Published online Jun 15, 2011. doi: 10.4291/wjgp.v2.i3.53
Revised: March 31, 2011
Accepted: April 7, 2011
Published online: June 15, 2011
Littoral cell angiomas (LCA) of the spleen are vascular tumors of unknown etiology arising from the littoral cells of the splenic red pulp sinuses. Usually a benign and incidental finding, LCA have been repeatedly reported in association with a variety of visceral malignancies and hold the potential for dissemination per se. We encountered a case of a 30 year old female who was diagnosed with solid pseudopapillary tumor of the head and distal pancreas by fine needle aspiration cytology. A distal pancreatectomy with splenectomy was performed in addition to a pylorus-preserving Whipple’s procedure and cholecystectomy. Histopathological examination confirmed solid pseudopapillary tumor of the pancreas and showed multiple well-circumscribed anastomosing vascular channels in the spleen. The diagnosis of LCA of the spleen was confirmed by immunohistochemistry that revealed co-expression of endothelial cell marker, CD31 and CD34, along with histiocytic marker, CD68 by the vascular lining cells. LCA has been previously reported in association with colorectal and pancreatic adenocarcinoma, malignant lymphoma, myelodysplasia and autoimmune disorders. We report the first case of LCA associated with solid pseudopapillary tumor of the pancreas.
- Citation: Bhavsar T, Wang C, Huang Y, Karachristos A, Inniss S. Littoral cell angiomas of the spleen associated with solid pseudopapillary tumor of the pancreas. World J Gastrointest Pathophysiol 2011; 2(3): 53-56
- URL: https://www.wjgnet.com/2150-5330/full/v2/i3/53.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v2.i3.53
Littoral cell angiomas (LCA) are rare vascular tumors of the spleen of uncertain biological behavior[1]. First described by Falk et al in 1991[2], the majority of LCA are asymptomatic incidental findings with no age or sex predilection[3,4]. Splenomegaly is a common feature of all the LCA and a few of them show symptoms of hypersplenism[5-8]. The unique feature of almost all LCA is its immunohistochemical reactivity to CD31 (endothelial marker) and CD68 (histiocytic marker); the latter suggesting the origin of the tumor as the splenic sinus lining littoral cells. LCA have been associated with a variety of visceral malignancies, including colorectal and pancreatic adenocarcinoma, malignant lymphoma[9] and myelodysplasia[10]. To the best of our knowledge, about 35 cases of LCA have been reported to date in the English literature.
