological treatment of AP[87]. Thus, fluid resuscitation, analgesia, supportive care, and management of the local and systemic complications are the key elements of the management of patients with acute pancreatitis. One of the most important components of therapy of AP is early intravenous fluid resuscitation[88]. In fact, the decrease in mortality observed over the last decade might be due to the prevention of pancreatic necrosis by maintenance of microcirculation due to more aggressive fluid resuscitation[89]. Two studies have shown a decrease in mortality by early and aggressive fluid resuscitation[90,91]. However, data on the amount of fluid needed to prevent necrosis or to improve outcome are contradictory and the volume must be adjusted to the patient’s age, weight, and pre-existing renal and/or cardiac conditions[92]. The importance of starting fluid resuscitation as early as possible and in fact already in the emergency room was shown by two retrospective studies[90,91]. However, the optimal type of fluid is still a matter of debate. Studies comparing isotonic saline and lactated Ringer’s solution and crystalloid vs colloid solutions, respectively, showed no differences between both groups regarding clinical outcome as determined by the frequency of pancreatic necrosis, length of hospital stay, or mortality[93,94]. Also, the optimal therapeutic goal of fluid resuscitation is not yet clear. A goal-directed fluid resuscitation algorithm based on changes in BUN measurements, as a mirror of renal function, showed no improvement in outcome in patients with AP[93]. Nonetheless, blood pressure, respiratory function, urine output, and-where appropriate-intraabdominal pressure should be closely monitored. One study showed a less severe course of post-ERCP pancreatitis when patients were treated according to a fluid resuscitation protocol based on vital signs and hematocrit[95]. While questions on the type of fluid, the optimal rate of administration, and the therapeutic goal to reach remain unanswered[96], the time-point appears to be very important - the earlier, the better[90,91].
Causative therapy: Elimination of any potential risk factor is another important approach to AP therapy. In case of suspected alcohol- or drug-induced AP, the intake of the causing agent must be stopped immediately. In case of biliary AP, the indication to perform an endoscopic retrograde cholangiography (ERC) and removal of stones within the bile duct depends on the degree of obstruction of the common bile duct and the presence of cholangitis. Biliary pancreatitis and cholangitis are clear indications for ERC and ERC should be performed as early as possible[49,97,98]. Immediate ERC is indicated in patients with biliary pancreatitis with common bile duct obstruction and cholangitis, arguable in patients with predicted severe pancreatitis but without cholangitis, and not indicated in predicted mild pancreatitis without cholangitis[49].
After biliary pancreatitis, cholecystectomy is recommended within the same hospital stay for mild pancreatitis or after an interval of 6 wk following an episode of severe pancreatitis[49].
Pain management: Given that most patients with AP suffer from severe pain, adequate analgesia is very important. In mild cases, non-opioid drugs might be satisfying, but in many cases, especially severe AP, parenterally administered narcotic agents are warranted and most patients will require the use of opioids to control the pain[99,100]. In contrast to historical reports, there is no evidence or a recommendation for restrictions on the type of pain medications being used[14].
Nutrition: For many years, resting the pancreas by giving the patient nothing per os was an important part of therapy. Nowadays, there is wide agreement that total oral abstinence from food combined with total parenteral nutrition is not beneficial to patients with severe AP, but may in fact be detrimental. A recent meta-analysis showed a statistically significant association of early enteral nutrition and reductions in systemic infections, pancreatic infections, length of hospital stay, and mortality[101]. Also, in patients with severe AP, enteral nutrition was significantly superior to total parenteral nutrition regarding mortality, infectious complications, and organ failure[102]. Gut barrier function is compromised in patients with acute pancreatitis, likely leading to bacterial translocation and potentially causing infected necrosis or even sepsis[103,104]. Because enteral feeding stabilizes gut barrier function, thereby reducing bacterial translocation, it is important early during the course of AP[14,105].
Therefore, whenever possible, i.e., when dissipating pain allows the patient to eat and infectious parameters do not continue to rise, oral food intake should be initiated as early as possible[49]. If oral food intake is not possible and the patient needs nutritional support, enteral tube feeding is preferred over total parenteral nutrition. However, the composition of an optimal diet has not yet been evaluated.
