Published online May 22, 2022. doi: 10.4291/wjgp.v13.i3.107
Peer-review started: November 25, 2021
First decision: January 12, 2022
Revised: January 23, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 22, 2022
Processing time: 174 Days and 7.8 Hours
The differential diagnosis of abdominal masses is somewhat troublesome, especially when there is a malignancy to be evaluated. We report herein a unique case of gastric adenocarcinoma concurrent with a pancreatic schwannoma. Correct assessment of intraoperative findings is essential for adequate tumor staging and to decide the proper management of a concurrent pancreatic lesion.
Computed tomography scan performed for gastric cancer staging revealed a solid and cystic pancreatic mass that had no signs of local invasiveness. Surgical resection of the pancreas was decided preoperatively since a radical approach of the gastric tumor could be performed. There were no signs of distant metastases, and the large pancreatic mass was in contact with the posterior gastric wall. Histopathological study revealed a pancreatic schwannoma, which is an uncommon neoplasm that arises from Schwann cells around peripheral nerves.
Therefore, pancreatic masses deserve special attention regarding the differential diagnosis in patients with gastric cancer. The presence of a large pancreatic mass should not preclude the potentially curative intent of the gastric cancer treatment.
Core Tip: We display here the first case of synchronous gastric cancer and pancreatic schwannoma, highlighting the relevance of the differential diagnosis in approaching pancreatic masses in the context of staging gastric neoplasm. Correct intraoperative staging was essential in treatment decision-making.
- Citation: Ribeiro MB, Abe ES, Kondo A, Safatle-Ribeiro AV, Pereira MA, Zilberstein B, Ribeiro Jr U. Gastric cancer with concurrent pancreatic schwannoma: A case report. World J Gastrointest Pathophysiol 2022; 13(3): 107-113
- URL: https://www.wjgnet.com/2150-5330/full/v13/i3/107.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i3.107
Accurate staging is essential in gastric cancer treatment decision-making, and any lymph nodes or masses observed in staging assessment should be investigated[1]. Schwannomas, also referred to as neurilemmomas, are rare neoplasms that arise from Schwann cells around peripheral nerves, usually epineurium of either autonomic sympathetic or parasympathetic fibers[2,3]. Pancreatic locations are unusual, with about 70 cases reported in the last 40 years, and most of them are benign. However, malignancy can be found in up to 15% of cases, especially in lesions greater than 6 cm[3-5]. Schwannomas are usually well-encapsulated firm masses, and two-thirds may undergo degenerative changes, which can be cystic formation, calcification, and hemorrhage, among others[2,6]. Due to these alterations, they can mimic cystic pancreatic lesions or metastasis of a different primary site tumor in radiologic investigation, including gastric cancer.
A 73-year-old woman presented with epigastric pain and weight loss.
She had a history of non-insulin-dependent diabetes mellitus, arterial hypertension, and elevated cholesterol level.
She did not report a history of other previous illnesses.
She was unaware of a family history of cancer.
Abdominal examination did not detect any marked change.
All laboratory data were normal, including hemoglobin of 12.2 g/dL. Serum amylase was 50 U/mL, serum CEA was 1.3 ng/mL, and CA19-9 was 12.7 U/mL.
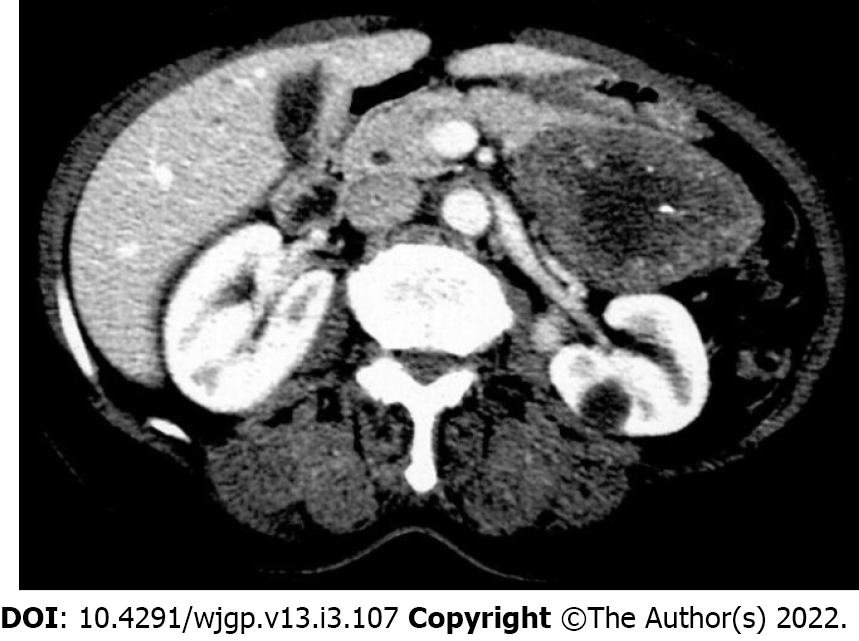
Upper gastrointestinal endoscopy revealed an ulcerated and infiltrative (Borrmann III) lesion measuring 4 cm in the lesser curvature extending to the posterior wall of the antrum and body region. Biopsy revealed a moderately differentiated adenocarcinoma. Preoperative evaluation using computed tomography (CT) scan showed a well-defined 8 cm × 5 cm solid and cystic tumor in the body and tail of the pancreas in close contact to the posterior wall of the gastric body. No sign of infiltration in the surrounding tissue was detected. No liver mass, peripancreatic lymph node swelling, or free peritoneal fluid was detected (Figure 1).