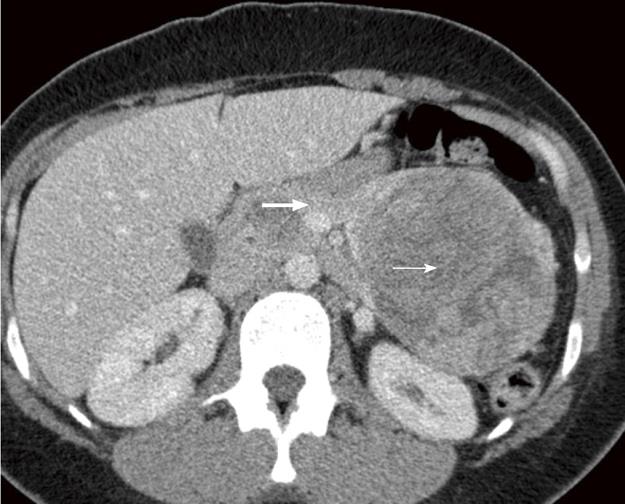
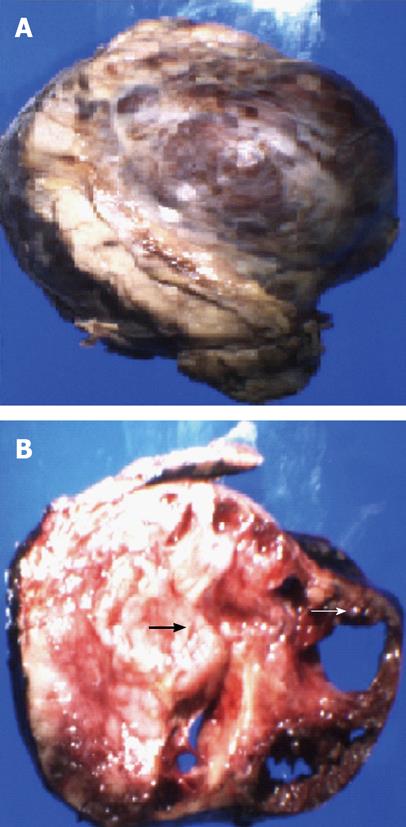
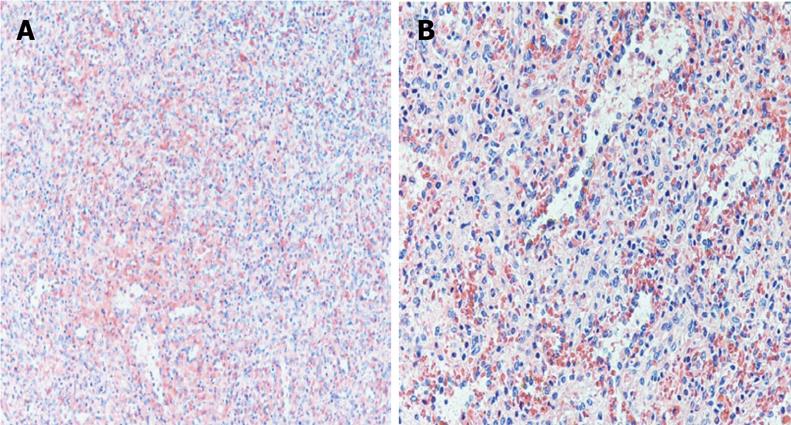
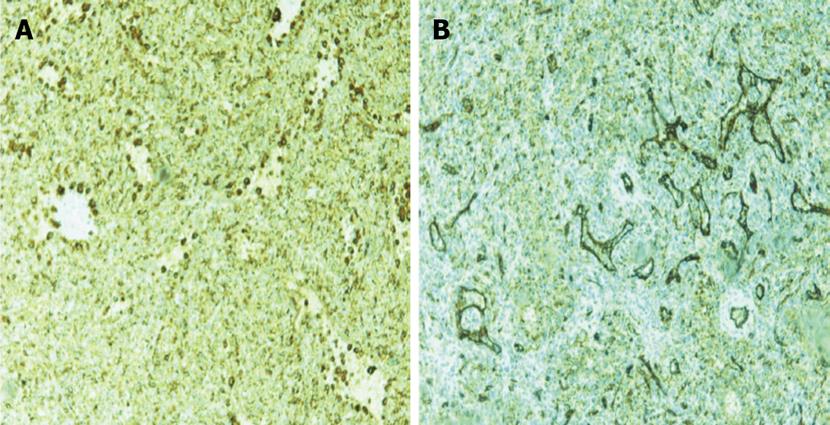
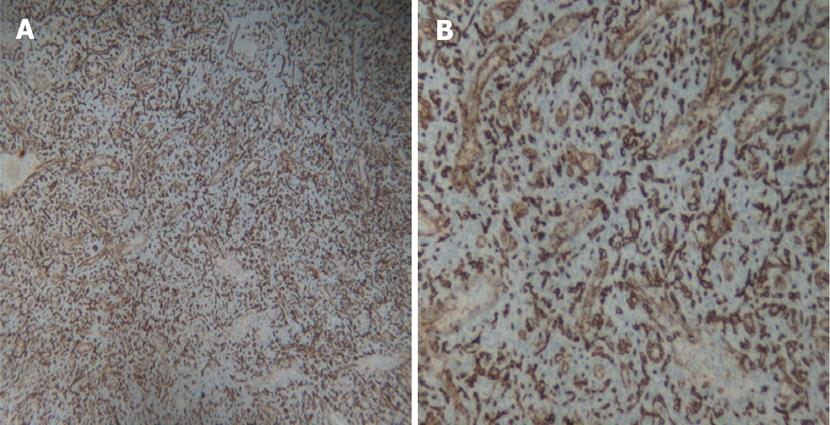
A 30 year old female with a history of sickle cell disease, SC trait presented with a 2 d history of gradual onset of back and bilateral lower extremity pain with fever and chills. The patient was diagnosed with solid pseudopapillary neoplasm of the pancreas by endoscopic ultrasonography-guided fine needle aspiration cytology. The diagnosis was confirmed by immunohistochemistry that showed a positive reactivity to CD56, synaptophysin, CD10 and alpha-1 antitrypsin. A CT-scan imaging of the abdomen identified an 11 cm tumor in the distal pancreas and a 2 cm tumor of the head of the pancreas with a bridge of preserved pancreatic tissue between the two tumors (Figure 1). A preoperative angiogram showed the dorsal pancreatic artery supplying the distal tumor and the patient underwent a distal pancreatectomy and splenectomy along with a Whipple’s procedure to prevent the overt diabetes. Gross examination of the pancreas showed a yellow-tan, lobulated, well-circumscribed mass located on the anterior aspect of the pancreatic tail (Figure 2) measuring 13 cm × 10 cm × 7.5 cm and a hemorrhagic, focally cystic red-brown tumor measuring 2 cm × 1.5 cm × 1 cm in the supero-anterior aspect of the pancreatic head. Histopathological examination of the tumor in the head and the distal pancreas revealed morphological changes of solid pseudopapillary tumor. Gross examination of the spleen showed a 113 g yellowish-brown nodular organ measuring 10 cm × 6.5 cm × 3 cm. Two dark-brown, well-circumscribed nodules were identified; one measuring 1.1 × 0.7 × 0.4 cm near to the hilum and other measuring 2.5 × 0.7 × 0.3 cm just underneath the capsule. Histopathological examination showed multiple, anastomosing vascular lesions that vaguely resembled splenic sinusoids lined by tall endothelial cells (Figure 3). The vascular lesions were well delimited from the surrounding splenic parenchyma. Immunohistochemistry revealed the co-expression of CD31 (Figure 4A), CD68 (Figure 4B) and CD34 (Figure 5) by the vascular lining cells, confirming the lesion as LCA of the spleen.