Antibiotic prophylaxis: There also has been a change regarding prophylactic antibiotic therapy in patients with AP. While in the 90ties, prophylactic antibiotics where thought to improve the outcome in patients with AP, there is no emerging evidence that prophylactic antibiotics reduce infectious complications or mortality[106-108]. Today, there is no clear evidence that supports antibiotic prophylaxis as a routine treatment in patients with severe AP[109-111]. Prophylactic antibiotics may reduce pancreatic infection in special subgroups of patients, but further well-designed and adequately-powered studies are needed to definitively answer the clinical usefulness of antibiotic prophylaxis in these patients[108]. Therefore, antibiotic prophylaxis is currently not recommended by international guidelines for the treatment of acute pancreatitis[14,49].
Acute pancreatitis is a frequent and potentially life-threatening disease. Numerous clinical prognostic scoring systems have been developed, and yet tools to discriminate between mild, moderate, and severe AP early during the course of the disease are not well advanced. Therapy is currently mostly symptomatic with fluid resuscitation, pain management, and early oral feeding. However, most of these therapeutic approaches are not well-defined. Vigorous fluid resuscitation remains a cornerstone of early management of acute pancreatitis. Cross-sectional imaging during the early phase of evaluation has not been associated with improvement in outcome. There is no role for prophylactic antibiotics in the management of the early phase of AP. Enteral nutrition in AP can reduce mortality, systemic infections, and multiorgan dysfunction compared to parenteral nutrition. Immediate ERC is indicated only in patients with biliary pancreatitis with common bile duct obstruction and cholangitis. These developments have contributed to an improved outcome for patients with acute pancreatitis, but further studies are still required to tackle the high mortality in this disease.
P- Reviewer: Bradley EL, Goral V, Pezzilli R, Sakata N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Spanier B, Bruno MJ, Dijkgraaf MG. Incidence and mortality of acute and chronic pancreatitis in the Netherlands: a nationwide record-linked cohort study for the years 1995-2005. World J Gastroenterol. 2013;19:3018-3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
| 2. | Satoh K, Shimosegawa T, Masamune A, Hirota M, Kikuta K, Kihara Y, Kuriyama S, Tsuji I, Satoh A, Hamada S. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2011;40:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas. 2012;41:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 5. | Oskarsson V, Sadr-Azodi O, Orsini N, Andrén-Sandberg Å, Wolk A. High dietary glycemic load increases the risk of non-gallstone-related acute pancreatitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 6. | Phillip V, Huber W, Hagemes F, Lorenz S, Matheis U, Preinfalk S, Schuster T, Lippl F, Saugel B, Schmid RM. Incidence of acute pancreatitis does not increase during Oktoberfest, but is higher than previously described in Germany. Clin Gastroenterol Hepatol. 2011;9:995-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994-2001. Pancreas. 2006;33:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002;56:S226-S230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 10. | Yadav D, Ng B, Saul M, Kennard ED. Relationship of serum pancreatic enzyme testing trends with the diagnosis of acute pancreatitis. Pancreas. 2011;40:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Saligram S, Lo D, Saul M, Yadav D. Analyses of hospital administrative data that use diagnosis codes overestimate the cases of acute pancreatitis. Clin Gastroenterol Hepatol. 2012;10:805-811.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1466] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 13. | Pavlidis P, Crichton S, Lemmich Smith J, Morrison D, Atkinson S, Wyncoll D, Ostermann M. Improved outcome of severe acute pancreatitis in the intensive care unit. Crit Care Res Pract. 2013;2013:897107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1150] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 15. | Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11:97-103. [PubMed] |
| 16. | Mounzer R, Whitcomb DC. Genetics of acute and chronic pancreatitis. Curr Opin Gastroenterol. 2013;29:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 17. | Papachristou GI. Prediction of severe acute pancreatitis: current knowledge and novel insights. World J Gastroenterol. 2008;14:6273-6275. [PubMed] |