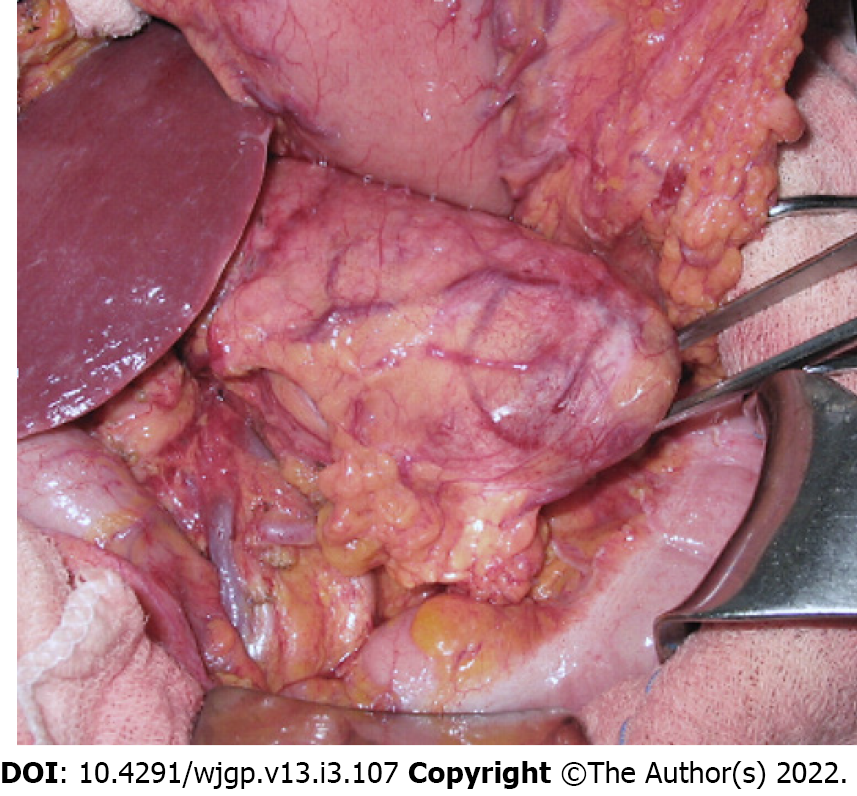
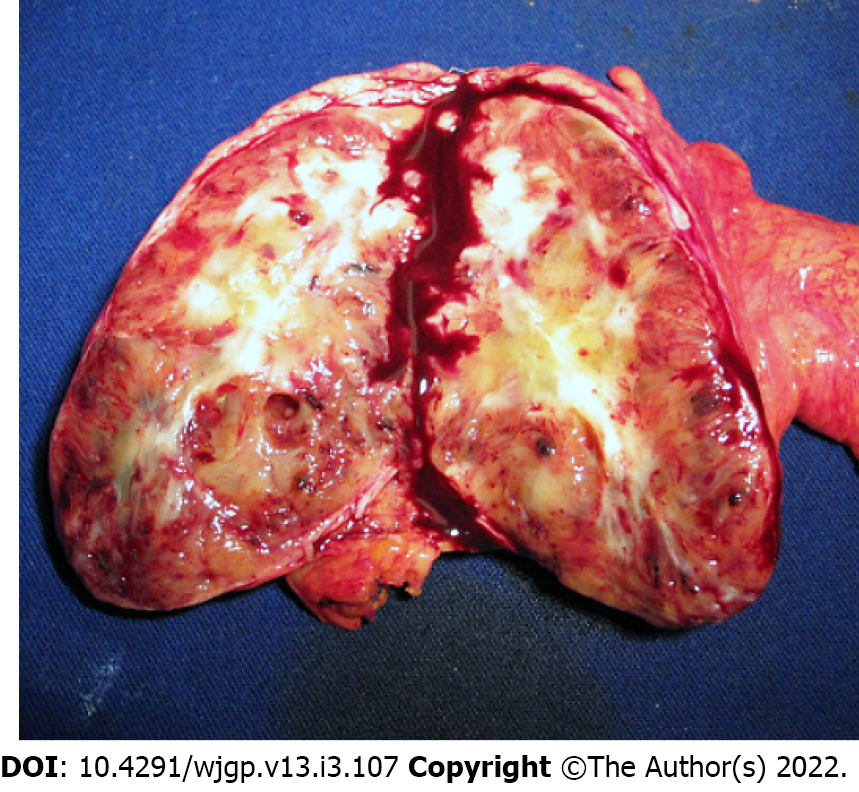
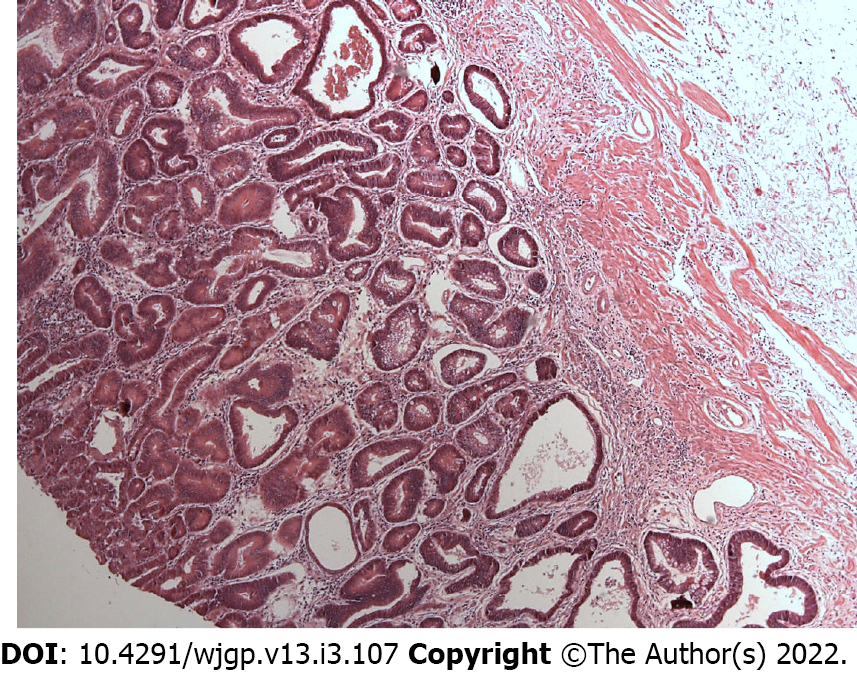
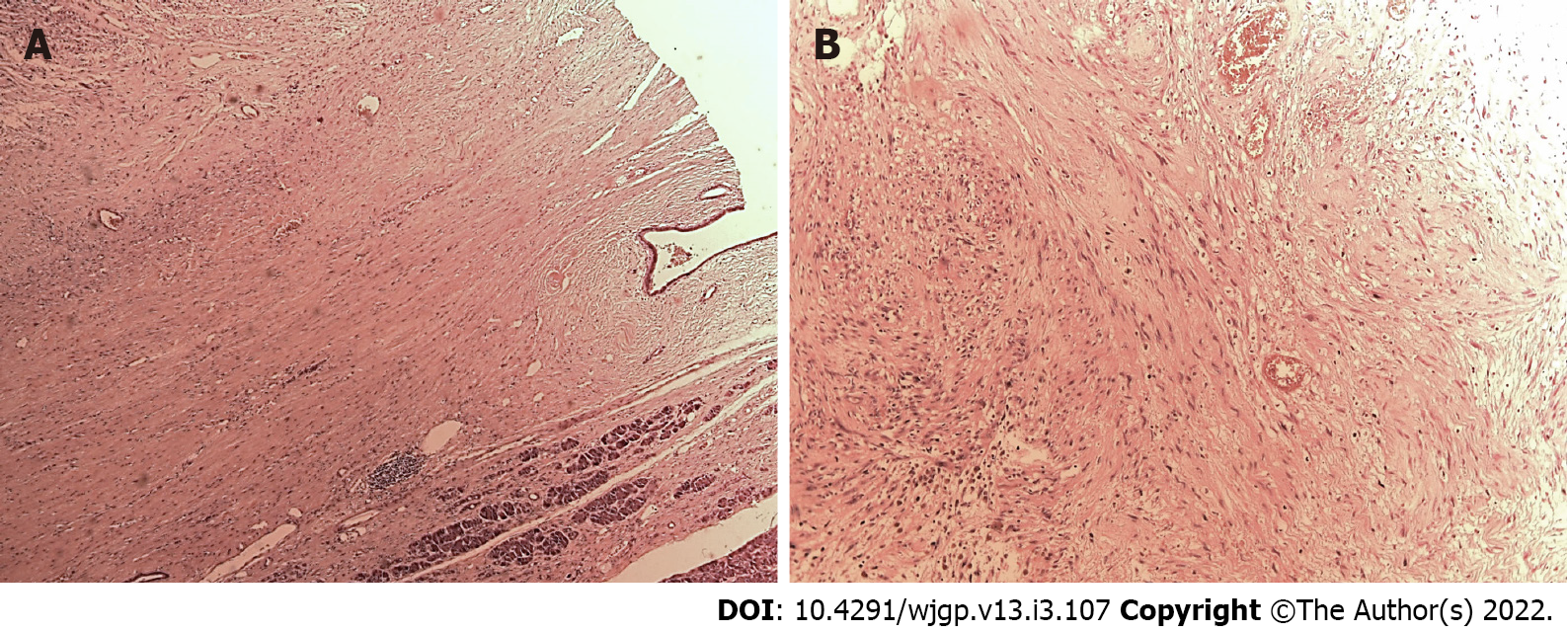
Laparotomy disclosed a localized gastric tumor in the body and a distinct solid, well-encapsulated tumor at the body of the pancreas without signs of inflammation or neoplastic infiltration. However, the lesion was in close contact to the posterior gastric wall (Figures 2 and 3). Due to the locoregional infiltration of the gastric tumor, absence of distant metastases, and proximity to a large pancreatic lesion, a total gastrectomy with D2 lymph node dissection plus distal pancreatectomy and splenectomy was performed. The final gastric cancer stage was pT2N0, with 0/73 lymph nodes examined (Figure 4). The cut surface of the excised 8 cm pancreatic tumor was pale yellow with hemorrhage foci. On microscopic examination, the lesion showed spindle cells with Antoni A and B patterns and was strongly positive for S100 protein (Figure 5).
Gastric adenocarcinoma and concurrent pancreatic schwannoma.
Total gastrectomy with D2 lymph node dissection, plus distal pancreatectomy and splenectomy.
The patient recovered without any complication, and she was discharged after 12 d. After 44 mo of follow-up, the patient has no evidence of recurrence.
In this case report, the patient presented unspecific symptoms including epigastric pain and weight loss. Therefore, it was not possible to define if these symptoms were related to the gastric cancer or if it was a symptomatic case of pancreatic schwannoma. Pancreatic schwannoma appear to be indolent, corroborating its benign nature, and around one-third of the pancreatic schwannomas are asymptomatic. Abdominal pain is the most displayed symptom, ranging from 30% to 57% of patients. Other symptoms are reported less frequently, such as back pain, jaundice, anorexia, vomiting, weight loss, anemia, abdominal mass, and gastrointestinal bleeding[7,8].
CT scan performed for gastric cancer staging showed a solid and cystic pancreatic mass, and it was necessary to make differential diagnosis with a primary pancreatic neoplasm or metastases from the gastric tumor. CT scan may be beneficial in pancreatic schwannoma initial evaluation, and most of them revealed low density or cystic masses, as presented in this case[9,10]. Moreover, magnetic resonance imaging appears to be more helpful in characterizing schwannomas by their typical encapsulation, hypointensity on T1-weighted images, and hyperintensity on T2-weighted images[11,12]. These characteristics are typical radiological features of Antoni A areas, suggesting that these should be classified as solid hypervascularized tumors of the pancreas. Meanwhile, type Antoni B tumor areas are characterized by a significant cystic component, in which differential diagnosis must be made from a large amount of pancreatic cystic neoplasms[9,12]. Fluorodeoxyglucose-positron emission tomography-CT usually demonstrates a hypermetabolic appearance[8,9]. Complementary magnetic resonance imaging and fluorodeoxyglucose-positron emission tomography-CT were not performed in this patient but would be helpful in better characterizing morphological tumoral features.
Endoscopic ultrasound-guided fine needle aspiration may be useful, but this method remains controversial due to high false-negative rate. In two reviews, only 44% and 50% of patients were correctly diagnosed with pancreatic schwannoma[4,8].
Intraoperative analysis is also a helpful tool in diagnosis, especially to ensure negative margins and correct resection of pancreatic neoplasms, as demonstrated in this case. One review showed that 47% of pancreatic schwannomas were correctly diagnosed, and 33% were reported as benign[8], showing that the intraoperative assessment of these tumors may aid the decision making in these cases.
Surgical treatment includes Whipple procedure (pancreaticoduodenectomy) or distal pancreatectomy with or without splenectomy, either because a definite diagnosis was not made pre- or intraoperatively or due to large tumor size[13,14] (Table 1). Enucleation should be considered a surgical option when preoperative histopathology confirms the diagnosis. However, a tumor size larger than 6.0 cm, vascular encasement, or visceral invasion should elicit suspicion of malignant transformation, and a more radical approach should be chosen[4].
| Ref. | Type of study | Number of patients | Case presented in the article | Literature review presented in the article | ||||||
| Moment of diagnosis | Surgery performed | Size (cm) | Mean size/range (cm) | Type of surgery performed | Malignancy, % | |||||
| Types of pancrea- | Enucleation | Surgical resection otherwise non-specified, % | ||||||||
| Paranjape et al[3], 2004 | Case report and review | 40 | Postoperative | Enucleation | 3.5 | 8.79 | 27 (67.5) | 4 (10.0) | 5 (12.5) | 5 (12.5) |
| Ma et al[4], 2017 | Case report and review | 68 | Postoperative | Whipple pancreaticoduodenectomy | 6 × 5 | 6.1 ± 5.7 (1-33) | 40 (59.0) | 8 (12.0) | 14 (21.0) | 8 (12.0) |
| Su et al[5], 2016 | Case report and review | 65 | Intraoperative frozen pathology | Central pancreatectomy | 1.6 × 1.1 × 1.1 | 5.83 ± 4.59 (1-20) | 40 (61.5) | 9 (13.8) | 13 (20.0) | 5 (7.7) |
| Gupta et al[6], 2009 | Case report and review | 37 | Postoperative | Whipple pancreaticoduodenectomy | 7.9 × 8.3 | - | 19 (51.3) | 6 (16.2) | 9 (24.3) | - |
| Moriya et al[7], 2012 | Case report and review | 47 | Intraoperative frozen pathology | Enucleation | 4 × 4 × 3 | 6.2 ± 5.1 (1-20) | 25 (53.0) | 7 (15.0) | 12 (26.0) | 5 (11.0) |
| Zhang et al[8], 2019 | Case report and review | 75 | Postoperative | Central pancreatectomy | 2.8 and 4.0 | 5.5 ± 5.0 (1.0-30.0) | 45 (60.0) | 11 (15.0) | 14 (19.0) | 4 (5.0) |
| Watanabe et al[9], 2018 | Case report | 1 | Postoperative | Subtotal stomach-preserving pancreaticoduodenectomy | 5.4 × 5.4 | - | - | - | - | - |
| Wang et al[11], 2019 | Case report | 1 | Postoperative | Distal pancreatectomy with splenectomy | 2.0 × 2.0 × 1.8 | - | - | - | - | - |
| Shi et al[14], 2021 | Case series and systematic review | 6 | Postoperative | Pancreaticoduodenectomy 5 (83%) and distal pancreatectomy 1 (17%) | 3.7 (range 2.0-6.4) | 4.3 ± 2.2 (1.4-10) | - | - | - | - |
| Kimura et al[15], 2021 | Case report | 1 | Postoperative | Distal pancreatectomy with splenectomy | 1.1 × 0.8 | - | - | - | - | - |
Gastrectomy with D2 lymph node dissection is a gold standard treatment considering the gastric neoplasm; however due to the pancreatic tumor size and the proximity to the posterior gastric wall harboring the tumor, it was decided to perform a partial pancreatectomy with splenectomy in addition to the gastric resection.
Microscopically, schwannomas are divided in two main subareas: Antoni A areas, displaying an organized hypercellular component, characterized by closely packed spindle cells with occasional nuclear palisading; and Antoni B areas, featuring a hypocellular component with loose myxoid stroma, often with degenerative changes[4,7]. Immunohistochemistry is crucial to the differential diagnosis since immunostaining is strongly positive for S-100 protein, vimentin, and CD56 and negative for cytokeratin AE1/AE3, desmin, smooth muscle myosin, CD34, and CD117[4,7,15]. In this case, diagnosis was confirmed by the presence of these typical findings in pathology: Antoni A and B areas as well as immunohistochemistry with strong S-100 (+) staining.
Pancreatic schwannomas usually have good prognosis, showing no rates of recurrence over a mean follow-up of 19 mo[4,8].
Therefore, we present the first case of synchronous gastric cancer and pancreatic schwannoma reported in the literature. Intraoperative staging examination was decisive in the adequate management of this patient. The presence of a large pancreatic mass should not preclude the potentially curative intent of the gastric cancer treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: De Raffele E, Italy; Wang YJ, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Ramos MFKP, Pereira MA, Yagi OK, Dias AR, Charruf AZ, Oliveira RJ, Zaidan EP, Zilberstein B, Ribeiro-Júnior U, Cecconello I. Surgical treatment of gastric cancer: a 10-year experience in a high-volume university hospital. Clinics (Sao Paulo). 2018;73:e543s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin N Am. 2004;15:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Paranjape C, Johnson SR, Khwaja K, Goldman H, Kruskal JB, Hanto DW. Clinical characteristics, treatment, and outcome of pancreatic Schwannomas. J Gastrointest Surg. 2004;8:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Ma Y, Shen B, Jia Y, Luo Y, Tian Y, Dong Z, Chen W, Li ZP, Feng ST. Pancreatic schwannoma: a case report and an updated 40-year review of the literature yielding 68 cases. BMC Cancer. 2017;17:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Xu SY, Sun K, Owusu-Ansah KG, Xie HY, Zhou L, Zheng SS, Wang WL. Central pancreatectomy for pancreatic schwannoma: A case report and literature review. World J Gastroenterol. 2016;22:8439-8446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Gupta A, Subhas G, Mittal VK, Jacobs MJ. Pancreatic schwannoma: literature review. J Surg Educ. 2009;66:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Moriya T, Kimura W, Hirai I, Takeshita A, Tezuka K, Watanabe T, Mizutani M, Fuse A. Pancreatic schwannoma: Case report and an updated 30-year review of the literature yielding 47 cases. World J Gastroenterol. 2012;18:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Siegelman ES, Lee MK 4th, Tondon R. Pancreatic schwannoma, an extremely rare and challenging entity: Report of two cases and review of literature. Pancreatology. 2019;19:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 9. | Watanabe T, Araki K, Ishii N, Igarashi T, Watanabe A, Kubo N, Kuwano H, Shirabe K. A Surgically Resected Pancreatic Schwannoma with Obstructive Jaundice with Special Reference to Differential Diagnosis from Other Cystic Lesions in the Pancreas. Case Rep Gastroenterol. 2018;12:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Yu RS, Sun JZ. Pancreatic schwannoma: CT findings. Abdom Imaging. 2006;31:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wang S, Xing C, Wu H, Dai M, Zhao Y. Pancreatic schwannoma mimicking pancreatic cystadenoma: A case report and literature review of the imaging features. Medicine (Baltimore). 2019;98:e16095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Novellas S, Chevallier P, Saint Paul MC, Gugenheim J, Bruneton JN. MRI features of a pancreatic schwannoma. Clin Imaging. 2005;29:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Shi Z, Cao D, Zhuang Q, You R, Li X, Li Z, Li Y, Huang X. MR imaging features of pancreatic schwannoma: a Chinese case series and a systematic review of 25 cases. Cancer Imaging. 2021;21:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kimura K, Adachi E, Toyohara A, Omori S, Ezaki K, Ihara R, Higashi T, Ohgaki K, Ito S, Maehara SI, Nakamura T, Fushimi F, Maehara Y. Schwannoma mimicking pancreatic carcinoma: A case report. World J Clin Cases. 2021;9:4453-4459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest. 1983;49:299-308. [PubMed] |