Published online May 15, 2013. doi: 10.4291/wjgp.v4.i2.28
Revised: February 16, 2013
Accepted: April 13, 2013
Published online: May 15, 2013
Processing time: 184 Days and 9.6 Hours
AIM: To investigate functional duodenal abnormalities in functional dyspepsia (FD) and the role of serotonin (5-hydroxytryptamine, 5-HT) in mucosal ion transport and signalling.
METHODS: Duodenal mucosal biopsies were obtained from 15 patients with FD and 18 healthy controls. Immunohistochemistry was used to study the number of 5-HT-containing cells and real-time polymerase chain reaction for expression of 5-HT receptors 1A, 1B, 2A, 2B, 3A, 3B, 3C, 3D, 3E, 4 and 7, as well as expression of the serotonin re-uptake transporter (SERT) gene SLC6A4 and tryptophan hydroxylase 1 (TPH1). Biopsies were mounted in Ussing chambers for evaluation of basal and 5-HT-stimulated short-circuit current (SCC).
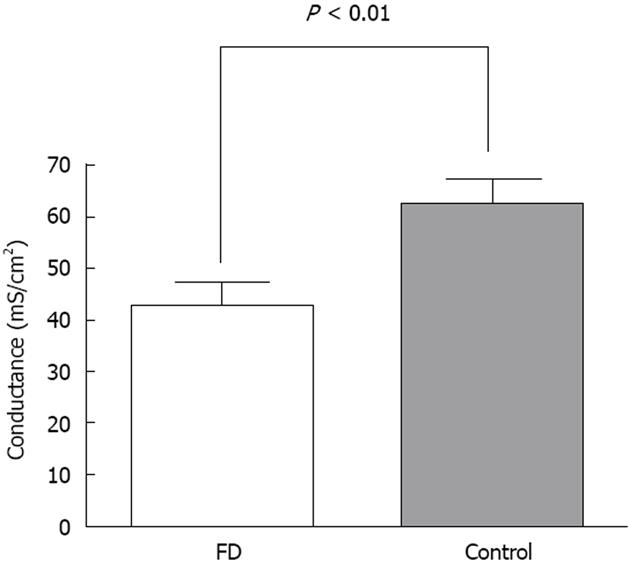
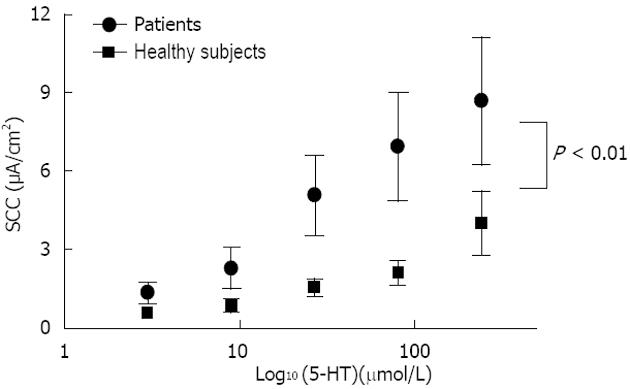
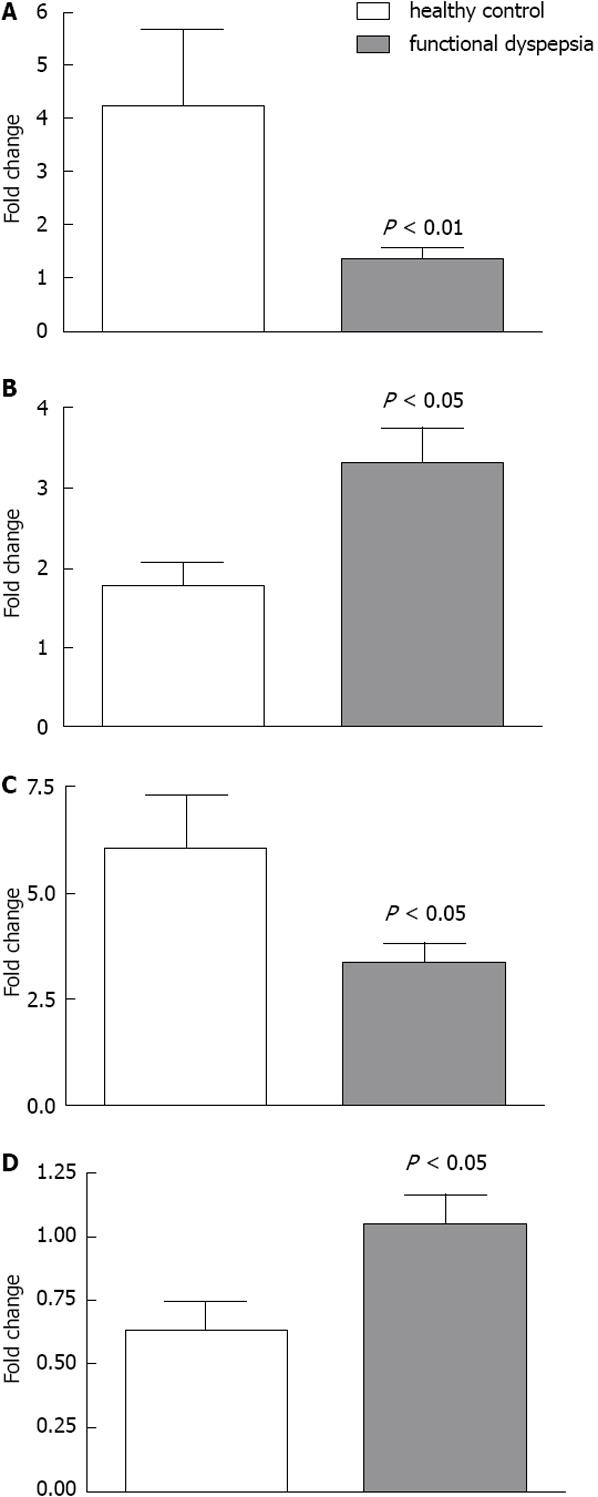
RESULTS: Conductance was lower in FD [42.4 ± 4.7 mS/cm2 (n = 15) vs 62.5 ± 4.5 mS/cm2 (n = 18), P = 0.005]. 5-HT induced a dose dependent rise in SCC in both FD (n = 8) and controls (n = 9), the rise was lower in FD (P < 0.001). Mean number of 5-HT stained cells per high power field was the same [34.4 ± 8.4 in FD (n = 15) and 30.4 ± 3.7 in controls (n = 18), P = 0.647]. The following genes were highly expressed: 5-HT receptor HTR3E, HTR4, HTR7, SERT gene (SLC6A4) and TPH1. Differences in expression levels were observed for HTR3E (higher expression in FD, P = 0.008), HTR7 (lower expression in FD, P = 0.027), SLC6A4 (higher expression in FD, P = 0.033) and TPH1 (lower expression in FD, P = 0.031).
CONCLUSION: Duodenal ion transport in response to exogenous 5-HT is abnormal in FD patients and associated with high expression of the HTR3E receptor and the serotonin transporter.
Core tip: The majority of patients with chronic symptoms from the gastro-duodenal region do not present signs of organic disease during routine examination, which commonly leads to the diagnosis of functional dyspepsia (FD). Our study strongly indicates the involvement of 5-hydroxytryptamine related duodenal mucosal mechanisms in FD pathogenesis. Future studies should further investigate alterations in up- and downstream effects related to HTR3E and HTR7 receptors.
- Citation: Witte AB, D’Amato M, Poulsen SS, Laurent A, Knuhtsen S, Bindslev N, Hansen MB, Schmidt PT. Duodenal epithelial transport in functional dyspepsia: Role of serotonin. World J Gastrointest Pathophysiol 2013; 4(2): 28-36
- URL: https://www.wjgnet.com/2150-5330/full/v4/i2/28.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v4.i2.28
The majority of patients with chronic symptoms from the gastro-duodenal region do not present signs of organic disease during routine examination, which commonly leads to the diagnosis of functional dyspepsia (FD). FD is known for its high prevalence, association with decreased quality of life[1] and difficult management, which pose a considerable economic burden[2-4]. Highly debated pathogenetic mechanisms include alterations in stomach function, e.g., hypersensitivity and emptying problems. Little is known about duodenal epithelial function in patients with FD and a broader understanding of the mucosal defence barrier, and insight in associated signalling systems is needed[5,6].
It is widely recognized that therapies that modulate serotonin 5-hydroxytryptamine (5-HT) activity are partly effective in patients with functional intestinal disorders. There are indications of increased transcription of tryptophan hydroxylase 1 (TPH1) and lowered serotonin re-uptake transporter (SERT) expression throughout the gut in isolated FD[7]. In addition, SERT polymorphisms appear to play a pathogenetic role in patients with postprandial distress syndrome[8].
5-HT in humans is mainly found in the duodenum, with the highest concentration in the lamina mucosa[9,10], and recent studies have revealed high expression levels of SERT, the 5-HT receptor HTR3 and HTR4 in the duodenum compared to other gut regions[11]. 5-HT is released by duodenal mucosal cells in response to luminal acidification[12] and together with other transmitters is involved in pathways mediating the response to luminal acid[13-15]. Furthermore, 5-HT has been shown to be a potent stimulator of electrogenic secretion in human duodenal mucosal biopsies[16].
We hypothesized that disturbances in the duodenal secretory reflex arches involving 5-HT play a pathogenic role in FD. Measurement of ion transport in human epithelia in vivo requires invasive methods; however a modified Ussing chamber for human endoscopic biopsies has been developed in our laboratory[17].
In the present study, 5-HT induced ion transport is measured in FD patients and healthy controls. Furthermore, duodenal mucosal biopsies are evaluated for expression of 5-HT receptors real-time polymerase chain reaction (RT-PCR) and the density of 5-HT-containing cells (immunohistochemistry).
The study was conducted in accordance with the Helsinki V declaration and approved by the Scientific Ethical Committee of Copenhagen. All participants received both written and oral information prior to the study and provided written informed consent.
Consecutive patients with dyspeptic symptoms referred for gastroscopy at Bispebjerg Hospital (Copenhagen, Denmark) who fulfilled the ROME III criteria[18] were invited to participate in the study. Healthy controls between 18-70 years were recruited. Two questionnaires, the gastrointestinal symptom rating scale and a non-validated Danish translation of a dyspepsia questionnaire developed by Tack et al[19,20], were used in order to confirm the diagnosis of FD, exclude gastroesophageal reflux disease as well as abdominal symptoms in the healthy controls. The gastrointestinal symptom rating scale is a validated instrument comprising 15 items for the assessment of gastrointestinal symptoms in irritable bowel syndrome (IBS) and peptic ulcer disease[21].
Seventeen patients with FD and 20 healthy controls were included in the study. Median age was 39 years (range 24-54 years) in the FD group (7 men, 10 women) and 24 years (range 21-50 years) in the healthy group (5 men, 16 women). With the exception of two subjects who used oral contraception, healthy controls were medication free. Four of the FD patients reported use of proton pump inhibitors on a non-regular basis. None had used proton pump inhibitor treatment one week prior to endoscopy, nor acetylsalicylic acid or non-steroid anti-inflammatory drugs. Over-consumption of alcohol was not present in any subject. One of the FD patients and three of the healthy subjects reported being smokers.
During gastroscopy, biopsies were obtained from the duodenum at the border between the duodenal bulb and the descending duodenum using standard biopsy forceps (Radial Jaw 4, outside diameter 2.4 mm, Boston Scientific, Denmark). In two FD patients and one healthy control a major part of the biopsies could not be obtained because the procedure was too distressing, while esophageal pathology was found in another healthy control. This meant that only 15 FD patients and 18 healthy controls were included, each with 8-10 biopsies available (out of 10 planned). Three of the biopsies were snap-frozen on dry ice for gene expression studies, one was stored in 4% buffered paraformaldehyde solution for subsequent immunohistochemical evaluation and up to four biopsies were placed in ice-cold Ringer solution for immediate mounting in Ussing chambers. Finally, one biopsy from the gastric antrum and one from the gastric corpus were stored in 4% buffered paraformaldehyde solution for subsequent histological analysis for Helicobacter Pylori detection.
Duodenal biopsies were transported to the laboratory in ice-cold bicarbonate-Ringer solution and 2-4 successfully mounted within 30 min in modified Ussing air suction chambers. Use of 10 times magnification through a stereomicroscope (Nikon, Tokyo) ensured correct mucosa-serosa orientation and appropriate fixation. Biopsies were fixed by constant air suction[17]. The exposed tissue area varied from 3.4 to 5 mm2, depending on the used insert, which was chosen to match the tissue size. The height of the (air) suction sleeve was 50 μm. Both sides of the tissue were bathed in bicarbonate-Ringer solution containing (in mmol/L) 140 Na+, 4 K+, 121 Cl-, 1 Ca2+, 0.5 Mg2+, 0.5 SO42- and 25 HCO3-. In addition, 11 mmol/L D-glucose was applied to the serosal side and 11 mmol/L D-sorbitol to the mucosal side. Temperature was maintained at 37 °C with the help of water jackets and oxygenation was ensured by constant input of gas-lift-circulating 95% O2 + 5% CO2. Short-circuit current (SCC) and slope conductance were continuously recorded by an automated voltage-clamp device (MFI 1-425) and measured as μA/cm2 and mS/cm2 respectively. Solution-resistance correction was performed immediately before mounting. Baseline values were recorded after an equilibration period of 15-30 min when stable values were reached. Further experiments were performed with different stimulating agents, but here we present results from the application of 5-HT, which was performed in nine FD patients and ten healthy controls as follows:
5-HT was applied in a dose-increasing manner ranging from 3 to 243 μmol/L (final concentration in the bathing solution) with 5-min intervals between applications. 5-HT was added to the serosal side as previously described[16]. For evaluation of tissue viability, transport capacity and correct tissue orientation, 4 mmol/L glucose and 0.1 mmol/L phloridzin, both purchased from Sigma, Denmark, were sequentially added to the mucosal side. A glucose response of > 5 μA/cm2 or response to phloridzin < 5 μA/cm2 was considered equivalent to full tissue viability. Some of the biopsies did not fulfil these criteria and were excluded from further analysis, resulting in a final number of eight FD patients and nine controls from whom 5-HT-stimulated values were obtained.
In addition to one un-mounted biopsy from each subject, all the mounted biopsies were gently collected upon conclusion of the Ussing chamber experiments and fixated in 4% buffered paraformaldehyde for a minimum of 24 h. They were then dehydrated, embedded in paraffin and cut into 10 μm sections, which were stained with hematoxylin/periodic acid Schiff for histological staining and examination. Protocols were blinded to the examiner. Assessment included possible pathology and the extent of epithelial damage, which was graded by a severity score ranging from 0 to 3; with 0 indicating no mucosal damage and 3 severe mucosal damages as previously described[16]. A Leitz Ortoplan microscope (Wetzlar, Germany) fitted with a cooled camera (Evolution MP, Media Cybernetics, Wokingham, Berkshire, United Kingdom) was used for examination and photography. For IHC analysis of anti-5-HT staining cells, sections of approximately 5 microns were deparaffinized and incubated for 5 min in 2% bovine serum albumin followed by 18 h at 4 °C with the primary antibody, monoclonal mouse anti-serotonin (M0758, DakoPatts) diluted 1:200. The immunoreactions were visualized by means of biotinylated rabbit anti-mouse immunoglobulins (E354, Dako) diluted 1:200, as the second layer, followed by streptavidin-peroxidase complex, VECTASTAIN PK-4000, diluted 1:100, for 30 min as the third layer. The sections were finally stained by means of 3,3’-diaminobenzidine for 30 min and counterstained with hematoxylin.
To determine the number, 5-HT-immunoreactive cells were counted in an objective manner from representative photomicrographs of the immunostained biopsies by means of Image-Pro Plus 6.0. Both the number of stained cells and the stained area per high power field were assessed. A field magnified 10 times was suitable to cover the biopsy area with only minor tissue deficiency, which correlates with a tissue area of 1.03 mm × 0.79 mm.
Gastric biopsies were immediately fixed in 4% buffered paraformaldehyde and after sectioning and staining with Giemsa (Merck, Darmstadt, Germany) examined for HP presence in the light microscope. Participants were considered HP positive if bacteria were found in either the antrum or the corpus fundus biopsies. These protocols were also blinded to the examiner.
Samples for PCR were stored at -80 °C until tissue collection was completed for one-series analysis. Total RNA was extracted from biopsies with commercially available kits (Qiagen, Hilden, Germany) and cDNA synthesized from 0.5-1.0 μg of RNA with SuperScript™ III reverse transcriptase (Invitrogen, Carlsbad, CA, United States) in accordance with the manufacturer’s instructions. In total, mRNA expression analysis was carried out on 10 FD patients and 16 controls.
For each gene examined, mRNA expression levels were measured by quantitative RT-PCR in ABI Prism 7500 Sequence Detection Systems (Applied Biosystems) with specific TaqMan Gene Expression Assays (Applied Biosystems), in accordance with the manufacturer’s instructions. Gene expression of HTR1A (Assay ID Hs00265014), 1B (Hs00265286), 2A (Hs01033524), 2B (Hs00168362), 3A (Hs00168375), 3B (Hs00175775), 3C (Hs00365674), 3D (Hs00699391), 3E (Hs00704511), 4 (Hs00410577) and 7 (Hs00989028), the SERT gene (SLC6A4, Hs00984355) and TPH1 (Hs00188220) were studied. RT-PCR reactions were performed in triplicate on each sample and, after normalization to internal endogenous controls (glyceraldehyde 3-phosphate dehydrogenase, Hs99999905), mRNA expression levels for each gene and in each sample were determined by the comparative CT method of relative quantification. Samples were only included in the analysis when gene expression was detected before cycle 35 in the PCR reaction (cycle threshold < 35) for a given gene, which resulted in slightly different N-values for each gene. Results were expressed in arbitrary units relative to a randomly chosen reference sample.
The effect of the drugs applied in the Ussing chambers was defined as SCC before versus after application. Mean results from the 2-4 biopsies from each subject were used for further analysis and are presented as mean ± SE. The unpaired t-test was used to compare the effects of glucose and the difference in basal SCC and slope conductance. The relationship between the concentration of 5-HT and induced SCC was investigated using a linear model, with treatment and log-transformed concentration as fixed effects. The number of 5-HT immune-reactive cells was compared between subject groups using the unpaired t-test. The Mann-Whitney U-test was employed to identify statistically significant differences between patients and controls in the RT-PCR gene expression analysis (mRNA). Commercially available software (PRISMA version 5.0 and SAS version 8.2) was used for all statistical analyses and two-tailed P < 0.05 was considered significant.
Mean basal SCC was 19.8 ± 3.0 μA/cm2 for FD patients (n = 15) and 21.4 ± 3.7 μA/cm 2 for controls (n = 18) with no significant difference between groups (P = 0.749). As shown in Figure 1, comparison of basal conductance revealed significantly lower values for FD patients compared to healthy controls (42.4 ± 4.7 mS/cm2 and 62.4 ± 4.5 mS/cm2 respectively, P = 0.005). Glucose control values after 5-HT stimulation yielded a mean magnitude of 12.5 ± 2.0 μA/cm2 for the FD group and 12.1 ± 2.5 μA/cm2 for controls (P = 0.906). 5-HT induced a dose dependent SCC rise in both healthy controls and FD patients (Figure 2). The 5-HT-induced rise in SCC was significantly lower in the latter (P < 0.001).
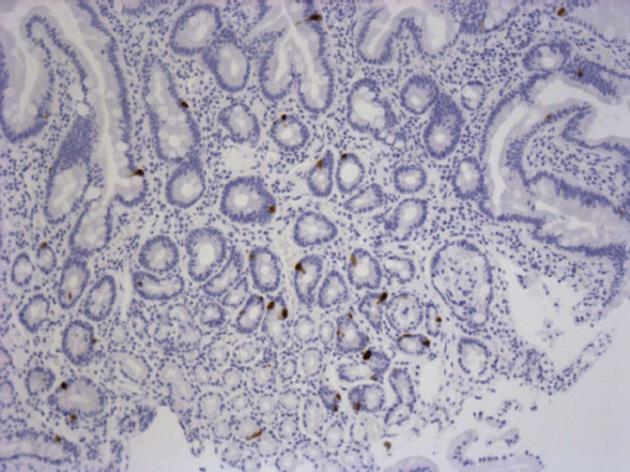
Histology revealed some variation with regard to biopsy depth; however, the surface epithelium and entire lamina propria were intact in all samples before and after mounting. Several biopsies also included the lamina muscularis mucosa and in some cases the submucosal layer contained part of Brunner’s glands. Epithelial damage before mounting ranged from 0-3 in both groups with a mean score of 0.7 in dyspeptic and 1.1 in healthy subjects (P = 0.179). 5-HT stained cells were found in the epithelium and Brunner’s glands, but only the number of cells in the epithelium was included in the evaluation since not all biopsies contained sub-mucosal tissue (Figure 3). In un-mounted biopsies, the mean number of cells per high power field was 34.4 ± 8.4 in the FD group and 30.4 ± 3.70 in controls (P = 0.647).
Helicobacter staining was positive in one FD patient and none of the healthy subjects.
Expression levels were undetectable for three genes, HTR1A, HTR3B and HTR3D, while there was only very low expression of HTR1B, HTR2A, HTR2B, HTR3A and HTR3C. The following genes were strongly expressed: HTR3E, HTR4, HTR7, SERT gene (SLC6A4) and TPH1. Genes that exhibited statistically significant differences in expression level between FD patients (n = 8-10) and healthy subjects (n = 13-16) include HTR3E (higher expression in dyspepsia, P = 0.008), HTR7 (lower expression in dyspepsia, P = 0.027), SLC6A4 (higher expression in dyspepsia, P = 0.033) and TPH1 (lower expression in dyspepsia, P = 0.031) (Figure 4).
5-HT increased the short circuit current in both healthy controls and FD patients in a dose-dependent manner, although the values were lower in FD patients (Figure 2). Slope conductance was significantly lower in FD patients.
Increased SCC is a direct result of secretion of negative ions to the duodenal lumen or flow of positive ions in the opposite direction and in the duodenum, bicarbonate secretion and hydrogen ion absorption are considered to be the most relevant fluxes. Disturbances in the response to duodenal acid have previously been described as related to dyspeptic symptoms[22,23]. Lower SCC values in our FD group might result from dysfunction in bicarbonate transport and subsequent impaired secretion.
Generally, the SCC values obtained in this study were distinctly lower than previously described by Engelmann et al[16], which could be explained by the fact that the studies were performed in different settings and electrophysiological measurements are easily affected by environmental factors. One should also bear in mind that the study by Engelmann et al[16] included patients with different dyspeptic conditions, which were not characterized by ROME III, and had no control group. In order to validate our results from the electrophysiological measurements, raw data were re-analysed by a second investigator blinded to the protocol and the analysis confirmed the results.
Reims et al[24] reported the applicability of Ussing chamber-measured epithelial electrical resistance for quantifying permeability changes in duodenal biopsies. In our case, biopsies from patients with FD had significantly lower conductance (thus higher resistance) compared to healthy controls, which may indicate decreased permeability. This result is rather surprising, because duodenal mucosal disease is generally associated with mucosal damage and impaired barrier function, thus one would expect higher permeability in patients with FD compared to healthy controls. Furthermore, increased epithelial permeability has been demonstrated in IBS sub-groups[25-27]. Routine histology did not reveal morphological alterations in our biopsies and there was no evidence of increased edge leakage in the healthy controls. One could argue that the resistance measured in our experiments reflects disturbance in electrolyte transport rather than nutrient or large molecule passage. Thus, it would be more reasonable to assume lower conductance of bicarbonate or chloride ions because of channel dysfunction or, for example, lowered tight junction capacity. With the present measurements of SCC and slope conductance we are unable to discriminate between an altered ionic passage through the cell compartment and/or the paracellular pathway. Alteration in potassium channels both apically and basolaterally in epithelial cells may account for the observed changes in conductance as well as in other channels involved in the bicarbonate secretory process. A molecular reconfiguration in the paracellular pathway as an explanation for the observed difference in conductance is also a possibility.
Expression of HTR3E was considerably increased in FD patients, as was that of SLC6A4. However, HTR7 and TPH1 were significantly less expressed in patients with FD (Figure 4).
SLC6A4 is the approved gene symbol for SERT. The transporter gene was highly expressed in the duodenal samples and there was a significant difference between experimental groups with higher expression in patients with FD. A relationship between SERT gene alterations and FD has been suggested in a number of reviews[8,28]. There are also several studies that have pointed out significant associations between SERT polymorphism and IBS[29-33]. To our knowledge, the only result that confirms the relevance of SERT polymorphisms in relation to dyspeptic symptoms comes from studies of postprandial distress syndrome[8], while at least two other investigations failed to detect significant associations[34-37]. Gene expression levels have previously been described in dyspeptic female patients[7], where decreased SERT expression was found in the patient group. Our results suggest the opposite, although the small sample size reduces reliability. As this issue remains unclear, further studies to detect a significant relation between alterations in SERT expression and FD are necessary. Both HTR3E and HTR4 have been reported to be prevalent in duodenal mucosa[11], which is supported by our study. Furthermore, we found elevated expression of the HTR3E sub-type in patients with FD. HTR3E polymorphism possibly related to increased HTR3E production has been described in IBS[38] and our results might thus account for involvement of this receptor in FD pathology. In IBS, HTR3 antagonists are already in clinical use, and the cation-selective ligand-gated ion channels have been found to be involved in motility and bicarbonate secretion. Interestingly, expressions of other HTR3 sub-types, i.e., A and B, were low in our samples. A strong genetic correlation between HTR3C and HTR3E in human duodenal tissue has recently been proposed[35] and patch clamp studies have revealed that HTR3C, HTR3D and HTR3E are non-functional when expressed alone[39]. HTR3A gene polymorphism has recently been associated with severe dyspeptic symptoms[40]. As HTR3A expression was generally low in our biopsies, differences between FD patients and controls might not have become apparent.
HTR7 is one of the 5-HT receptors about which very little is known, mainly due to the lack of specific ligands. A role in upper intestinal smooth muscle relaxation[41,42], activation of afferent neurons[43] as well as high expression in the stomach and ileum[44] has been revealed previously, and we found high expression in duodenal samples. Our results also indicate significant differences in expression level between patients with FD and controls. HTR7 has been proposed as a novel target for treatment of FD and related disorders[45] and further elucidation of its pathophysiological role would definitely be valuable.
TPH1 gene abnormalities have recently been suggested in IBS[46] and lower mRNA expression levels have been observed in such patients[30]. We found lower expression values in our FD group, indicating that decreased 5-HT availability might be part of the disease pathology. In contrast, Foxx-Orenstein et al[7] presented results demonstrating higher expression in duodenal mucosa of female patients with FD. A recent study of TPH1 expression in gastric mucosal biopsies from children with FD found no difference between these patients and controls[47].
As we did not observe any significant differences in the content of 5-HT-positive cells between FD patients and controls, further studies including a larger number of subjects may be needed to conclusively address this issue. 5-HT content in duodenal mucosa has been studied immunohistochemically in patients with post-infectious FD and found to be significantly reduced in patients 6 mo or more after the infection, but not in recovered controls[48].
The low number of subjects in the patient and control groups and differences in terms of age and smoking status (which can affect the mucous layer and bicarbonate secretion) are a limitation of this study.
In conclusion, our study strongly indicates the involvement of 5-HT related duodenal mucosal mechanisms in FD pathogenesis. Future studies should further investigate alterations in up- and downstream effects related to HTR3E and HTR7 receptors.
We thank Heidi Paulsen, Lise Strange and Anne-Marie Møller for technical assistance as well as Sofia Zetterstrand for help with the statistical analysis.
The majority of patients with chronic symptoms from the gastro-duodenal region do not present signs of organic disease during routine examination, which commonly leads to the diagnosis of functional dyspepsia (FD).
It is widely recognized that therapies that modulate serotonin 5-hydroxytryptamine (5-HT) activity are partly effective in patients with functional intestinal disorders.
Duodenal ion transport in response to exogenous 5-HT is abnormal in FD patients and associated with high expression of the HTR3E receptor and the serotonin transporter. Their study strongly indicates the involvement of 5-HT related duodenal mucosal mechanisms in FD pathogenesis. Future studies should further investigate alterations in up- and downstream effects related to HTR3E and HTR7 receptors.
The authors present a a study about an involvement of 5-HT in FD. Due to the prevalence of FD, it is important to reveal the mechanisms involved as a basis for the development of new therapies.
P- Reviewer Pehl C S- Editor Gou SX L- Editor A E- Editor Yan JL
| 1. | Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, Ronkainen J. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Alander T, Svärdsudd K, Agréus L. Functional gastrointestinal disorder is associated with increased non-gastrointestinal healthcare consumption in the general population. Int J Clin Pract. 2008;62:234-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Ford AC, Moayyedi P. Managing dyspepsia. Curr Gastroenterol Rep. 2009;11:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20 Suppl 1:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | van Boxel OS, ter Linde JJ, Siersema PD, Smout AJ. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010;105:803-811; quiz 802, 812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Foxx-Orenstein A, Camilleri M, Gershon MD, Linden DR, Mawe GM, Lewis JT, Jensen KL, Talley NJ, Szurszewski JH, Zinsmeister A. Alterations in intestinal serotonin expression in dyspepsia and irritable bowel syndrome. Gastroenterology. 2007;132:A72. |
| 8. | Oshima T, Toyoshima F, Nakajima S, Fukui H, Watari J, Miwa H. Genetic factors for functional dyspepsia. J Gastroenterol Hepatol. 2011;26 Suppl 3:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Meyer T, Brinck U. Differential distribution of serotonin and tryptophan hydroxylase in the human gastrointestinal tract. Digestion. 1999;60:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120-1130. [PubMed] |
| 11. | van Lelyveld N, Ter Linde J, Schipper ME, Samsom M. Regional differences in expression of TPH-1, SERT, 5-HT(3) and 5-HT(4) receptors in the human stomach and duodenum. Neurogastroenterol Motil. 2007;19:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kellum JM, Jaffe BM. Release of immunoreactive serotonin following acid perfusion of the duodenum. Ann Surg. 1976;184:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1-C19. [PubMed] |
| 14. | Nylander O, Pihl L. Luminal hypotonicity increases duodenal mucosal permeability by a mechanism involving 5-hydroxytryptamine. Acta Physiol (Oxf). 2006;186:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Smith AJ, Chappell AE, Buret AG, Barrett KE, Dong H. 5-Hydroxytryptamine contributes significantly to a reflex pathway by which the duodenal mucosa protects itself from gastric acid injury. FASEB J. 2006;20:2486-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Engelmann BE, Bindslev N, Poulsen SS, Larsen R, Hansen MB. Functional characterization of serotonin receptor subtypes in human duodenal secretion. Basic Clin Pharmacol Toxicol. 2006;98:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Larsen R, Mertz-Nielsen A, Hansen MB, Poulsen SS, Bindslev N. Novel modified Ussing chamber for the study of absorption and secretion in human endoscopic biopsies. Acta Physiol Scand. 2001;173:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 19. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 762] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 20. | Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 410] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 21. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1035] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Lee KJ, Demarchi B, Demedts I, Sifrim D, Raeymaekers P, Tack J. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol. 2004;99:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Reims A, Strandvik B, Sjövall H. Epithelial electrical resistance as a measure of permeability changes in pediatric duodenal biopsies. J Pediatr Gastroenterol Nutr. 2006;43:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 28. | Camilleri M. Pharmacogenomics and serotonergic agents: research observations and potential clinical practice implications. Neurogastroenterol Motil. 2007;19 Suppl 2:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Pata C, Erdal ME, Derici E, Yazar A, Kanik A, Ulu O. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97:1780-1784. [PubMed] |
| 30. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 31. | Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Park JM, Choi MG, Park JA, Oh JH, Cho YK, Lee IS, Kim SW, Choi KY, Chung IS. Serotonin transporter gene polymorphism and irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Sikander A, Rana SV, Sinha SK, Prasad KK, Arora SK, Sharma SK, Singh K. Serotonin transporter promoter variant: Analysis in Indian IBS patients and control population. J Clin Gastroenterol. 2009;43:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Camilleri CE, Carlson PJ, Camilleri M, Castillo EJ, Locke GR, Geno DM, Stephens DA, Zinsmeister AR, Urrutia R. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterol. 2006;101:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | van Lelyveld N, Linde JT, Schipper M, Samsom M. Candidate genotypes associated with functional dyspepsia. Neurogastroenterol Motil. 2008;20:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Toyoshima F, Oshima T, Nakajima S, Sakurai J, Tanaka J, Tomita T, Hori K, Matsumoto T, Miwa H. Serotonin transporter gene polymorphism may be associated with functional dyspepsia in a Japanese population. BMC Med Genet. 2011;12:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Park CS, Uhm JH. Polymorphisms of the Serotonin Transporter Gene and G-Protein β3 Subunit Gene in Korean Children with Irritable Bowel Syndrome and Functional Dyspepsia. Gut Liver. 2012;6:223-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, Rance KH, Trinh H, Balmer G, Kelly FM, Yusaf SP. Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009;108:384-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Mujakovic S, ter Linde JJ, de Wit NJ, van Marrewijk CJ, Fransen GA, Onland-Moret NC, Laheij RJ, Muris JW, Grobbee DE, Samsom M. Serotonin receptor 3A polymorphism c.-42C & gt; T is associated with severe dyspepsia. BMC Med Genet. 2011;12:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Janssen P, Prins NH, Moreaux B, Meulemans AL, Lefebvre RA. Characterization of 5-HT7-receptor-mediated gastric relaxation in conscious dogs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G108-G115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Tonini M, Vicini R, Cervio E, De Ponti F, De Giorgio R, Barbara G, Stanghellini V, Dellabianca A, Sternini C. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology. 2005;129:1557-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Meuser T, Pietruck C, Gabriel A, Xie GX, Lim KJ, Pierce Palmer P. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002;71:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422-23426. [PubMed] |
| 45. | Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil. 2005;17:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Jun S, Kohen R, Cain KC, Jarrett ME, Heitkemper MM. Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:233-239, e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883-891. [PubMed] |