| 18. | Leser HG, Gross V, Scheibenbogen C, Heinisch A, Salm R, Lausen M, Rückauer K, Andreesen R, Farthmann EH, Schölmerich J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991;101:782-785. [PubMed] |
| 19. | Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius H. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [PubMed] |
| 21. | Lesina M, Wörmann SM, Neuhöfer P, Song L, Algül H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol. 2014;26:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Gullo L, Migliori M, Oláh A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223-227. [PubMed] |
| 24. | Halonen KI, Leppaniemi AK, Puolakkainen PA, Lundin JE, Kemppainen EA, Hietaranta AJ, Haapiainen RK. Severe acute pancreatitis: prognostic factors in 270 consecutive patients. Pancreas. 2000;21:266-271. [PubMed] |
| 25. | Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit VK. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013;2013:367581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Sánchez-Lozada R, Acosta-Rosero AV, Chapa-Azuela O, Hurtado-López LM. [Etiology on determining the severity of acute pancreatitis]. Gac Med Mex. 2003;139:27-31. [PubMed] |
| 27. | Sánchez-Lozada R, Camacho-Hernández MI, Vega-Chavaje RG, Garza-Flores JH, Campos-Castillo C, Gutiérrez-Vega R. [Acute pancreatitis: five year experience at the Hospital General de Mexico]. Gac Med Mex. 2005;141:123-127. [PubMed] |
| 28. | Fan ST, Choi TK, Lai CS, Wong J. Influence of age on the mortality from acute pancreatitis. Br J Surg. 1988;75:463-466. [PubMed] |
| 29. | Birgisson H, Möller PH, Birgisson S, Thoroddsen A, Asgeirsson KS, Sigurjónsson SV, Magnússon J. Acute pancreatitis: a prospective study of its incidence, aetiology, severity, and mortality in Iceland. Eur J Surg. 2002;168:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Otsuki M, Matsuno S, Shimosegawa T, Williams JA, Go VL. International symposium: mechanism of pancreatitis--between bedside and laboratory. Pancreas. 2002;24:391-407. [PubMed] |
| 31. | Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7:S15-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Arata S, Takada T, Hirata K, Yoshida M, Mayumi T, Hirota M, Yokoe M, Hirota M, Kiriyama S, Sekimoto M. Post-ERCP pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Nitsche CJ, Jamieson N, Lerch MM, Mayerle JV. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Fortson MR, Freedman SN, Webster PD. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134-2139. [PubMed] |
| 36. | Wang DB, Yu J, Fulcher AS, Turner MA. Pancreatitis in patients with pancreas divisum: imaging features at MRI and MRCP. World J Gastroenterol. 2013;19:4907-4916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144:1292-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | DiMagno MJ, DiMagno EP. New advances in acute pancreatitis. Curr Opin Gastroenterol. 2007;23:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Löhr JM, Dinter D, Diehl SJ, Haas SL, Veeser M, Pfützer R, Retter J, Schönberg SO, Düber C, Keim V. Rapid progression of a splenic aneurysm due to segmental arterial mediolysis: a rare cause of acute pancreatitis. Pancreatology. 2013;13:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |
| 41. | Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22:274-278. [PubMed] |
| 43. | Cobb JP, O’Keefe GE. Injury research in the genomic era. Lancet. 2004;363:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, Schaapherder AF, Gooszen HG. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 45. | Phillip V, Schuster T, Hagemes F, Lorenz S, Matheis U, Preinfalk S, Lippl F, Saugel B, Schmid RM, Huber W. Time period from onset of pain to hospital admission and patients’ awareness in acute pancreatitis. Pancreas. 2013;42:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Keim V, Teich N, Fiedler F, Hartig W, Thiele G, Mössner J. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas. 1998;16:45-49. [PubMed] |
| 48. | American Gastroenterological Association (AGA) Institute on "Management of Acute Pancreatits" Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132:2019-2021. [PubMed] |
| 49. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1039] [Article Influence: 86.6] [Reference Citation Analysis (6)] |
| 50. | Martínez J, Sánchez-Payá J, Palazón JM, Suazo-Barahona J, Robles-Díaz G, Pérez-Mateo M. Is obesity a risk factor in acute pancreatitis? A meta-analysis. Pancreatology. 2004;4:42-48. [PubMed] |
