INTRODUCTION
The microbiome is an significantly complicated biological system of microbial species that it is contained in our body, their genome, and the surroundings they live[1]. Hence, the microbiome alludes to each microorganisms and their action, which contains of microbial metabolites and hereditary components[2]. Totality of genomes of a microbiota, regularly utilized to depict the entity of microbial traits (= functions) encoded with the aid of using a microbiota[3]. These microbial elements account for more than one hundred trillion microorganisms that encode more than three million genes and a big range of metabolites. Therefore, for the reason that our body interacts symbiotically with organisms, it performs primary immune and metabolic capabilities[4], being influenced by dietary style, quality of life and environmental factors[5].
In that produces secretions that are distributed throughout the body through the bloodstream or lymph, it produces both hormonal factors and humoral factors and regulates the innate immune system in a metabolism-dependent manner. This presence is necessary for digestion and energy production, and independent of metabolism, such as signaling factors. Metabolites and products that are formed during processes like fermentation such as short chain fatty acids (SCFAs), conversion of bile acids, conversion of choline and L-carnitine to trimethylamine (TMA), can contribute to the occurrence of conditions like increased insulin resistance and increased inflammation, increased intestinal permeability and diseases such as atherosclerosis cardiovascular diseases and stroke[6].
Moreover, dysbiosis by changing the production of small molecules that are secreted in the digestive tract, such as ghrelin, YY peptide, GLP-1, glucagon and other molecules that are related to the feeling of satiety or play a role in accelerating the emptying of the stomach and reducing appetite can affects on the intestinal leakage and also causes the desire to consume more food and obesity. Obesity is associated with several metabolic dependent diseases in different organs: Cardiovascular diseases, type 2 diabetes, non-alcoholic fatty liver disease, colorectal, breast, endometrial, kidney, esophagus, ovary, prostate, pancreas and stomach, esophageal reflux, asthma, arthritis and depression and anxiety[7] and Alzheimer’s disease[8].
Dysbiosis by changing homeostasis leads to changes in the absorption of iron and selenium, iodine and minerals, and changes in the absorption of vitamins play a role in the weakness of the immune system and changes in thyroid activity and can lead to thyroid cancer[9] and several other diseases[10]. In addition, recent years have provided a increasingly growing frame of experimental and clinical evidences highlighting how gut microbiota dysbiosis may play an initiating and probable perpetuating role in a number of ocular diseases, hence establishing the concept of an eye-gut axis[11].
Two percent to five percent of individuals through inflammatory bowel illness have eye problems[12]. A few considers have illustrated the presence of a gut-eye hub in which intestine microscopic organisms impact resistance at removed locales, counting the eye[13]. In this consider we are going examine more fine points of interest of the response of the immune system and defense additives in connection to the intestinal and eye microbiome. In addition, we would have a look at the connection between dysbiosis, inflammation and maturing and eye illnesses along side the part of the microbiome situations, nourishment, probiotics, prebiotics, postbiotics and utilitarian nourishments, vitamins and supplements and anti-microbials on eye wellbeing.
DIGESTIVE MICROBIOME VARIETY AND FACTORS AFFECTING IT
The microbiome varies from person to person and changes in anybody determines the composition of the intestine microbiome because of components related from pregnancy to birth, maturing, environment, use of anti-microbials and eating habits[14].
The microbiome regulates the physiological capacities of the have, contains digestion system and the improvement and support of resistant homeostasis. The human gut microorganisms differs in classification and function in each of the anatomical regions of the gastrointestinal (GI) tract, because these areas have different characteristics in terms of physiology, pH, oxygen tension, digestive flow rate, substrate availability and host secretions[15].
Molecular science advances have given the microbial phyla show within the intestine microbiome and their evaluation by analyzing DNA and RNA extracted from stool tests. It has been found that the intestinal microbiome incorporates some phyla such as Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria and Verrucomicrobia where the two phylums Firmicutes and Bacteroidetes represent the bigger part of the intestinal microbiome. The Firmicutes phylum contains the most species of Lactobacillus, Bacillus, Enterococcus and Ruminococcus, with Clostridium forming, majority of this phylum primarily contains Prevotella and Bacteroides genera. The phylum Actinobacteria is normally found in the microbiome and was primarily composed of the class Bifidobacterium[4].
Intestine microbial imbalance influences the host and contributes to the pathogenicity or progression of a wide range of harmful effects in different organs[16]. For the establishment of the immune system and physiological improvement, intestinal colonization of microbiota is fundamental in early life[17].
Gestational age at birth, mode delivery (caesarean or physiological), nourishing and duration of breastfeeding, as well as life-style, geographic area, hereditary traits of the infant, utilize of anti-microbials[18], eat less and utilization of drugs influence the intestine microbial populace[19].
THE RELATIONSHIP AMONG DYSBIOSIS, INFLAMMATION AND EYE DISEASES
Pathological properties that make intestinal dysbiosis and alter microbial metabolites disrupt the immune system metabolism, important to lead to inflammatory and immune system diseases. Dysbiosis refers to modification within the quali-quantitative composition of microbiome communities. The commensal microorganisms in intestine track directs the development of the mucosal immune system though the harmful microbiome may cause immune diseases, i.e. an awkwardness through the commensal and pathogenic microbiome that can incline to inflammatory diseases[20].
The digestive tract microbiome impacts the homeostasis and immune system either regionally and systemic. Expanded intestinal permeability (leaky gut) and microbial translocation results in systemic inflammatory aggravation[21]. Enterocytes, or intestinal epithelial cells, create a barrier that selectively regulates the exchanges among he internal and external surroundings of the lumen, toxins, antigens, as well as the absorption of nutrients, vitamins and water via intercellular and paracellular transport mechanisms[22]. Intestinal mucosal membrane permeability is associated with paracellular trafficking of microbial agents and increased immune response tolerance against non-self antigens[23]. Intestinal dysbiosis may also additionally become to end result in altering zonulin, the modulator of physiological intestinal permeability[24] which causes moieties of luminal contents to pass through the epithelial lining. This phenomenon would then trigger proinflammatory cytokine factors that further increase intestinal membrane permeability, allowing for a massive influx of microbial antigens and substances that prompt T cells. Then, depending of the genetic predisposition of the host, those T cells are possibly to remain in the GI tract and cause inflammatory bowel diseases (Crohn's disease, irritable bowel syndrome and other undefined inflammatory bowel diseases) or migrate to specific organs and igniting infection, low-grade inflammation[25,26] and overt GI illnesses[27]. In addition, intestinal dysbiosis can cause the production of an inflammatory lipopolysaccharide (LPS), that is a glycolipid withinside the outer membrane of Gram-negative microorganisms and it is a common endotoxin composed of the O antigen[28]. The release of LPS inside the intestinal area and its passage into the blood explains LPS-associated toxicity, i.e., endotoxemia[29]. LPS can enter the circulatory system directly or with the aid of an increased intestinal permeability[30]. Lipid A stimulates signaling cascades and a wide range of pro- inflammatory pathways, for example nuclear factor kappa-B (NF-kB) and prolongs oxidative stress after coupling to Toll-Like receptor (TLR) 4[31,32]. Increased oxidative stress may lead to systemic and retinal disease[33]. It is noteworthy that the first part of the GI tract, the oral region, serves as a gateway for foreign bacteria and bacteria that reside in the mouth and potentially affect distant organs such as the eye can concurrently lead to age-related macular degeneration (ARMD), glaucoma, and diabetic retinopathy (DR)[34]. For example, it has been observed that in the presence of an exposed periodontal pathogen community, it can gain access, possibly evade autophagy, and stably proliferate in retinal pigment epithelial (RPE) cells as one of the mechanisms leading to DR and ARMD[35].