Since the identification of LCA by Falk et al in 1991[2], these vascular tumors have been periodically reported[5-8] in the literature. Two forms of LCA have been described; the more commonly encountered diffuse multiple nodular form as in our case and the rare solitary form[11].
The differential diagnosis of splenic neoplasm with a radiological imaging similar to LCA is extensive and includes hemangiomatosis, lymphangiomatosis, hamartoma, hemangiopericytoma, hemangioendothelioma, angiosarcoma[1], lymphoma, metastasis and sarcoidosis.
Clinically, LCA can present as an abdominal mass, mostly due to splenomegaly, with symptoms of hypersplenism with ensuing anemia and/or thrombocytopenia, pulmonary hypertension and pyrexia of unknown origin[5-8], or can be an incidental finding. However, in our case the patient did not have a splenomegaly associated with solid pseudopapillary tumor of the pancreas. More dramatically, LCA has been reported to present as splenic rupture and hemoperitoneum[12,13].
Radiological studies by CT scan, MRI, sonography or nuclear medicine studies, although not conclusive[14], can contribute to diagnosing LCA. A CT-scan imaging shows LCA as hypoattenuating nodules of varying size. Delayed phase imaging on CT-scan reveals the nodules to be isodense to surrounding splenic parenchyma due to delayed filling of the nodules. MRI of the spleen shows hypodense lesions on T1 and T2 weighted scan due to the hemosiderin content of the tumor[1]. However, no hypodense nodules in the spleen were evident on CT-scan imaging in our case. Sonography is rarely helpful as findings vary greatly from isoechoic to hypo- and hyper-echoic lesions[15]. Tc-99m labeled RBC scintigraphy can differentiate splenic lesions from splenic hemangiomas[16].
The pathogenesis of LCA remains unclear but, given its association with autoimmune disorders such as Crohn’s disease and inborn metabolic diseases such as Gaucher’s disease, immune system dysfunction has been postulated as a possible pathogenic mechanism[17,18]. Supporting this hypothesis, other reports have suggested that chronic infection and systemic immunosuppression may contribute to the development of LCA[12,19]. Interestingly, once thought of as a benign and incidental lesion, one third of the reported cases are associated with malignancies of visceral organs including adenocarcinoma of colorectum (most common), kidney, liver[20], lung[21], pancreas, hepatocellular carcinoma and malignant lymphoma[9]. It has also been associated with myelodysplasia[10] and aplastic anemia[22]. Interestingly, this is the first report of a LCA of the spleen associated with a solid pseudopapillary tumor of the pancreas. The strong association of LCA with various malignancies necessitates splenectomy in most of the cases. The splenectomy in our case, however, was a part of the distal pancreatic tumor excision.
Two subtypes of LCA, angiosarcoma and hemangioendothelioma, have been reported to have malignant potential. In two rare cases[23,24], distant metastasis with neoplastic cells consistent with the morphology of LCA have been identified after splenectomy. There was no evidence of any distant metastasis of the LCA in our case.
The definite diagnosis of LCA is made at pathology after splenectomy which remains the gold-standard of the treatment[13]. Grossly, the spleen shows nodules with blood/blood products of variable color, usually dark-red to brown/black depending upon the chronicity of blood in these lesions[16]. Histopathology reveals proliferation of anastomosing vascular channels lined by tall endothelial cells with papillary fronds extending into the vascular channels. Some exfoliated cells may be seen in the vascular spaces and atypical cells and mitoses are rare. LCA shares morphological and immunohistochemical features with hemangiomas at other locations such as immunoreactivity for vascular endothelial marker CD31 and factor VIII. Even although they are usually negative for markers highlighting the red pulp sinusoidal epithelium such as CD8 and CD34, the LCA in our case expressed the endothelial marker, CD34 (Figure 5). The expression of endothelial marker CD31 and histiocytic marker CD68 by the vascular cells is unique and diagnostic of LCA[1], as in our case.