| 51. | Lankisch PG, Weber-Dany B, Hebel K, Maisonneuve P, Lowenfels AB. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of nonsevere disease. Clin Gastroenterol Hepatol. 2009;7:702-705; quiz 607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Oskarsson V, Mehrabi M, Orsini N, Hammarqvist F, Segersvärd R, Andrén-Sandberg A, Sadr Azodi O. Validation of the harmless acute pancreatitis score in predicting nonsevere course of acute pancreatitis. Pancreatology. 2011;11:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81. [PubMed] |
| 54. | Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294-299. [PubMed] |
| 55. | Imrie CW, Benjamin IS, Ferguson JC, McKay AJ, Mackenzie I, O’Neill J, Blumgart LH. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br J Surg. 1978;65:337-341. [PubMed] |
| 56. | Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591-597. [PubMed] |
| 57. | Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, Dennison AR. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9:601-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 516] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 59. | Cho YS, Kim HK, Jang EC, Yeom JO, Kim SY, Yu JY, Kim YJ, Do KR, Kim SS, Chae HS. Usefulness of the Bedside Index for severity in acute pancreatitis in the early prediction of severity and mortality in acute pancreatitis. Pancreas. 2013;42:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [PubMed] |
| 61. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 62. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 341] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 960] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 64. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol. 2011;197:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Tang W, Zhang XM, Xiao B, Zeng NL, Pan HS, Feng ZS, Xu XX. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol. 2011;80:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas. 2000;20:367-372. [PubMed] |
| 67. | Lankisch PG, Mahlke R, Blum T, Bruns A, Bruns D, Maisonneuve P, Lowenfels AB. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol. 2001;96:2081-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 68. | Gan SI, Romagnuolo J. Admission hematocrit: a simple, useful and early predictor of severe pancreatitis. Dig Dis Sci. 2004;49:1946-1952. [PubMed] |
| 69. | Knoefel WT, Kollias N, Warshaw AL, Waldner H, Nishioka NS, Rattner DW. Pancreatic microcirculatory changes in experimental pancreatitis of graded severity in the rat. Surgery. 1994;116:904-913. [PubMed] |
| 70. | Bassi D, Kollias N, Fernandez-del Castillo C, Foitzik T, Warshaw AL, Rattner DW. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg. 1994;179:257-263. [PubMed] |
| 71. | Mann O, Kaifi J, Bloechle C, Schneider CG, Yekebas E, Kluth D, Izbicki JR, Strate T. Therapeutic small-volume resuscitation preserves pancreatic microcirculation in acute experimental pancreatitis of graded severity in rats. Pancreatology. 2009;9:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009;104:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Lipinski M, Rydzewski A, Rydzewska G. Early changes in serum creatinine level and estimated glomerular filtration rate predict pancreatic necrosis and mortality in acute pancreatitis: Creatinine and eGFR in acute pancreatitis. Pancreatology. 2013;13:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 75. | Wu BU, Bakker OJ, Papachristou GI, Besselink MG, Repas K, van Santvoort HC, Muddana V, Singh VK, Whitcomb DC, Gooszen HG. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med. 2011;171:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Pongprasobchai S, Jianjaroonwong V, Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S, Pausawasdi N, Srikureja W, Chainuvati S, Prachayakul V. Erythrocyte sedimentation rate and C-reactive protein for the prediction of severity of acute pancreatitis. Pancreas. 2010;39:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Hjalmarsson C, Stenflo J, Borgström A. Activated protein C-protein C inhibitor complex, activation peptide of carboxypeptidase B and C-reactive protein as predictors of severe acute pancreatitis. Pancreatology. 2009;9:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, Deus JR. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol. 2013;25:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832-840. [PubMed] |
| 80. | Rau BM, Kemppainen EA, Gumbs AA, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007;245:745-754. [PubMed] |
| 81. | Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, Larvin M, McMahon MJ. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. Br J Surg. 2003;90:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Pindak D, Parrak V, Pechan J, Vavrecka A, Kuzela L, Fuchs D, Irsakova J. The clinical value of the procalcitonin in prediction of severity and outcome in acute pancreatitis. Hepatogastroenterology. 2003;50 Suppl 2:ccviii-ccccix. [PubMed] |
| 83. | Kim BG, Noh MH, Ryu CH, Nam HS, Woo SM, Ryu SH, Jang JS, Lee JH, Choi SR, Park BH. A comparison of the BISAP score and serum procalcitonin for predicting the severity of acute pancreatitis. Korean J Intern Med. 2013;28:322-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Modrau IS, Floyd AK, Thorlacius-Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. Am J Gastroenterol. 2005;100:1593-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Lankisch PG, Blum T, Bruns A, Dröge M, Brinkmann G, Struckmann K, Nauck M, Maisonneuve P, Lowenfels AB. Has blood glucose level measured on admission to hospital in a patient with acute pancreatitis any prognostic value? Pancreatology. 2001;1:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Rajaratnam SG, Martin IG. Admission serum glucose level: an accurate predictor of outcome in gallstone pancreatitis. Pancreas. 2006;33:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Pezzilli R. Pharmacotherapy for acute pancreatitis. Expert Opin Pharmacother. 2009;10:2999-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol. 2012;107:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Wall I, Badalov N, Baradarian R, Iswara K, Li JJ, Tenner S. Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas. 2011;40:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 91. | Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, Burchard PR, Gordon SR, Gardner TB. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 92. | Haydock MD, Mittal A, Wilms HR, Phillips A, Petrov MS, Windsor JA. Fluid therapy in acute pancreatitis: anybody’s guess. Ann Surg. 2013;257:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 94. | Du XJ, Hu WM, Xia Q, Huang ZW, Chen GY, Jin XD, Xue P, Lu HM, Ke NW, Zhang ZD. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas. 2011;40:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Reddy N, Wilcox CM, Tamhane A, Eloubeidi MA, Varadarajulu S. Protocol-based medical management of post-ERCP pancreatitis. J Gastroenterol Hepatol. 2008;23:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Sarr MG. Early fluid “resuscitation/therapy” in acute pancreatitis: which fluid? What rate? What parameters to gauge effectiveness? Ann Surg. 2013;257:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983. [PubMed] |
| 98. | Tse F, Yuan Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst Rev. 2012;5:CD009779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Pezzilli R, Uomo G, Gabbrielli A, Zerbi A, Frulloni L, De Rai P, Castoldi L, Cavallini G, Di Carlo V. A prospective multicentre survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis. 2007;39:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Ebbehøj N, Friis J, Svendsen LB, Bülow S, Madsen P. Indomethacin treatment of acute pancreatitis. A controlled double-blind trial. Scand J Gastroenterol. 1985;20:798-800. [PubMed] |
| 101. | Li JY, Yu T, Chen GC, Yuan YH, Zhong W, Zhao LN, Chen QK. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One. 2013;8:e64926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, Zhu Y, Xia B. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med. 2012;51:523-530. [PubMed] |
| 103. | Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, Barclay GR, Larvin M, McMahon MJ. Calcitonin precursors: early markers of gut barrier dysfunction in patients with acute pancreatitis. Pancreas. 2003;27:239-243. [PubMed] |
| 104. | Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7:26-35; discussion 35-6. [PubMed] |
| 105. | Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2003;10:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta-analysis. Pancreas. 2001;22:28-31. [PubMed] |
| 107. | Bassi C, Larvin M, Villatoro E. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2003;CD002941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;CD002941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 109. | Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2011;46:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 110. | Jiang K, Huang W, Yang XN, Xia Q. Present and future of prophylactic antibiotics for severe acute pancreatitis. World J Gastroenterol. 2012;18:279-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647-656. [PubMed] |