RELATIONSHIP BETWEEN DYSBIOSIS AND DR
A 2010 study looked at the effect of gut microbiota on diabetes establishing an association between Firmicutes and Bacteroidetes in Danish diabetics as in comparison to non-diabetic people. In this way, their ratio was found to be notably decreased, while the ratio of Bacteroidetes increased withinside the intestines of patients with type 2 diabetes[36].
An imbalance of the intestine microbiome has been linked to many eye illnesses which include uveitis[37], glaucoma[38] and ARMD[39]. In addition, Beli et al[40] reported that by altering gut microbiome through intermittent fasting can prevent retinopathy and extend lifespan in mice. The prevalence of diabetes and retinopathy associated with intestine microbe dysbiosis is now convincingly related.
In a study by Huang et al[41], 16S rRNA gene sequencing was used to achieve a complete composition of intestine microbiota in stool samples from seventy five individuals, who include diabetic sufferers without overt retinopathy (DM) as well as patients with retinopathy (DR) with different grades have been analyzed. The severity of healthy people with the same age and sex without latest history of probiotic, prebiotic or antibiotic use were also selected as the healthy control (HC) group. In every group, they additionally compared different genera and families of bacteria in the DM and DR and HC group and with every different.
In Huang et al’s study[41], fundus by ophthalmoscopy confirmed a grossly ordinary structure in the DM and HC groups, but confirmed retinal vascular lesions, microaneurysms, hemorrhages, cotton wool spots, and lipid secretions in DR group. Optical coherence tomography pictures confirmed ordinary histological form in DM and HC groups, while macular edema, retinal thickening and retinal detachment were evident in DR group. With reliable diagnostic accuracy, they recognized 16 phyla in all samples, in which Firmicutes, Actinobacteriota, Proteobacteria and Bacteroidota were the maximum dominant phyla in every group with greater than 99% abundance. In general, through evaluating the relative frequency of every phyla some of the 3 groups, only Firmicutes and Desulfobacterota phyla confirmed a great difference[41]. In particular, the abundance of Firmicutes and associated Clostridiaceae was decreased in DM and DR groups than in healthy individuals. In addition, Bacteroidetes were greater considerable in DR than in DM and HC groups[42].
Further, it was reported that Clostridium has a negative relationship with fasting glucose, insulin and plasma triglycerides and a positive relationship with adiponectin and excessive-density lipoprotein, which are all intently associated with diabetes. Therefore, it is speculated that the prevalence of diabetes and retinopathy can also be related to decrease of those useful bacteria[41].
Desulfobacterota are organisms that could reduce sulfur compounds through the different DsrAB sulfite reduction pathway[43] and take part in butyrate degradation through carrying out a β-butyrate oxidation pathway, suggesting that Desulfobacterota are concerned withinside the catabolic balance of reactions[44]. Therefore, it can be hypothesized that Desulfobacterota can promote LPS to induce an inflammatory damage or exacerbate abnormalities of energy metabolism, each of them are involved in diabetes[41].
Blautia and Lactobacillus genera were more abundant in individuals with diabetes and were greater abundant in DM group than DR group[38,44,45].
Wang et al[46] reported that Blautia was positively correlated with tauroursodeoxycholic acid (TUDCA) levels. TUDCA, a farnesoid X receptor (FXR) antagonist, regulates glycolipid metabolism through its receptor for FXR and the bile acid G protein-coupled membrane receptor (TGR5). TGR5 is present in primary retinal ganglion cells (RGCs), and activation of TGR5 can prevent retinopathy and prolong the life span of db/db mice, so diabetic sufferers with higher Blautia expand less retinopathy.
Lactobacillus is a common probiotic with immunomodulatory and antioxidant properties that play a role in the mechanism of DR[47].
This study also showed that some drugs used to treat diabetes have critical outcomes on changes in the intestine microbiome. For example, Wu et al[48] suggested that metformin notably impacts the intestine microbiome (consisting of extended Bifidobacterium) to enhance glucose tolerance and enhance antidiabetic effects. Eubacteriaceae showed a positive correlation with fasting glucose levels and have been notably abundant in diabetic sufferers, specifically in the DR group. In addition, an increase in the levels of Bifidobacterium and Lactobacillus and a decrease in the levels of Escherichia Shigella and Faecalibacterium were found in the DM and DR groups as in comparison to the HC group[49].
THE RELATIONSHIP AMONG DYSBIOSIS, EYE MICROBIOME, IMMUNITY AND FACTORS AFFECTING ITS CHANGES
Ocular microbiota, microorganisms present in the eye are common and pathogenic. Commensal microbiota play a crucial function in regulating host physiology, inducing and growing the immune system.
The floor of the eye is constantly in contact with the outside environment and diverse pathogens. Common microorganism isolated from eye spots are gram-positive strains of bacteria such as coagulase-negative Staphylococcus, Propionibacterium, Streptococcus, Diphtheroid and Micrococcus. In the normal floor of the eye, some strains which are considerable in intestinal flora which include Escherichia coli, Enterococcus, Bacillus and Lactobacillus are found less. Fungal isolates and Gram-negative strains such as Haemophilus, Neisseria, Pseudomonas are much rarer, however, they can be isolated from the floor of the eye without causing symptoms such as inflammation or infection. Physiological conditions of the disease along with retinal vascular damage can lead to microbial invasions that are circulating withinside the blood.
Retinal vascular leakage is one of the early pathological manifestations of DR. DR has been shown to be affected also by the microbiome. Each disorder has a unique relationship with intraocular microbiome, which shows that there is a relationship among intraocular microbiota and eye health and disease[50].
Age and sex hormones are amongst the modulators in eye microbiota. Dry eye syndrome (DES) is described as a condition related to the lack of homeostasis and hyperosmolarity withinside the tear layer, causing inflammation in the eye surface and vision impairment, which is promoted by inflammatory cytokines and the infiltration of inflammatory cells into the conjunctiva and lacrimal glands. The most common cause of dry eye is meibomian gland disorder which is a modified autoimmune-progressive disease. Despite some report of beneficial effect by oral and systemic antibiotics, a review conducted by the American Academy of Ophthalmology concluded that there was only scant evidence to indicate their efficacy against side effects. A Cochrane review comprising about 3500 cases of dry eye cases ended up suggesting there was little evidence that over-the-counter drops provided any significant benefit as compared to placebo[50]. In 2007 it was shown a significant quali-quantitative change between the corneal microbiome population of subjects with dry eye as opposed to those without, specifically identifying Klebsiella oxytoca and Bacillus spp. most of the bacteria were coagulase negative Staphylococci[51].
This overgrowth correlated with a reduction of mucin-generating goblet cells in the eye as well as observed in dysbiotic GI tract. Dysbiosis is involved in various diseases related to systemic inflammation, inflammatory bowel disease and several eye diseases such as uveitis, glaucoma and ARMD[52]. Some microbial species of the intestinal microbiota can degrade glycans in the host's mucous secretions and mucin secreted by goblet cells or destroy epithelial cells[53], Cholinergic neurotransmitters stimulate mucin reflex secretion. Lacrimal gland secretion is regulated by nerves, androgens and growth factors from the family of epidermal growth factors[52].
Based at the outcomes of targeted 16S rRNA gene sequencing, there are presently 52 identified bacterial phyla stated, with about five to seven phyla recognized to reside withinside the mammalian GI tracts. Among those, 4 most important phyla Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria dominate and occupy as much as 97% of the whole bacteria. A entire and balanced bacterial environment forms due to ideal interactions some of the different bacterial phyla[54].
In a 2022 study by Watane et al[55], it was found that all individuals with dry eye disease had a decrease in the phyla Firmicutes and an increase in the phyla Proteobacteria, Actinobacteria, and Bacteroidetes compared to control individuals. People with Sjogren's dry eye compared to control people, an increase in phylogenetic diversity was observed without relation to age or co- immune diseases. The above emphasized the link between gut associated lymphoid tissue, mucosal associated lymphoid tissue and corneal associated lymphoid tissue, to mention only some stations. All the above paved the way to a rationale of gut-eye microbiota intervention. The oral supplementation of omega-3 fatty acids was tested for their prebiotic properties and endowed by some initial positive reports. However, probably due also to uneven i7clusion criteria, this approach has so far conflicting results despite more recent large studies[56,57]. Interestingly, a combination of fish oil, lactoferrin protein, zinc, vitamins C and E, lutein, amino acid γ-aminobutanoic acid, and Enterococcus faecium wb2000 bacteria on dry eye disease significantly improved clinical symptoms during an 8-week trial[58]. In addition to promoting intestinal probiotic bacteria, curcumin extract has been proposed to inhibit hyperosmolarity in human corneal epithelial cells and also reduce the expression of inflammatory cytokines in a mouse model[59]. Regarding clinical interventions with oligosaccharides, a randomized controlled trial using a combination of probiotics and fructo-oligosaccharides as a tear substitute, significantly improved symptoms in dry together with a beneficial change of ocular surface microbiota[60]. It has also been shown experimentally that oligosaccharide-enriched human milk protects corneal epithelial thickness to the same extent as cyclosporine in a mouse model of dry eye disease[61].
The is also interest in compounds with polyphenols for their known antioxidant, anti-inflammatory, antibacterial properties, which are used to decline along the aging process[62] and proving beneficial changes in other tissues[63]. However, more fine biochemical studies are required in after some promising positive data from blueberry or grape seed extracts for corneal diseases[64,65].
On the other hand, it has been recently reported that elevated polipyphenolic concentration of green tea may negatively affected the tear film quality with lipid oxidation and detrimental decrease in electrolyte tear concentration[66].
The prebiotic properties of quercetin in these settings have been shown to regulate immunity by increasing the tear volume, restoring the smooth surface of the cornea and increasing the density of goblet cells when used topically in mouse dry eye models[67]. In addition to these effects, resveratrol also increased levels of Akkermansia species, bifidobacteria and lactobacilli in mouse models[68].
Kim et al[69] has reported few years ago about the efficacy of a probiotic mixture based on Lctobacillus reuteri, Lctobacillus casei, Sreptococcus thermophiles, Lctobacillus acidophilus and Bfidobacterium bifidum in successfully treating an autoimmune model of dry eye, thus corroborating the gut ecosystem relevance in the disease and as a potential therapeutic target.
Accordingly, in the same year Chisari et al[70] carried out a pilot study dealing with elderly eye microbiota in subjects suffering from dry eye. The protocol implied the use of oral supplementation of a mixture of Saccharomyces boulardii and also Enterococcus faecium and the results clearly showed a significant benefit on severity reduction of the disease and symptoms paralled by improved tear tests and eye microbiota.
The above data proved also to be beneficial withinside the knowledge and treatment of Sjogren’s syndrome (SS), where the reduced salivary gland secretion fuels an increased bacterial dental plaque formation. In these patients, prebiotics and probiotics have shown to inhibit cross-reactive T cells against gut peptides potentially yielding a beneficial ocular microbiome shift[71] .In another study a relationship between the quali-quantitative gut microbiome changes and soluble inflammatory markers was established, where the low Bifidobacterium counting was inversely associated to a clear pro-inflammatory pattern in the blood (high IL-17 and TNF-α)[72].
Uveitis shares with DES some pathophysiological aspects, but it is less common. Terminologically, uveitis refers to an inflammatory process of the center layer of blood vessels and eye pigment. Regarding the strength of the immune privilege concept in the context of uveitis, this word should be used cautiously. This is because this assumption may be dependent on whether the inflammation takes place in front of or behind the blood-retinal barrier (BRB). In that, the retina with its greater immunomodulatory microenvironment, lacks of immunosuppressive mediators and major histocompatibility complex (MHC) class II+ dendritic cells may not prevent an inflammatory uveoretinitis. It appears that there is a convincing evidence that rather than a single microorganism triggering an antigenic cross-reactivity, this disease is leaning on multiple microbiota species[73].
In sufferers with illne sses such as diabetes, dyslipidemia, conjunctivitis, autoimmune ailments such as Behçet's disease and rheumatoid arthritis, SS, meibomian gland disorder and DES, show a modified ocular microbiota[53].
INFLAMMATION, POTENTIAL MECHANISMS OF INTRAOCULAR INFLAMMATION, IMMUNE AND GENETIC FACTORS AND INTRAOCULAR MICROBIOME RELATIONSHIP
Despite the eye is enforced by what is termed as immune priviledge, i.e., a defensive reaction system to immune challenge based on the sequestration of tissue self-antigens in the back of blood-tissue barriers, ocular tissues are susceptible to infection and inflammatory conditions. There are two types of intraocular inflammation, acute inflammation and chronic inflammation. Acute inflammation due to pathogenic microbes after surgery or accident and causes changes withinside the permeability of the BRB and the infiltration of immune system cells which include macrophages and polymorphonuclear leukocytes. Chronic eye inflammation includes a combination of predisposing genetic (such as HLA haplotypes, A29, B27, B51, DR4 and DQ4) and environmental factors generating an enhanced leukocyte trafficking among the gut and the eye. In fact, the production of autoantibodies against endogenous antigens, the dysregulation of regulatory T cells, and the infiltration of macrophages and T lymphocytes underlie chronic intraocular inflammation, especially uveitis[74].
Uveitis occurs as an acute inflammation or chronic intraocular inflammation of an infectious or noninfectious basis and accounts for 10%-15% of severe visual impairment worldwide and as much as 15% of blindness in the United States[75].
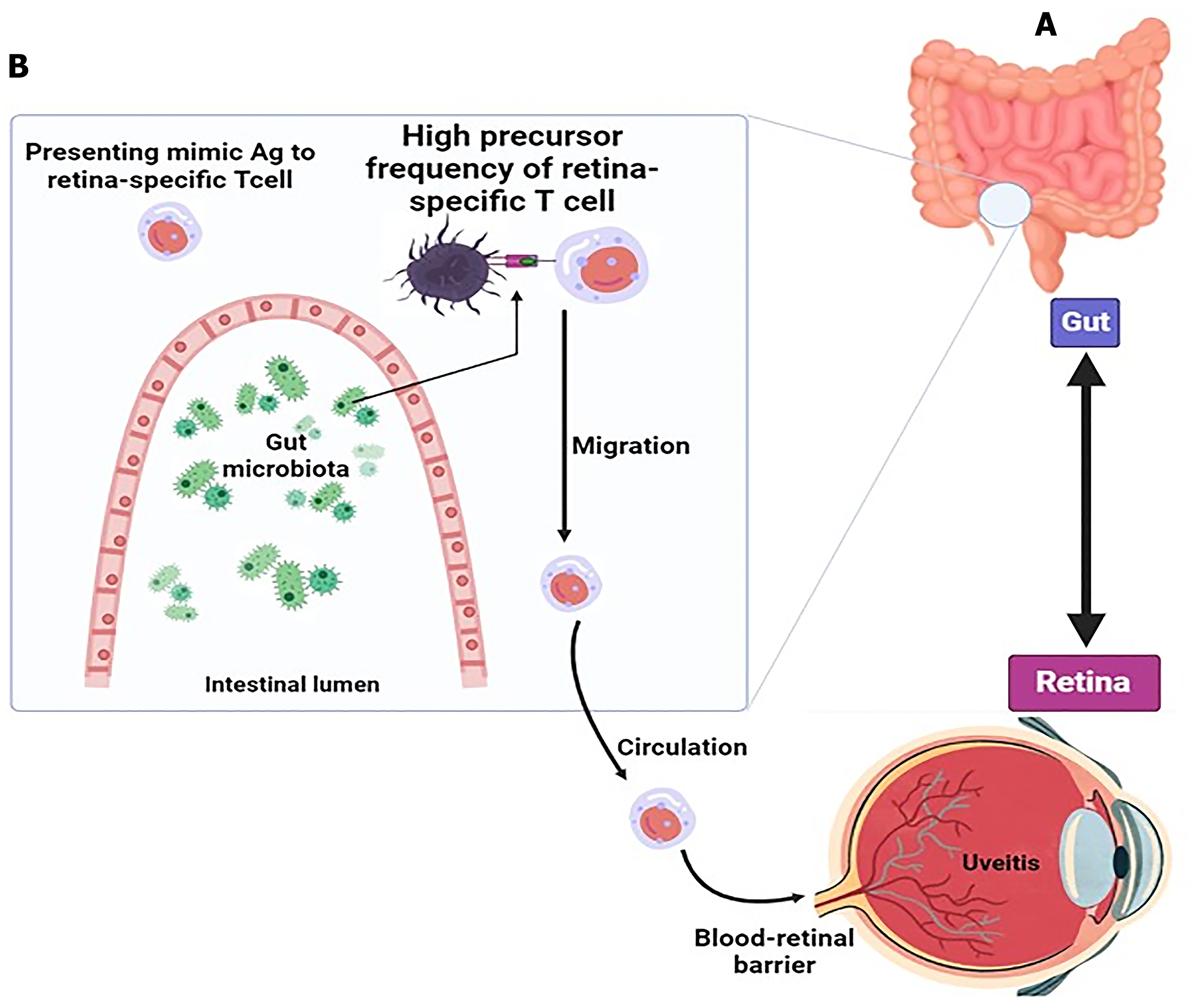
Dysbiosis can cause overgrowth of pathogenic microorganisms that affect antigen-presenting cells (APCs; Figure 1). . In humans, uveitis is related to a MHC class I allele, human leukocyte B27 (HLA-B27), an antigen expressed on the surface of white cells. Individuals how have acute anterior uveitis contain specific intestinal microbial changes. Immature APCs engulf the microbe to mature after which migrate to the nearest lymph nodes to be recognized by CD4+ T cells, giving rise by antigenic mimicry to autoreactive lymphocytes specific for the neuroretina[76,77].
Figure 1 Activation of specific autoreactive T cells by microbiota and development of uveitis.
A: Gut connection with retina in uveitis; B: Mechanism of retina-specific T cell activation by gut microbiome. Uveitis is caused by the retina-specific autoreactive T cells activated by microbiota. The gut lipopolysaccharide exhibits many retina-specific autoreactive T lymphocytes in the periphery. Antigen-presenting cells present gut microbiota-derived products that to these T cells may resemble Ag. Retina-specific T cells become activated, migrate to the eye, and pass through the blood-retina barrier leading to inflammation of the retina. Citation: Horai R, Caspi RR. Microbiome and Autoimmune Uveitis. Front Immunol 2019; 10: 232. Copyright ©The Author(s) 2019. Published by Frontiers Media SA[166].
Retinal-specific T cells that are activated in this state disrupt the BRB and penetrate into the eye to secrete pro-inflammatory cytokines and chemokines and absorb inflammatory and cellular mediators. About 20 years ago, researchers found that a peptide from cow's milk casein protein (Cas) and PDSAg peptide from retinal protein S antigen have antigenic mimics[78]. Recently, the same group found an increased antibody response to Cas and PDSAg as well as an increased T cell response in the sera of patients with uveitis compared to HCs[79]. While this area still represents a therapeutic challenges, there are some worth considering data. For example, orally administered berberine has been shown to inhibit BRB breakdown induced by experimental autoimmune uveitis (EAU). This was also related to a robust modulation of the spleen transcriptome and increasing the number of immunomodulatory-endowed gut bacteria[80]. Another gut microbiota-targeted therapeutic option is represented by SCFA whose direct ingestion may yield a beneficial modulation of the gut mucosal immunity. Nakamura et al[81] had demonstrated that the ingestion of the SCFA propionate significantly decreased the severity of EAU probably also due to a Regulatory T cells (Treg) increase.
Intestinal commensals are also concerned in the pathogenesis of several noninfectious eye diseases including ARMD and glaucoma. ARMD is a proliferative retinal degeneration with chronic low-grade inflammation of the inner eye. In genome-wide studies, the association of genetic variants in various pathways of complement and inflammatory elements including complement factor (CF) H, CFI, ARMS2, TIMP3 and MMP9 are shown. Also in the lipid pathway such as APOE, LIPC, ABCA1 and CETP were correlated with this disease[82,83]. At least 71% of ARMD is genetically heritable[84]. This may be attributed to the interaction of genetics with environmental factors including smoking, obesity, diet and sun exposure[82]. Commensal microorganism could play a role in ARMD patients as a potential environmental influence. There are several reasons for it[85]: (1) Microbes are very effective on low-grade chronic inflammation by regulating host immunity and regulating lipid metabolism, which is involved in the pathogenesis of ARMD; (2) Drusen withinside the eyes of ARMD patients consists of anti-inflammatory components including apolipoprotein E and complement components plus amyloid β, vitronectin, immunoglobulins and C1Q; and (3) Microorganisms in the gut can send signals that have an effect on distant organs including the eye. Microbial communities in the gut, mouth, nose and pharynx have also demonstrated the potential function of mucosal floor microbes withinside the pathogenesis of ARMD and glaucoma[41,86,87]. In particular, Zhang et al[88] found in ARMD patients with clinical features as in comparison to healthy individuals that the number of Firmicutes showed a great decrease and increased levels of Proteobacteria and other bacteria (i.e., Escherichia-Shigella), which metagenomic analysis showed to be related to LPS biosynthesis.
The receptors of the innate immune system, such as TLRs, cause an immune response withinside the retina. These receptors are placed in the retina and have an extracellular domain and an intracellular domain, the extracellular domain is hooked up to bacterial structural components which include lipids, peptidoglycans and their LPS with leucine repeats, and the cytoplasmic intracellular domain signaling. Stimulation of TLRs signaling increases NF-kB or activation of interferon factors or activation of mitogen protein kinase pathways, which express more inflammatory mediators and It can play an important function in the pathogenesis of uveitis[46].
Moreover, studies show that the predominant species of bacteria Anaerotruncus and Oscillibacter, Ruminococcus torques and Eubacterium ventriosum are more abundant in people with ARMD, in where the amino acid production pathways of arginine are increased and lead to the destruction of glutamate[35].
The commonality between heat shock proteins (HSPs; HSPs of the gut microbial population and highly conserved human HSPs makes them cross-react with human HSPs, which triggers autoimmune diseases and RGC damage[89,90].
In a 2019 study by Henein and Khaw[91], it was found that the levels of HSP-27 and HSP-60 specific T cells and antibodies were higher in each primary open angle glaucoma (POAG) and normal tension glaucoma patients when compared to age-matched healthy individuals. The pathophysiological reasons of these changes are multifactorial and involving also multigenetic mechanisms. The underlying dangers of elevated intraocular pressure (IOP) and POAG have been defined in relation to the role of tyrosine kinase signaling, lipid metabolism, mitochondrial function, and developmental processes. Age and increased IOP are the most important risk factors for glaucoma, but mechanisms beyond pressure-induced neurodegeneration are involvealsod. It seems that the proximity of immune cells along the digestive tract and the dysfunction of the retinal blood barrier in stressful conditions are other possibilities of this case. Data are accumulating on the growing evidence there exists a commensal microbiome as an integral component of the retinal environment which can also additionally suffer from subtle intraocular microenvironment changes. These may be modifying the retinal immune reaction regulation and overcoming the suppressive mechanisms leading to retinal degeneration. A ”dual-hit” hypothesis has been postulated, i.e., from gut to retina. The TLRs-mediated recognition by the microbial pathogen-associated molecular pattern in the resident microglia in the retina give raise to a surge of proinflammatory secretome and MHC II. Once the retina undergoes a chronic innate immunity activation, the infiltrative homing of peripheral primed T cells takes place with breaking the BRB and this is suggested also to occur for blood-brain barrier[92].
Experimental data with POAG rats have clearly shown that this condition was invariably associated with a decresed microbial diversity and an altered gut microbial metabolomics (e.g., significantly increased Akkermansia, Romboutsia, Bacteroides and Verrucomicrobia phylum) which correlated with the status of RGCs. These data confirmed the similar clinical findings together with a major decrease of Lactobacillus in patients with POAG[93].
Experimental and clinical studies also agree in suggesting that increased bacteria in glaucoma were all active mucus degraders, especially in a fiber-poor diet[94], and this, on its turn, favoring inflammatory-related genera inversely related to RGC density. In recent years, dysbiosis in glaucoma has also staged as a key player in guiding the peripheral immune system while also having part in the T cells activation. In aid of the hypothesis of a bacteria-sensitization of T-cell response in glaucoma progression, a completely elegant review by Chen et al[95] highlighted how even transient IOP elevations were consistent enough to break the BRB and generating a retinal infiltration from CD4+ T-cells triggering glaucomatous neurodegeneration-prone HSP-specific CD4+ T-cell responses.
As one of the intestine metabolites, also bile acid composition showed in POAG a higher level of some specific bile acids (3A,7A-dihydroxycholanoic acid)[40] as compared to health control and, as also somehow similarly noted in Parkinson’s disease[96].
An interesting bridging hypothesis has been suggested early this year by Pezzino et al[97] who reported the data linking obesity and elevated body mass index associated to high IOP and glaucoma. Obesity index being the best predictor of worsening IOP either in an Asian[98]and in a European population[99].
As a matter of fact, obesity is associated to a proinflammatory cytokine profile and to gut and oral dysbiosis both facilitating bacterial translocation with also endotoxic activity and associated to glaucoma[100].
Indeed a further recent work where authors found that TMA, a gut bacteria metabolite, concentration in acqueous humor of glaucomatous patients was significantly high thus potentially promoting the progression of glaucoma by impairing smooth muscle-based trabecular meshwork filtration and enhancing hypoxia[101].
In addition, studies show that there is an association between Helicobacter pylori (H. pylori) and glaucoma, as determined by measuring increased antibody titers in POAG patients. Intestinal dysbiosis confers susceptibility to H. pylori infection, and this has indeed been reported in a recent meta-analysis[102]. It is interesting to note that the successful eradication of H. pylori can lead to a partial improvement of IOP[103]. The by-products of intestinal microorganisms play a role in the stimulation of microglial cells in the central nervous system (CNS), their regulation and function, and their maturation and differentiation[91].
In a study using fermented corn supernatant rich in probiotic bacteria, changes in the gut microbiome in mice modulated the retinal immune response and protected RGCs[104].
Another study showed that increased levels of arginine play a role in causing retinal degeneration and changes in intestinal microbiota[105].
Changes in gut microorganisms, for instance a loss of bacteria that affect fatty acid length, have been linked to ARMD[35]. Their production byproducts may regulate intraocular inflammation by transiting lymphocytes from the gut to the eye[106].
Inflammatory processes associated with intestinal dysbiosis in the elderly have been shown to cause premature senescence of retinal cells, leading to neovascularization and impaired vascular repair through inflammatory factors and angiogenesis[107,108].
Another retinal disorder involving the gut microbiome is retinitis pigmentosa (RP). This study found that changes in gut microorganisms composition were associated with RP[109].
Different cells withinside the innate and adaptive immune system express different elements that act to activate G protein-coupled receptors and regulate gene expression. SCFAs are histone deacetylase inhibitors. Other metabolites of nuclear receptors are receptors consisting of pregnane X receptor, vitamin D receptor and liver X receptors[110].
RELATIONS WITH AGING AND GUT MICROBIOME AND EYE DISEASES
The intestinal epithelium, which contains factors including IgA secretory immunoglobulins, the intestinal layer, and antimicrobial peptides, is necessary to hold homeostasis. Aging influences the composition of the gut microbiome by changing this barrier. With increasing age, these changes increase and the diversity and richness of intestinal microbes decreases[111].
Aging causes stem cells to decline and the body loses its capacity to repair tissues and cells[112]. During aging, because of host elements which includes genetics, nutrition, intestinal growing older and immune system, living environment, drug exposure, etc., it causes a gradual change of intestinal microorganisms[113]. Proteolytic bacteria increase and saccharolytic bacteria decrease[114], resulting withinside the onset of many age-associated diseases, which includes neurodegenerative diseases[115].
Low-grade infection is manifested with the aid of using expanded expression of inflammatory cytokines and transcription elements consisting of IL-6, TNF-α, and NF-κB[116].
Aging is determined and moderated through the surroundings[117]. In a 2021 Yoon et al[118] study, it become determined that intestine dysbiosis is related to age and inflammation and continual illnesses in the aged and contributes to the pathophysiology and exacerbation of dry eye disease. In univariate evaluation on this look at, the genera of Firmicutes, Proteobacteria and Cyanobacteria, Firmicutes/Bacteroidetes and Alistipes, Bacteroides, Prevotella, Paraprevotella and Helicobacter had been related to the degree of dry eye.
Zhang et al[88] in 2023, In the case of age-associated macular degeneration (AMD), that's the primary reason of vision loss in humans over 50 years of age, suggested that the intestine microorganisms performs a function withinside the pathogenesis of eye diseases and isn't the same as healthy people.
In 2017, Wen et al[119] confirmed that age- and gender-associated variations have an effect on the conjunctival microbiome and might modify ocular floor immune homeostasis via adjustments in its shared microbiome. In this observe, there has been a selected distinction in the variety of microorganisms, their metabolic features and the frequency of antibiotic resistance genes among the young and old groups. They stated that growing older impacts the ocular floor microbiome. Propionibacterium acnes (P. acnes) turned into located in 88% of healthy volunteers, at the same time as Staphylococcus epidermidis turned into located in 73%. Epidermidis reduced considerably from male to woman, whilst Escherichia coli extended drastically in the female group. Staphylococcus haemolyticus, Micrococcus luteus and Escherichia coli withinside the aged in comparison to the younger groups, and an extensive lower in Ochrobactrum anthropi, Mycoplasma hyorhinis and P. acnes from young to old.
In Chen et al’s study[120] on the role of microbiome dysfunction in the pathogenesis of many neurodegenerative diseases and the relationship between the gut microbiome and glaucoma, it is reported that the gut microbiome of glaucoma patients has a higher level of Dysgonamonadaceae, and a decrease level of Barnesiellaceae. This microbiome pattern causes SCFAs to be produced more in stool and blood samples. Deletion of glaucoma patient-particular intestine microbiome microorganism decreased RGC and retinal microglia activation and inflammatory cytokine overproduction, whilst SCFAs remedy decreased microglia activation. Studies have also shown that estrogen is protective in models of RGC injury and indeed, a still poorly addressed area is the association between menopause and glaucoma. In this regard a mixed phytochemical[121] and probiotics intervention[122] can be envisaged.
Further discount of intestine microbiome microorganism in those patients decreased retinal microRNAs which include MiR-122-5p, which turned into related to inhibition of retinal inflammation via neurodegeneration. In addition, they confirmed that transplantation of fecal microbiome from glaucoma patients exacerbated microglia activation and will increase retinal inflammation and adjustments the expression of retinal microRNAs which results in retinal inflammatory response[120].
In 2021, Alam et al[121] confirmed that food-borne Listeria monocytogenes (L. monocytogenes), which causes gastroenteritis, septicemia, meningitis, and chorioamnionitis, is associated with excess mortality in old age and altered gut microbiota in old age. It increases after infection and causes dysbiosis. Major bacterial phyla including Bacteroidetes and Firmicutes, they are no longer significantly increased at some stages of aging or infection, while Verrucomicrobia, Clostridaiceae and Lactobacillaceae are reduced in aged mice. L. monocytogenes infection spreads Porphyromonadaceae and Prevotellaceae species, even Ruminococcaceae and Lachnospiraceae in adult mice, while Blautia and Alistipes reduced after infection in adult and old mice. Butyrate-producing and immunomodulatory microorganism including Pseudoflavonefractor and Facalybacteriomethans were enhanced in aged mice, which contribute to up-regulation of intestinal inflammatory mRNA in aged mice. Histological evaluation of gastric tissues confirmed many lesions in aged mice with Listeria infection. Common species along with Lactobacillus, Clostridial and Ackermansia were more in mice with inflammation, but their frequency decreased in old mice. Changes in these microorganisms reduce and destroy beneficial substances inside the intestine. Therefore, aging can also affect the composition of gut microorganisms and increase the risk of invasive monocytogenes infection.
Parker et al[122] in 2022 show that fecal microbiota transfer, from among younger and elderly mice changed the recipient microbiota composition with a composition such as the donor, enriched for specific bacterial species, and changed the metabolic ability of the ensuing intestine microbial composition. They are show that elderly donor microbiota transfer to younger mice drives inflammation and lack of integrity withinside the intestinal epithelial barrier extended systemic and tissue markers of inflammaging, and upregulated inflammation withinside the retina and brain. The transferred elderly microbiota and ensuing recipient microbiota composition have been outstanding through enrichment for Prevotella, Lacnospiraceae and Facecalibaculum species and depletion of long-chain fatty acid synthesis. Conversely, switch of elderly mice with younger donor microbiota, which become outstanding through enrichment Bifidobacteria, Eubacteria, and Akkermansia species, and enrichment for B vitamin biosynthesis and also lipid synthesis pathways reversed inflammatory modifications in the elderly intestine, brain, and retina[123]. They also showed that transplantation of an elderly donor's microbiota affected extended intestinal concentrations of TNF and broader surrogate biomarkers of epithelial barrier permeability (I-FABP, LBP), which contribute to intestinal barrier integrity and promote systemic inflammatory responses.
Transplantation of young donor microbiota reduced the number of Iba-1 + microglia within the CNS of elderly recipients, whereas aged donor microbiota expanded Iba-1+ cells within the CNS of younger recipients. These dramatic changes in microglial activation could recapitulate expansion and/or contraction in the total number of microglial cells, or modulation of Iba-1 + expression in resident populations, or both[124-126].
Intestinal dysbiosis has been associated with an increased risk of infection. Imbalances in gut microbial community structure associated with adverse inflammatory responses are increasingly associated with disease processes that affect many body systems. Overall, given the studies reviewed in this section on the relationship between aging, the immune system, and the microbiome, how dysbiosis affects human aging and disease is becoming clear. It is becoming more apparent that the link between gut dysbiosis and age-related diseases may lie in the way the gut microbiome interacts with the gut mucosa and the systemic immune system, given that these networks share a common reciprocal relationship[127].
MICROBIOME CONTROL AND TREATMENT GOALS AND EYE DISEASES
Natural supplements effective on dry eyes, ARMD and other eye diseases
A study by Rowan et al[128] showed that clostridials were associated with a "high glycemic diet" in patients with ARMD, while Bacteroidetes were associated with a "low glycemic diet" and protected against disease. In the study by Fernandes et al[129] and Oubaha et al[130] it was also reported that hyperglycemia causes changes in the intestinal barrier that lead to destruction of immune system homeostasis, a chronic systemic inflammatory process, and even retinal vascular involvement.
Overall, human and animal model studies have shown that gut microbial composition is influenced by dietary vitamins and omega-3 polyunsaturated fatty acids[131] in ARMD[132].
Scientific evidence shows that nutritional carotenoids have an important effect on the regulation of intestinal microbiota and human health, and this is due to pro-vitamin a activity and antioxidant function. Beta-carotene, α-carotene, lycopene, astaxanthin, lutein, zeaxanthin, beta Cryptoxanthin, α-cryptoxanthin, γ-carotene, and fucoxanthin are common human dietary carotenoids found in fruits and vegetables and seafood. Carotenoid consumption reduces the risk of cancer, cardiovascular diseases, eye diseases and many other diseases. Diet has the potential for dietary therapeutic strategies for microbial design and stability[133].
Epidemiological studies have shown that diet is a modifiable ARMD risk factor, and nutrient modification is a particularly appealing adjuvant treatment for ARMD. Recently, the age-related eye disease study part 2 was concluded and demonstrated further benefit with the addition of lutein and zeaxanthin as a replacement for the β-carotene of the previous generation formulation. Lutein is a carotenoid found in green leafy vegetables, avocados, and eggs, and is purported to have protective effects against ARMD as well as benefits for visual and cognitive health. Recent studies have indicated significant variation in serum lutein among individuals and that GI microbial profile may potentially contribute to lutein status[133,134].
A 2019 study showed that Dialister, Ruminococcus, Gemmiger and Phascolarctobacterium together with Bacteroides eggerthii, Ruminococcus torques differ in serum lutein levels and are increased in people with ARMD. This suggested a relationship between these microbes and Lutein is availability[135].
In a 2022 study, Grant et al[136] showed that consumption of fish, fruits, vegetables and a low glycemic index resulted in a healthier retina in old age.
Zhang et al[137], stated that omega-3 fatty acid supplementation could target AMD.
Epidemiological studies have shown that consuming fermented foods is related to a reduced risk of type 2 diabetes, metabolic syndrome, and heart disorder at the side of improved weight management. Microorganisms in those foods are recommended to make contributions to those health advantages. Among these, the starter culture organisms of yogurt are Streptococcus thermophilus and Lactobacillus delbruecki of the bulgaricus subspecies, in addition to Bifidobacterium and Lactobacillus strains, which are added due to their probiotic properties. In contrast, for other fermented foods, including sauerkraut, kimchi, and miso, fermentation is initiated through independent microbes present in the raw materials[138]. A higher knowledge of the relationship among gut microbes could offer new therapeutic targets for eye disorders[139].
By healing the gut surroundings and balancing the microbial population[140,141], probiotics have created a new therapeutic goal for various eye diseases[142].
Lactobacillus paracasei (L. paracasei) KW3110 has been used in experimental and clinical trials. Consumption of this bacterium suppresses cell death[143] and has anti-inflammatory effects on retinal epithelial cells and decreases cytokines and chemokines[144].
Prebiotics were defined by a panel of experts in 2016[145]. Fructo-oligosaccharides, inulin and galacto-oligosaccharides have beneficial effects on human health due to fermentation. They increase lactobacillus and bifidobacteria, which with useful products cause absorption of calcium, less protein fermentation, reduction of pathogenic bacteria, strengthening of intestinal lining and immune system[146].
Studies have shown the relationship between intestinal microbiota in SS and dry eye severity. Gut microbiota is less diverse in dry eye patients[147].
Chisari et al[70] study showed that faecium LMG S-28935 and Saccharomyces boulardii MUCL 53837 improve mental symptoms by increasing tear secretion and can reduced tear breakup time in another study, bifido increased tear secretion and tear breakup time in addition to changing eye microbiota in dry eye patients[62].
In the study by Kawashima et al[58] facium WB2000 with fish oil improved subjective symptoms by increasing tear secretion in dry eye patients.
In the study by Tavakoli et al[148] in 2022, MULTIBIOTIC™ probiotics (Medlab Pty Ltd., Botany, NSW, Australia) and maltodextrin (placebo) were used, which reduced dry eye symptoms.
Given the connection between the ocular mucosa and the gut, we hypothesize that inflammation of the ocular surface will also improve, thereby reducing the signs and symptoms of dry eye. Modulation of intestine microbiome modulates proteins expressed by lacrimal glands by increasing IL-10 and decreasing IL-1β and IL-6[147,148].
Research in functional foods uses postbiotics, a combination of metabolic substances released by microorganisms on cells such as L. paracasei KW3110[143], exopolysaccharides, enzymes such as glutathione peroxidase, SCFAs[149] directly or indirectly health increase the host[150].
In a 2018 study by Morita[143], administration of L. paracasei KW3110 improved eye fatigue induced by visual display terminal loads at high levels of eye fatigue by reducing retinal cell death.
Postbiotics perform their function by modulating the intestinal microbiome and increasing epithelial barrier functions, modulating local and systemic immune responses, modulating systemic metabolic responses and systemic signaling through the nervous system, and protect the cell wall against the destruction of enzymes, GI and immune attack protection[151].
Diet affects the richness and diversity of microbiota, polyphenols in wine and tea and vitamin D can balance beneficial bacteria .Dietary fibers, inulin, galacto-oligosaccharides, arabinoxylan and oligofructose promote beneficial microbes and reduce harmful species[152].
The association of vitamin D and various eye diseases, including myopia, ARMD, glaucoma, DR, and DES (DES) has been investigated in several studies. Thyroid eye disease (TED), uveitis, retinoblastoma (RB), and cataracts discuss vitamin D metabolism in the eye. We searched two research databases for articles examining the association between vitamin D deficiency and various eye diseases. There is evidence of an association between vitamin D and myopia, AMD, DR and DES. But the available evidence for the association with other eye diseases such as glaucoma, TED and RB is limited. Vitamin D affects the homeostasis of mineral metabolism and has antioxidant and anti-inflammatory properties. It also plays a role in anti-angiogenesis, cell cycle modulation, including cell proliferation, differentiation and apoptosis. VDR and vitamin D regulatory enzymes are present in ocular tissues. Ocular tissues can activate and regulate vitamin D, indicating the importance of vitamin D in maintaining eye health[153].
RELATIONSHIP BETWEEN ANTIBIOTIC USE AND EYE DROPS, MICROBIOME AND EYE DISEASES
Antibiotics cause the destruction of beneficial microbial species and the overgrowth of pathogenic species such as C difficile and changes in digestive metabolism[154,155].
Antibiotics kill sensitive species. The study of patients treated with broad-spectrum antibiotics shows that the microbial load of stool samples during treatment with b-lactams was associated with an increase in bacteroidetes compared to Firmicutes. Drugs are metabolized in the intestine by large amounts of cytochrome p450 cyp enzymes, especially chromogranin, which increase certain microbial species[156]. Studies show that Eubacterium aerofaciens, Desulfomonas pigra and Streptomyces coelicolor A3, has cytochrome CYP family genes. C-type cytochromes in Geobacter sulfurreducens are active in electron transfer to iron (III) oxides. Eubacterium lentum contains cytochromes a, b and c and a pigment that binds to carbon monoxide[157].
According to studies, the administration of some antibiotics in mice (ampicillin, vancomycin, metronidazole, and gentamicin) in corneal abrasions caused an abnormal gene expression profile, which caused a decrease in corneal nerve density. Specifically, CCR2-corneal macrophages were reduced in antibiotic-treated mice. However, after discontinuation of antibiotics, stool transplantation, and probiotics, corneal macrophages were restored and corneal nerve fibers were regenerated. The author concluded the importance of probiotic supplementation whenever an antibiotic treatment for corneal damage is instituted[158].
One note of caution for uncontrolled use of antibiotics come from an experimental work by Möhle et al[159] where the daily use of antibiotics caused a decreases hippocampal neurogenesis and memory retention. To obtain a complete restoration of these abnormalities it was needed a synergy of gut flora reconstitution, plus wheel running exercise or probiotics.
A 2022 study from Columbia University found out that the microbiota of unilateral POAG patients treated with oreservative-added eye drops showed that alpha diversity and relative abundance of Gram-negative microbes are significantly higher as opposed to the gram-positive predominant microbes observed in the control eye and in HCs. Moreover, the metagenomic study suggested also that the treated glaucoma patients displayed an increased synthesis of LPS. This finding may be tentatively explained by the presence of the preservative benzalkonium chloride which acts as a detergent of potential bacterial overgrowth into the eye drops bottle[160].
Indeed, a similar study has very recently proven that eye drop therapy does not modify the eye microbioma but a very few (about 10%) were using preservative containing topical treatment[161].
Lim et al[162] raised some concern in the use of topical prostaglandins (PG) in glaucoma in that he unveiled that the eyelid microbiome of patients under PG was different as compared to naïve-POAG individuals, it creates problems about the eyelid surrounding of individuals using these drugs suggesting that this change may require some careful long term investigation.
In a study in Finland, children were treated with macrolides including erythromycin, roxithromycin, azithromycin and clarithromycin. These antibiotics can lead to a decrease in the diversity of intestinal microbiota, changes in metabolic activity and the abundance of antibiotic-resistant organisms, and have effects such as antibiotic diarrhea and recurrent C difficile infections[163].
Evidence also shows that exposure to antibiotics affects the cognitive immune system and the nervous system of the digestive system[164]. The vascular and neural mechanisms of retinal toxicity by aminoglycosides such as gentamicin are unclear. Gentamicin is a strong acid whose toxicity may be influenced by pH or by destroying the acidic environment of the lysosome and disrupting RPE metabolism[165].
CONCLUSION
In this review, we have described the relationship between the microbiome and health and eye diseases by reviewing prominent studies. We described how intestinal microbial imbalances are associated with various diseases and investigated the relevance of common gut and eye microbes on the mechanism of immune development and homeostasis in the development of eye diseases. The eye is affected by gut microbiota in association with changes in the immune system with an inflammatory component. We study eye surface microbes in pathological conditions, injury and surgery and their imbalance and changes in relation to eye diseases, ARMDs, RP, glaucoma, DR, dry eye and we checked uveitis. The retina is a very delicate organ with an inability to regenerate cells in old age. The use of antibiotics and chemotherapy drugs also has a toxic impact on the cells of the eye area, which, with the mechanism of action on the extracellular matrix and lysosomes and metabolism, causes many side effects, including dry eyes, mild redness of the eyes, itching, hemorrhage, retinal infection, neuritis in some cases. The function of microbiota in modulating homeostasis processes creates new methods for therapeutic purposes and pathology of extraintestinal diseases, including eye diseases. In addition, the intestine microbiota plays an essential role in nutrient absorption and metabolism. It produces vitamins K and B, which are essential for bacteria. In addition, it seems that the intestine microbiota can influence the metabolism of polyphenols and influence their biological activity. People with old age are severely affected by changes in the intestinal microbiome, eyes and chronic inflammatory diseases including diabetes, obesity, rheumatoid arthritis, Alzheimer's, Parkinson's and multiple sclerosis, intestinal inflammation and cardiovascular diseases and other diseases such as Behçet's syndrome and Sjogren's, stated that these diseases are specifically inflammatory diseases of the immune system related to the changes in the intestinal microbiome. Studies that show the effect of probiotics, prebiotics, and synbiotics on the gut and eye microbiota, and which modulate inflammation and homeostasis, have been reviewed. Changes in intestinal microbes and the use of beneficial microbes investigated in studies, the use of probiotics, prebiotics, postbiotics and fermented foods and foods containing essential vitamins and supplements, the misuse of antibiotics and even transmission fecal microbiota in elderly people, it seems that they have the potential to be used in the treatment of eye diseases and preventive strategies.