In conclusion, LCA are primary vascular neoplasms of the spleen and are usually an incidental finding. Even though the vast majority of these are benign, malignant association and potential have been documented prompting close evaluation and surveillance in patients with LCA for development of other malignancies. We report the first case of an incidental LCA of the spleen associated with a solid pseudopapillary tumor of the pancreas.
Peer reviewers: Jens Hoeppner, MD, Department of Surgery, University of Freiburg, Hugstetter Str. 55, Freiburg 79106, Germany; Mohammad Al-Haddad, MD, Indiana University School of Medicine, 550 N. University Blvd, UH 4100, Indianapolis 46033, United States; Marco Bustamante, MD, Gastroenterology, Hospital Universitario Peset Avda. Gaspar Aguilar 90, Valencia 46017, Spain
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
| 1. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Levy AD, Abbott RM, Abbondanzo SL. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology. 2004;230:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Najera L, Dotor AM, Santoja C. Littoral cell angioma of the spleen. A case report and review of the literature. Rev Esp Pato. 2006;39:49–53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Dascalescu CM, Wendum D, Gorin NC. Littoral-cell angioma as a cause of splenomegaly. N Engl J Med. 2001;345:772-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Collins PJ, Ettler H, Amann J, Rajgopal C. Soft-tissue images. Splenic littoral cell angioma. Can J Surg. 2003;46:204-205. [PubMed] |
| 7. | Ziske C, Meybehm M, Sauerbruch T, Schmidt-Wolf IG. Littoral cell angioma as a rare cause of splenomegaly. Ann Hematol. 2001;80:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Espanol I, Lerma E, Fumanal V, Palmer J, Roca M, Domingo-Albos A, Pujol-Moix N. Littoral cell angioma with severe thrombocytopenia. Ann Hematol. 2000;79:46-49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Bisceglia M, Sickel JZ, Giangaspero F, Gomes V, Amini M, Michal M. Littoral cell angioma of the spleen: an additional report of four cases with emphasis on the association with visceral organ cancers. Tumori. 1998;84:595-599. [PubMed] |
| 10. | Erçin C, Gürbüz Y, Hacihanefioğlu A, Turgut Karakaya A. Multiple littoral cell angioma of the spleen in a case of myelodysplastic syndrome. Hematology. 2005;10:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Arber DA, Strickler JG, Chen YY, Weiss LM. Splenic vascular tumors: a histologic, immunophenotypic, and virologic study. Am J Surg Pathol. 1997;21:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Harmon RL, Cerruto CA, Scheckner A. Littoral cell angioma: a case report and review. Curr Surg. 2006;63:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Willcox TM, Speer RW, Schlinkert RT, Sarr MG. Hemangioma of the spleen: presentation, diagnosis, and management. J Gastrointest Surg. 2000;4:611-613. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bhatt S, Huang J, Dogra V. Littoral cell angioma of the spleen. AJR Am J Roentgenol. 2007;188:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Giovagnoni A, Giorgi C, Goteri G. Tumours of the spleen. Cancer Imaging. 2005;5:73-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Johnson C, Goyal M, Kim B, Wasdahl D, Nazinitsky K. Littoral cell angioma. Clin Imaging. 2007;31:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Gupta MK, Levin M, Aguilera NS, Pastores GM. Littoral cell angioma of the spleen in a patient with Gaucher disease. Am J Hematol. 2001;68:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Suvajdzić N, Cemerikić-Martinović V, Saranović D, Petrović M, Popović M, Artiko V, Cupić M, Elezović I. Littoral-cell angioma as a rare cause of splenomegaly. Clin Lab Haematol. 2006;28:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Fadare O, Hileeto D, Mariappan MR. Pathologic quiz case: multiple splenic lesions in a bacteremic patient. Littoral cell angioma of the spleen. Arch Pathol Lab Med. 2004;128:1183-1185. [PubMed] |
| 20. | Lin CH, Yu JC, Shih ML, Peng YJ, Hsieh CB. Littoral cell angioma of the spleen in a patient with hepatocellular carcinoma. J Formos Med Assoc. 2005;104:282-285. [PubMed] |
| 21. | Collins GL, Morgan MB, Taylor FM. Littoral cell angiomatosis with poorly differentiated adenocarcinoma of the lung. Ann Diagn Pathol. 2003;7:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Tholouli E, Roulson JA, Byers R, Burton I, Liu Yin JA. Littoral cell angioma of the spleen in a patient with severe aplastic anaemia. Haematologica. 2003;88:ECR33. [PubMed] |
| 23. | Rosso R, Paulli M, Gianelli U, Boveri E, Stella G, Magrini U. Littoral cell angiosarcoma of the spleen. Case report with immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1995;19:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Ben-Izhak O, Bejar J, Ben-Eliezer S, Vlodavsky E. Splenic littoral cell haemangioendothelioma: a new low-grade variant of malignant littoral cell tumour. Histopathology. 2001;39:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |