Published online Dec 12, 2020. doi: 10.4291/wjgp.v11.i6.114
Peer-review started: July 21, 2020
First decision: September 24, 2020
Revised: October 7, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 12, 2020
Processing time: 143 Days and 5.8 Hours
The intestinal barrier is a complex and well-controlled physiological construct designed to separate luminal contents from the bowel wall. In this review, we focus on the intestinal barrier’s relationship with the host’s immune system interaction and the external environment, specifically the microbiome. The bowel allows the host to obtain nutrients vital to survival while protecting itself from harmful pathogens, luminal antigens, or other pro-inflammatory factors. Control over barrier function and the luminal milieu is maintained at the biochemical, cellular, and immunological level. However, disruption to this highly regulated environment can cause disease. Recent advances to the field have progressed the mechanistic understanding of compromised intestinal barrier function in the context of gastrointestinal pathology. There are numerous examples where bowel barrier dysfunction and the resulting interaction between the microbiome and the immune system has disease-triggering consequences. The purpose of this review is to summarize the clinical relevance of intestinal barrier dysfunction in common gastrointestinal and related diseases. This may help highlight the importance of restoring barrier function as a therapeutic mechanism of action in gastrointestinal pathology.
Core Tip: Intestinal barrier dysfunction is an underlying pathophysiological feature in many gastrointestinal diseases. Understanding barrier dysfunction may help drive a deeper understanding of gastrointestinal pathology and identify novel way(s) to manage and/or treat disease. Here, we summarize the evidence supporting intestinal barrier dysfunction in common immune-mediated gastrointestinal diseases and its clinical relevance.
- Citation: Muehler A, Slizgi JR, Kohlhof H, Groeppel M, Peelen E, Vitt D. Clinical relevance of intestinal barrier dysfunction in common gastrointestinal diseases. World J Gastrointest Pathophysiol 2020; 11(6): 114-130
- URL: https://www.wjgnet.com/2150-5330/full/v11/i6/114.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v11.i6.114
The gut contains about 0.2 kg, or approximately 40 trillion cells, of bacteria (commonly referred to as the microbiome) and maintains a highly specialized architecture to manage the host’s interaction with food-borne and bacterial antigens[1]. While the epithelial monolayer and multiprotein junctional complexes [e.g., tight junctions (TJs)] comprise the primary physical barrier, other cell types, such as mucin-producing Goblet cells and Paneth cells, have antimicrobial functions[2,3]. The bowel’s defense system is further supported by proximal lymphoid tissue, which is critical to maintaining homeostasis with respect to microorganisms beneficial to the host as well as responding to pathogenic invaders. This interconnected system facilitates a robust immune response and allows broad coverage over a large surface area. In fact, it is estimated that the intestinal lining contains more immune cells and produces more antibodies than any other organ[4].
Barrier maintenance is challenged by the rapid turnover of intestinal epithetical cells–occurring every 4-5 d[5]. Homeostasis is readily maintained, in part, due to cell shedding and stem cell proliferation within the intestinal crypts; however, loss of intestinal barrier function may expose antigens of the microbiome to immune cells inside luminal epithelium. This can activate an immune response and cause pathology. The purpose of this review is to characterize and quantify the clinical relevance of impaired intestinal barrier function in common gastrointestinal diseases. Specifically, we will review Crohn’s disease (CD), ulcerative colitis (UC), diarrhea-predominant irritable bowel syndrome (IBS-D), and an emerging pharmacotherapy-associated condition known as immune checkpoint inhibitor-related colitis. In addition to reviewing the current state of knowledge, this review may help identify knowledge gaps in the understanding of impaired intestinal barrier function and highlight the relevance of restoring barrier function as a therapeutic mechanism of action.
Functional in vivo assessment of bowel permeability relies on the measurement of orally administered sugars, or some other indigestible probe, that reflects non-mediated absorption through the paracellular and/or transcellular route. Selection of an appropriate test probe involves knowledge of the molecules’ properties, route(s) of permeation, and potential confounding variables-which have been described elsewhere[6]. A summary of clinically available probes is listed in Table 1.
| Functional probe | Proposed test site | Sample site | Ref. |
| 51Cr-EDTA | Whole intestine | Urine | Bjarnason et al[14], 1983 |
| Lactulose/mannitol1 | Small intestine | Plasma/urine | Rao et al[11], 2011 |
| PEG400 | Whole intestine | Urine | Ma et al[132], 1990 |
| Sucralose2 | Colon | Urine | Anderson et al[133], 2004 |
| Sucrose2 | Gastroduodenal | Urine | Meddings et al[134], 1993 |
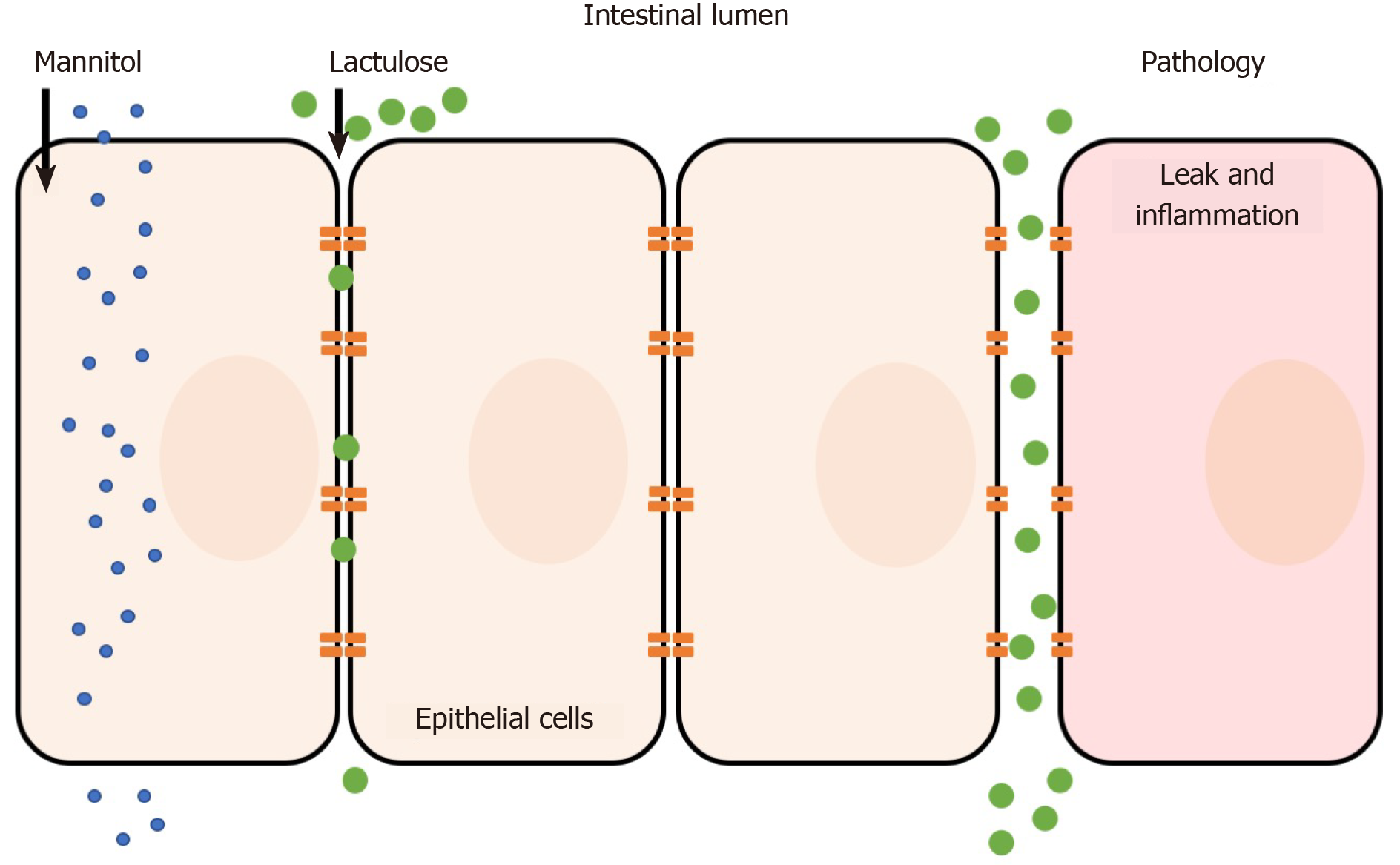
The lactulose/mannitol (L/M) test is the most widely used method to evaluate small intestinal permeability in vivo. The basis for a two-sugar test relies on the para- and transcellular absorption pathways of lactulose and mannitol. Mannitol is a monosaccharide approximately 6.5 Å in molecular diameter and is readily absorbed by passive diffusion through the membrane of bowel epithelium (transcellular transport). In contrast, the larger disaccharide lactulose (9.5 Å) is minimally absorbed because of its size; however in abnormal situations (e.g. pathology) lactulose can fit through intercellular spaces and becomes absorbed via the paracellular route (Figure 1)[7-9]. Following absorption into the blood stream, both sugars are then renally secreted by glomerular filtration without active re-absorption and are thus readily measurable in blood and urine. Because of the paracellular vs transcellular absorption pattern of lactulose and mannitol, the L/M ratio is small (or close to zero) under normal physiological conditions. In disease, alterations to lactulose and/or mannitol excretion are thought to reflect pathological features of leakiness (lactulose) normalized to surface area (mannitol)[10]. Hence, the L/M ratio is higher under pathological conditions due to increased paracellular absorption of lactulose. Both sugars cannot be metabolized by bacteria in the small bowel (but are by colonic bacteria) and are unaffected by differences in liver metabolism, which ensure its integrity is maintained for measurement[6]. The L/M test consists of concomitant oral administration of lactulose and mannitol (dissolved in water) followed by timed blood or urine collection (up to 24 h). Urinary excretion from 0 to approximately 5 h reflects small intestine permeability, although methodological enhancements of the L/M tests suggest that shorter time frames (e.g., 0-2 h) may be ideal[11]. L/M can also be measured in serum to reduce complications of timed urine collections. For example, one dual sugar test was shown to have similar results when analyzing either serum or urine[12]. The serum-based test was simpler because it required less sample volume, needed a short waiting time after sugar ingestion (1 h compared to 5 h for urine collection), and was more cost-effective to analyze than urine[12]. The L/M test has also shown intra-individual and analytical repeatability, making it a simple and reliable measurement method[13].
The primary results of the test are expressed as lactulose-to-mannitol ratio (e.g. lymphocyte-to-monocyte ratio (LMR) in urine: %lactulose/%mannitol where “%” represents the percentage of the ingested dose) with a higher LMR indicating increased small intestine permeability. Other sugars have been investigated (e.g. L-arabinose, L-rhamnose, raffinose) but there appears to be no widely-accepted practical benefit over L/M[6].
Chromium-51-ethylenediaminetetra-acetate (51Cr-EDTA) is a radiolabeled form of the indigestible EDTA. 51Cr-EDTA is orally administered and transverses the lumen paracellularly, similar to large sugars, where it is readily excreted in urine[14]. 51Cr-EDTA is believed to cross both the small and large intestine, in contrast to lactulose and mannitol, which predominately transverses the small intestine only[15]. Moreover, when combined with a sugar probe to correct for small intestinal absorption, 51Cr-EDTA allows assessment of colonic permeability because it is not metabolized by the microbiome of the colon. Routine usefulness of the test, however, appears to be limited by reliability and practicality (e.g., up to 24 h urine collection, requires gamma counter, short shelf-life of 51Cr)[6,14].
Confocal laser endomicroscopy is an in vivo real-time imaging method that can detect and quantify mucosal abnormalities at the microscopic level following administration of a contrast agent. It has several clinical applications such as in the identification of dysplasias/neoplasias, diagnostic work-up of Inflammatory bowel disease (IBD), and other surgical use cases. Confocal laser endomicroscopy has been also experimentally applied to assess intestinal permeability[16,17]. The main advantage is that it can quantify permeability at specific intestinal site(s), which may be cross-referenced with histological or endoscopic findings. However, the technique remains experimental and there appears to be no well-validated methodology or robust reproducible findings in the literature[18]. Technical and analytical challenges may have also contributed to its lack of wide spread acceptance.
IBD is a group of chronic gastrointestinal disorders primarily consisting of CD and UC. Genetic susceptibility and environmental triggers underly the etiology of pathology which converge to activate an immune response associated with compromised intestinal barrier[19-21]. For example, nucleotide-binding oligomerization domain 2 (NOD2), expressed in ileal Paneth cells, is induced in response to the presence of bacterial components and activates an innate immune response; genetic analysis has confirmed the relationship between mutations in NOD2 and CD and it is estimated that 30%-50% of CD patients in the Western hemisphere harbor one mutant allele of NOD2[22-24]. Two pathogenic alleles increases the risk of CD by 20-40 times[23,24]. These data suggest that certain susceptibility factors may contribute to dysfunctional/overactive innate and adaptive immune pathways that may not be able to self-regulate as it would under normal or homeostatic conditions. Substantial data implicate abnormal bowel barrier function as an underlying pathological feature of IBD.
CD is a chronic relapsing-remitting inflammatory disease of the gastrointestinal tract, the cause of which remains generally unknown. The disease affects the gastrointestinal tract discontinuously from mouth to anus, but most commonly the disease is located both in ileum and colon (40%), followed by a disease in the small bowel only (30%), and in the colon only (25%). CD can lead to severe disability and significant reduction in quality of life[25,26]. Abnormal intestinal barrier function has been a well-established pathological feature of CD for nearly 40 years[27-31]. Impaired intestinal function may be multifactorial but structural barrier damage appears to be an characteristic pathological feature (Table 2).
| Disease | Disease activity | Functional probe | Intestinal permeability change | Ref. |
| CD | Not reported | C/M | ↑ | Secondulfo et al[135], 2001 |
| Not reported | 51Cr-EDTA | ↑ | Jenkins et al[136], 1987 | |
| Low activity and high activity | Iohexol | Low activity: ↑; high activity: ↑ | Gerova et al[55], 2011 | |
| Low activity and high activity | L/M | Low activity: ↑; high activity: ↑ | Benjamin et al[57], 2008 | |
| Remission, low activity, and high activity | L/M | Remission: ↔; low activity: ↑; high activity: ↑ | Welcker et al[82], 2004 | |
| Remission | L/M | ↑ | Wyatt et al[52], 1993 | |
| Low activity | L/M; L/R; R/M | L/M: ↑; L/R: ↑; R/M: ↔ | Katz et al[30], 1989 | |
| Low activity | L/M; L/R; L/PEG; PEG/M; PEG/R | ↔ | Munkholm et al[45], 1994 | |
| UC | Not reported | 51Cr-EDTA | ↑ | Jenkins et al[136], 1987 |
| Remission, low activity, and high activity | C/M; C/R; L/M; L/R; | Remission: ↔ or ↑1; low activity: ↔ or ↑1; high activity: ↑ | Welcker et al[82], 2004 | |
| Low activity and high activity | Iohexol | Low activity: ↑; high activity: ↑ | Gerova et al[55], 2011 | |
| Remission | L/M; S; Su | ↑ | Büning et al[83], 2012 | |
| Remission | L/M/S/Su/E/R | ↔2 | Wegh et al[81], 2019 | |
| IBS-D | Active | 51Cr-EDTA | ↑ | Gecse et al[103], 2012 |
| 51Cr-EDTA | ↑ | Dunlop et al[102], 2006 | ||
| L/R | ↑ | Mujagic et al[105], 2014 | ||
| L/M | ↑ | Shulman et al[98], 2014 | ||
| L/M | ↑ | Vazquez-Roque et al[100], 2012 | ||
| L/M | ↑ | Zhou et al[104], 2009 | ||
| L/R | ↑ | Zhou et al[137], 2010 |
The intestinal barrier, as it relates to bowel epithelial function, consists of a polarized monolayer of epithelial columnar cells connected by TJs, an intercellular feature designed to create separation between gut and lumen. Four groups of proteins comprise TJs: Occludin tricellulin, junctional adhesion molecule, and claudins[32-35]; The latter appear to have a particular crucial role in barrier function including seal or pore-forming properties and may act as paracellular channels for small ions[36-39]. The relevance of TJ abnormalities in CD has been demonstrated in sigmoid colon biopsies, which revealed a reduction in the number of TJ strands, reduced depth of TJ meshwork, and strand breaks[40]. Expression of sealing claudin-3, -5 and -8 and occludin were diminished, indicating altered TJ structure[40-42]. The pore-forming claudin 2 also appears to be upregulated[40,43]. These data were generated from patients with mild-to-moderate CD, suggesting that impaired TJ structure may characteristic that occurs early in disease progression. This is consistent with the hypothesis that permeability abnormalities are a primary disorder, as evidenced by healthy first-degree relatives of CD patients who display detectable permeability defects and who also have a higher risk of developing CD compared to healthy non-relatives[44-47]. Zeissig et al[40] (2007) also discovered that epithelial apoptosis was increased in mild-to-moderate CD, a feature previously thought to be specific to UC[40]. These data implicate that both changes to TJ structure and epithelial apoptosis may serve as a mechanistic explanation(s) consistent with barrier dysfunction. This hypothesis is also consistent with less pronounced changes to TJ structure and epithelial apoptosis in patients in remission, suggesting that epithelial structure may correlate with clinical symptoms, although permeability alterations have been observed in areas evident of lesions in addition to areas absent of macroscopic injury[48,49].
Increased intestinal permeability in patients with CD is well-recognized and has also been shown to associate with symptomatic status in patients with IBD. In one prospective study, patients with symptomatic IBD had a significantly higher median Confocal Leak Score (CLS) (19.0) than patients with asymptomatic IBD (7.3; P < 0.001) or control subjects (5.9, P < 0.001)[50]; no differences in CLS were found between control subjects and asymptomatic IBD patients, suggesting that low barrier function may be a causative mechanism underlying IBD symptoms. This was confirmed on a symptom level when regression analysis revealed that every increase in CLS of 1.9 correlated with an additional diarrheal motion per day[50]. Other studies have found a relationship between increased intestinal permeability in clinically inactive patients and also demonstrated a correlation with pending disease relapse[51,52]. Wyatt et al[52] (1993) prospectively followed 72 CD patients in symptomatic remission and following a L/M test, discovered the permeability index (PI) was significantly higher in patients than in controls at baseline (0.046 vs 0.018, respectively)[52]. After one year, the relapse rate was 70% in CD patients who had an abnormal PI compared to 17% of patients who had a normal PI. The sensitivity of the PI as predictor of relapse was 81% and specificity was 73%. Other studies are consistent with these findings and have estimated relative risk of relapse within 1 or 2 years in patients with elevated intestinal permeability to be approximately 3-18[7,17,51,53]. This may be explained, in part, by the association between increased intestinal permeability, inflammation, and disease activity (i.e. Crohn’s disease activity index)[54,55]. In addition, increased epithelial gaps as measured by confocal laser endomicroscopy appear to predict hospitalization or surgery in patients with IBD[56]. These data point to elevated bowel permeability that may allow for a greater probability of interaction between certain pathogens of the microbiome with the epithelial immune system of the bowel.
Elevated intestinal permeability may also indicate greater disease involvement. For example, more patients with ileo-colonic disease (57.8%) had an abnormal PI as measured by L/M compared to 26.7% and 15.6% of patients with colonic and small intestinal disease, respectively[57]. The authors also found that patients with stricturing disease had a significantly elevated LMR compared to patients with non-fistulizing non-stricturing disease. These data highlight the clinical relevance of intestinal permeability measures in the context of disease involvement, symptoms of the IBD disease, and as trigger for relapse that may suggest restoring barrier function may be a useful approach to maintain remission[7,52,53,58,59]. It should be noted that not all studies have found intestinal permeability abnormalities in CD[45]. Although the reasons for this observation are not clear, differences in study design, study population, selected probe, sample size, and analytical methods, among others, may contribute to conflicting literature reports.
As noted earlier, increased intestinal permeability may or may not occur in the presence of histological findings. Protein antigen uptake in macroscopically normal segments of distal ileum of patients with CD was reported to be increased despite being histologically unaffected[60]. Early histological lesions appear to occur in the follicle-associated epithelium (FAE) of lymphoid follicles and Payer’s Patches located in the mucosa, which can occur even in the presence of normal overlying epithelium[61,62]. Indeed, Keita et al[63] (2008) discovered enhanced intercellular and transcellular uptake of E. Coli across FAE in ileal CD, but not UC, indicating defective barrier function at immunological inductive sites[63]. This appears to be related to microtubule-, microfilament-dependent internalization and transcytosis of bacteria associated with enterocyte cytoskeletal changes under conditions of stress–further linking structural changes to increased permeability[64]. In vivo studies support bacterial translocation secondary to increased paracellular permeability as the mechanistic basis for chronicity and/or acute relapses in IBD[65]. These examples may help explain the relevance of barrier function and associated functional tests in the absence of endoscopic and/or histological findings.
Most available treatments in CD aim to control symptoms (e.g., thiopurines, and steroids) or reduce the immune response, for example by inhibiting tumor necrosis factor-α (TNF-α) (e.g. infliximab, adalimumab, golimumab, and certolizumab pegol)[66]. Although anti-TNF-α agents have proven clinically beneficial, approximately 1 in 3 do not respond to induction therapy, and of those who do respond, approximately 1 in 3 become secondary non-responders during maintenance therapy[67-71]. Anti-TNF-α agents are also associated with several side effects (e.g. risk of infection, malignancies, skin lesions, immune reactions, peri-operative complications, and decreased fertility)[72-75]. Persistent diarrhea in patients with CD despite mucosal healing (Mayo scores of 0-1 or segmental endoscopic severity CD scores of 0-5) is not uncommon suggesting that achieving mucosal healing alone through conventional management may be insufficient in some patients. This may warrant developing therapies targeted to intestinal barrier dysfunction and dysbiosis[76]. We suggest that next generation treatments for CD should focus on restoring bowel barrier function as a way to induce and maintain remission which may also prevent some of the side effects of long-term general immunosuppressive therapy.
Similar to CD, the pathogenesis of UC appears to be related to both genetic and environmental susceptibility factors. UC differs in that it continuously affects the colon and perirectal region with shallow and indiscrete ulcers associated with inflammation limited to the mucosa. Structural characterization of the epithelia in UC appears less robust compared to the available evidence available in CD; however, some notable defects have been reported. Freeze-fracture electron microscopy revealed epithelial cell TJ strand count was reduced by approximately 30% in UC biopsies compared to controls[77]. This paralleled the authors findings that found a 50% and 80% reduction in total and epithelial wall resistance, respectively, using alternating current impedance analysis, which indicates a leak-flux imbalance that may help explain diarrhea in UC[77]. Similar studies corroborate these reports and also suggest that apoptotic foci are a source of epithelial leaks that appear more pronounced with increasing inflammation[78]. Indeed, Th2 cytokines, e.g., interleukin (IL)-13, have been shown to be important effector molecules relevant to epithelial dysfunction that affect tight junctions and cellular apoptosis[79]. Cytokine-mediated altered expression of TJs appears to occur at the signal transduction level[80].
In contrast to CD, patients with UC and in remission generally do not always appear to have notable permeability defects (Table 2). Wegh et al[81] (2019) reported that when using a 5-sugar test, intestinal permeability in patients with UC in clinical remission was not different compared to historic values (e.g., lactulose/rhamnose approximately 0.02-0.06), although the study did not include a true control arm[81]. Urinary sucrose excretion and sucralose/erythritol ratio also appeared normal. This was consistent with normal or slightly abnormal inflammatory markers in the study population, suggesting that functional permeability tests may have little relevance in subclinical disease particularly in the absence of abnormal biomarkers. Similar findings were observed by Welcker et al[82] (2004) when using multiple functional probes[82]. In a larger study including patients with UC in remission and healthy first degree relatives however, an elevated LMR was observed significantly more often in UC patients in remission (28.1%) compared with healthy controls (6.1%; P < 0.001)[83]. This is somewhat paradoxical as UC affects the colon, and gastroduodenal and colonic permeability were found to be normal using sucrose and sucralose as functional markers, respectively. However, healthy first degree relatives also had an higher prevalence of elevated LMR compared to controls (20% vs 6.1%, respectively, P = 0.01), possibly indicating that abnormal permeability may be a risk factor rather than a reliable marker of disease, at least in patients in remission[83].
Despite histological and in vitro evidence indicating colonic barrier abnormalities, clinical data that reliably demonstrate altered barrier function in active UC appears less clear compared to CD. This may be related to the observation that intestinal permeability is in UC less prevalent than in CD[55]. Welcker et al[82] (2004) for example reported increased permeability in patients with low and high activity UC[82]. However, the interpretation of this evidence is potentially limited by the use of multiple functional tests (multiplicity) and low sample size (e.g., n = 4 in patients with highly active UC). Further research is needed to clarify the magnitude, clinical relevance, and true anatomical location(s) of impaired barrier function, particularly in active UC.
The therapeutic goals of UC are similar to CD: Induce and maintain remission. Biologic agents including anti-TNF-α agents, Janus kinase inhibitors, IL-12/IL-23 inhibitors, and integrin receptor antagonists are considered when conventional therapies (e.g. aminosalicylates, oral immunomodulators, and corticosteroids) are inadequate. Because these agents are immune-targeting, they carry traditional down side risks of potentially long-term immunosuppression and can have notable contraindications (e.g. in patients with advanced heart failure or neurological conditions)[84].
IBS is primarily characterized by recurring abdominal pain and bowel movement changes without discernable gross pathology[85]. Patients experience mixed symptoms such as pain, bloating, abnormal stools, and dyspepsia and are categorized by stool pattern: Diarrhea predominant (IBS-D), constipation predominant (IBS-C), or mixed (IBS-M). IBS is highly prevalent (global prevalence: Approximately 10%) and negatively affects quality of life, work productivity, and can even cause severe disability[86-88].
The pathophysiology of IBS appears multifactorial. Historically, environmental and psychosocial triggers, genetic modifiers, intestinal dysmotility, microbiota disturbances, and bile acid malabsorption, among others, had been proposed[89-93]. However, more recently IBS is thought to be a disease of the gut-brain axis with the trigger being an interaction between the microbiome and certain immune cells[94]. Mast cells, for example, have been shown to correlate with intestinal permeability in patients with IBS-D and are a key mediator of downstream inflammation that are also linked to symptoms such as pain[95,96]. Emerging evidence continues to support mast cells as central to the initiation and symptomatic presentation of IBS.
Gastroenteritis (especially related to Campylobacter jejuni infection) has been shown to precede or cause IBS symptoms, particularly in IBS-D, suggesting that alterations to the intestinal milieu and subsequent immune stimulation may be a primary driver contributing to IBS-D pathology[91,97]. Spiller et al[97] (2000) was among the first to report increased gut permeability (approximately 4-fold increase in LMR compared to controls) in a small cohort of patients with post-dysenteric IBS[97]; immunohistology also revealed elevated CD3, CD4, and CD8 lymphocyte counts in the lamina propria, consistent with the known relationship between the immune system and gut permeability. Subsequent studies have confirmed these observations[91,98-100]. Thus, impaired intestinal barrier function and subsequent microbial infiltration have become central to the proposed gut disease model in certain forms of IBS[101]. The pathogenesis of IBS also appears to link a relationship between the microbiome, gut, and brain, as neural networks have been shown to be influenced by the composition of the gut microbiome[94]; therefore the gut-brain axis is highly relevant in the pathophysiology of IBS.
Increased intestinal permeability appears greatest in IBS-D. Dunlop et al[102] (2006) showed that proximal small intestinal permeability, as measured by 51Cr-EDTA, was significantly higher in patients with postinfectious IBS-D compared to patients with IBS-C and healthy control subjects[102]. Patients with IBS-D but without a history of acute gastroenteritis had the highest median excretion of 51Cr-EDTA in the study, further implicating the diarrhea-predominant phenotype with impaired intestinal barrier function; colonic permeability has been shown to correlate with stool frequency, suggesting site-specific defects in barrier function may be related to disease severity[103]. Similar observations have been reported in the literature[104,105]. IBS-C was not included in this review because intestinal permeability appears to be normal in this patient population[106].
The relationship between intestinal permeability and symptoms was evaluated by Gecse et al[103] (2012) who used 51Cr-EDTA as an intestinal permeability probe in patients with IBS-D[103]. The number of stools per week was significantly correlated with permeability (Pearson r = 0.62; P = 0.0057). Interestingly, 51Cr-EDTA excretion was only elevated compared to control when measured between 5 and 24 h after ingestion, suggesting that colonic, but not small intestine, permeability may explain the study findings and indicates that site-specific permeability may be a feature of IBS-D[103]. 51Cr-EDTA excretion was similar to patients with UC, suggesting that impaired bowel function in IBS-D may be more similar to IBD than IBS-C. It should also be noted that molecular markers of impaired intestinal barrier function help further establish the mechanistic association with IBS-D. These data indicate a strong link between intestinal barrier dysfunction, IBS-D, and disease severity. This shows that there is a good correlation between IBS-typical symptoms and the degree of increased bowel permeability. These data also indicate that the pathophysiology of IBS-D seems to differ from IBS-C.
Other measures of symptomatic disease severity, including visceral and thermal hypersensitivity to pain [visual analog score (VAS) and bowel severity (FBDSI score)], have been shown to correlate with intestinal permeability. Zhou et al[104] (2009) reported that IBS-D patients with a LMR of ≥ 0.07 had significantly higher VAS intensity ratings to nociceptive and thermal pain than those IBS patients and controls with a LMR of < 0.07[104]. The authors used nociceptive and thermal stimuli in areas apart from the gut because many patients with IBS frequently complain of pain in body regions somatotopically distinct from the gut. The authors hypothesized their findings may be related to sensitization of the myenteric plexus and the common spinal segments of the central nervous system, thus establishing a quantitative link between bowel permeability and pain symptoms. If true, restoring bowel permeability may also have an effect on pain.
In total, these data suggest that a therapy designed to restore intestinal barrier dysfunction may be a promising therapeutic approach in IBS-D. Similar to other gastrointestinal diseases discussed in this review, notable architectural defects in the intestinal architecture have been reported in IBS-D including decreased expression of transmembrane (e.g., occludin and claudin-1) and intercellular TJs (e.g. ZO-1)[107-109]. It should be noted that mood disorders are also correlated with IBS; epidemiological evidence indicates that mood disorders develop after IBS in approximately 50% of patients, implicating gut mechanisms as drivers of non-gastrointestinal symptoms. Interestingly, one study found that the LMR correlated with IBS, interference with activities and work and retrospectively measured anxiety and depression[98]. If true, correcting intestinal pathology (e.g., intestinal barrier dysfunction) could also have a significant impact on mood disorders secondary to IBS[93,110,111].
Alosetron (5-HT3 receptor antagonist), rifaximin (antibacterial), and eluxadoline (mu-opioid receptor agonist) are among the few drugs with marketing authorization for IBS-D in the United States market. Alosetron was removed from the market from 2000-2002 due to safety reasons but subsequently re-introduced with a more restrictive label. The efficacy of approved agents also appears limited. For example, a meta-analysis of five rifaximin trials indicated a small improvement in global IBS symptoms when patients were treated with rifaximin (42.2%) compared to placebo (32.4%)[112]. Other agents commonly used include antidiarrheals (e.g., loperamide), bile acid sequestrants (e.g. cholestyramine), antispasmodics (e.g., dicyclomine), and antidepressants (tricyclic agents, e.g., amitriptyline)[113]. Nonpharmacological interventions such as probiotics and diet modification are also considered[114,115]. The lack of robust, and well-controlled randomized trials makes it difficult to precisely quantify the efficacy and safety of all available options; however, consensus opinion and available data indicate that most patients do not achieve complete symptom relief[113,116]. Given the newly established pathophysiologic relationship between the microbiome and interactions with certain immune cells, it seems promising to develop interventions that repair and maintain bowel permeability as next generation treatments for IBS-D.
Immune checkpoint inhibitors (ICIs) are a class of monoclonal antibodies designed to interrupt critical signaling pathways between T cells and antigen-presenting cells. At least 6 approved ICIs have proven clinically effective across more than several dozen oncology indications. Despite their robust efficacy, ICIs are associated with a wide range of toxicities. The most common type of adverse events is a group of immune-mediated conditions including colitis, hepatitis, and thyroiditis, and generally appear early following treatment initiation. As these conditions resemble autoimmune diseases, this seems to suggest an abnormal or exaggerated immune response, possibly in relation to antigen exposure, such as the microbiome. Among immune-mediated diseases, gastrointestinal toxicity (ICI colitis) is the most common adverse event associated with ICI therapy that occurs usually in 6-8 wk after initiation of treatment[117]. Diarrhea and colitis occurs in up to approximately 54% and 22% of patients treated with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) therapy, respectively[118]. programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) inhibitors appear to have lower rates of diarrhea and colitis (up to 30% and 7% respectively), although the incidence of these toxicities is high in anti-CTLA4 and anti PD-1/PD-L1 combination therapy (45%), with approximately 10% of cases reported as grade 3 or higher[117,118].
The endoscopic and histologic features of ICI-related colitis appear to resemble IBD[117,119,120]. For example, in 39 patients with ipilimumab-induced colitis, immune infiltrate was highly prevalent: Neutrophilic infiltrate occurred in 46% of patients, lymphocytic infiltrate in 15% of patients and a mixed neutrophilic–lymphocytic infiltrate 38% of patients[120]. Edema, erythema, erosions and ulceration are also common endoscopic features. The same study by Marthey et al[120] (2016) found that 97% patients with ipilimumab-induced colitis had erythema or ulceration in the sigmoid colon or rectum and 66% had extensive colitis[120]. The pathogenesis of ICI-related colitis, therefore, appears to relate to colonic inflammation although the precise mechanism(s) are not well characterized. Given clinicopathological similarities IBD, it is reasonable to hypothesize that impaired bowel barrier function may play a role in the development of colitis. This is supported by a retrospective study that found pre-existing IBD increases the risk of gastrointestinal adverse events following ICI therapy[121]. Samaan et al[117] (2018) proposed several barrier function-related etiopathogenic hypotheses including pre-existing intestinal dysbiosis or epithelial stress/mucositis secondary to chemotherapy[117]. Thus, normalizing bowel barrier function may be a promising therapeutic mechanism to treat or even prevent ICI-related colitis.
While the relationship between impaired bowel barrier function and bowel pathology appears self-evident, increasing evidence has also revealed a connection between the bowel and the brain–colloquially referred to as the gut-brain axis. This axis consists of complex, bidirectional pathways relevant to normal gastrointestinal functions (e.g. satiation), but also to higher executive functions (e.g. decision making) and emotions (e.g. fear and stress)[122]. Communication between the bowel and the brain is regulated at the neuronal, endocrine, and immunological level. Hence, as the lumen interfaces with the microbial environment, including signaling molecules such as quorum-sensing molecules and bile acids within the lumen, communication to the brain can reflect interaction(s) between the host and the microbiome[123]. Substantial nonclinical and clinical evidence has revealed a well-documented connection between impaired bowel barrier function, microbial dysbiosis, and neurological diseases[123,124]. For example, a recent nationwide longitudinal study revealed the hazard ratio of developing dementia among patients with IBD was 2.54[125]. In Parkinson’s disease, there is increasing evidence that pathogenic microbial peptides are able to access the enteric nervous system and/or systemic circulation via a highly permeable bowel wall, which could act as a disease trigger or driver of neuronal destruction, possibly by way of retrograde axonal and transneuronal transport of α-synuclein via the vagus nerve[126]. Increased bacteria/endotoxin exposure has been shown to correlate with sigmoid mucosa α-synuclein in Parkinson’s disease, consistent with findings of increased intestinal permeability in patients with Parkinson’s disease[127]. Altered intestinal permeability has been linked to other extraintestinal disease such as nonalcoholic fatty liver disease, type 1 diabetes, arthritis, and other autoimmune diseases[128-131]. This may imply that restoring intestinal barrier function may have therapeutic benefits beyond bowel pathology alone.
Multiple genetic, environmental, and host-related risk factors play a pivotal role in the development of gastrointestinal pathology, yet the initiation of pathology coupled with the complex interplay between the microbiome and host makes the precise pathophysiology of disease difficult to assess at the individual level. Impaired intestinal barrier function appears to be a central characteristic of common gastrointestinal diseases as summarized in this review. The field of gastroenterology continues to evolve as a more precise characterization of intestinal barrier function in the context of gastrointestinal disease is uncovered. This will improve our ability to understand the complex role of intestinal barrier abnormalities and design therapeutic interventions to restore barrier function. Novel therapies may be developed to target bowel permeability which address a primary disease trigger without the side effects of traditional long-term immunosuppressive therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujimori S, Velikova TV S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2328] [Cited by in RCA: 2895] [Article Influence: 321.7] [Reference Citation Analysis (0)] |
| 2. | McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med. 2015;21:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Kopp ZA, Jain U, Van Limbergen J, Stadnyk AW. Do antimicrobial peptides and complement collaborate in the intestinal mucosa? Front Immunol. 2015;6:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 628] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1322] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 6. | Travis S, Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond). 1992;82:471-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 222] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 542] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 8. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 629] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 11. | Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919-G928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 12. | Katouzian F, Sblattero D, Not T, Tommasini A, Giusto E, Meiacco D, Stebel M, Marzari R, Fasano A, Ventura A. Dual sugar gut-permeability testing on blood drop in animal models. Clin Chim Acta. 2005;352:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | van Elburg RM, Uil JJ, Kokke FT, Mulder AM, van de Broek WG, Mulder CJ, Heymans HS. Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr. 1995;20:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bjarnason I, O'Morain C, Levi AJ, Peters TJ. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983;85:318-322. [PubMed] |
| 15. | Elia M, Behrens R, Northrop C, Wraight P, Neale G. Evaluation of mannitol, lactulose and 51Cr-labelled ethylenediaminetetra-acetate as markers of intestinal permeability in man. Clin Sci (Lond). 1987;73:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, Bethge J, Böttner M, Klose J, Milla PJ. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014; 147: 1012-20. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 18. | Rasmussen DN, Karstensen JG, Riis LB, Brynskov J, Vilmann P. Confocal Laser Endomicroscopy in Inflammatory Bowel Disease--A Systematic Review. J Crohns Colitis. 2015;9:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1106] [Article Influence: 122.9] [Reference Citation Analysis (1)] |
| 20. | Shouval DS, Rufo PA. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017;171:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3338] [Article Influence: 185.4] [Reference Citation Analysis (11)] |
| 22. | Yamamoto S, Ma X. Role of Nod2 in the development of Crohn's disease. Microbes Infect. 2009;11:912-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3899] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 24. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3472] [Article Influence: 144.7] [Reference Citation Analysis (1)] |
| 25. | Floyd DN, Langham S, Séverac HC, Levesque BG. The economic and quality-of-life burden of Crohn's disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60:299-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Feagan BG, Bala M, Yan S, Olson A, Hanauer S. Unemployment and disability in patients with moderately to severely active Crohn's disease. J Clin Gastroenterol. 2005;39:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 394] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 30. | Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 209] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn's disease of the terminal ileum and colon. Digestion. 1983;27:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1844] [Cited by in RCA: 1873] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 33. | Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1851] [Cited by in RCA: 1898] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 34. | Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 599] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 35. | Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1051] [Cited by in RCA: 990] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 36. | Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1125] [Cited by in RCA: 1178] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 38. | Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969-4976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 651] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 39. | Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Müller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 949] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 41. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 391] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 42. | Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 44. | Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology. 1996;110:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Monsén U, Bernell O, Johansson C, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with Crohn's disease. Scand J Gastroenterol. 1991;26:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH Jr. A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537-547. [PubMed] |
| 49. | Peeters M, Ghoos Y, Maes B, Hiele M, Geboes K, Vantrappen G, Rutgeerts P. Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig Dis Sci. 1994;39:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 2017; 153: 723-731. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 51. | Vogelsang H. Do changes in intestinal permeability predict disease relapse in Crohn's disease? Inflamm Bowel Dis. 2008;14 Suppl 2:S162-S163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 449] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 53. | D'Incà R, Di Leo V, Corrao G, Martines D, D'Odorico A, Mestriner C, Venturi C, Longo G, Sturniolo GC. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am J Gastroenterol. 1999;94:2956-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Pironi L, Miglioli M, Ruggeri E, Levorato M, Dallasta MA, Corbelli C, Nibali MG, Barbara L. Relationship between intestinal permeability to [51Cr]EDTA and inflammatory activity in asymptomatic patients with Crohn's disease. Dig Dis Sci. 1990;35:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Gerova VA, Stoynov SG, Katsarov DS, Svinarov DA. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J Gastroenterol. 2011;17:2211-2215. [PubMed] [DOI] [Full Text] |
| 56. | Turcotte JF, Wong K, Mah SJ, Dieleman LA, Kao D, Kroeker K, Claggett B, Saltzman JR, Wine E, Fedorak RN, Liu JJ. Increased epithelial gaps in the small intestine are predictive of hospitalization and surgery in patients with inflammatory bowel disease. Clin Transl Gastroenterol. 2012;3:e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Benjamin J, Makharia GK, Ahuja V, Kalaivani M, Joshi YK. Intestinal permeability and its association with the patient and disease characteristics in Crohn's disease. World J Gastroenterol. 2008;14:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Hilsden RJ, Meddings JB, Hardin J, Gall DG, Sutherland LR. Intestinal permeability and postheparin plasma diamine oxidase activity in the prediction of Crohn's disease relapse. Inflamm Bowel Dis. 1999;5:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Söderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Morson BC. The early histological lesion of Crohn's disease. Proc R Soc Med. 1972;65:71-72. [PubMed] |
| 62. | Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Keita AV, Salim SY, Jiang T, Yang PC, Franzén L, Söderkvist P, Magnusson KE, Söderholm JD. Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn's disease. J Pathol. 2008;215:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Nazli A, Wang A, Steen O, Prescott D, Lu J, Perdue MH, Söderholm JD, Sherman PM, McKay DM. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect Immun. 2006;74:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Porras M, Martín MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis. 2006;12:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Na SY, Moon W. Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease. Gut Liver. 2019;13:604-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 67. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1186] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 68. | Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 728] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 69. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 767] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 70. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3047] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 71. | Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, Van der Woude J, Baert F, Eliakim R, Katsanos K, Brynskov J, Steinwurz F, Danese S, Vermeire S, Teillaud JL, Lémann M, Chowers Y. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 72. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 73. | Andersen NN, Jess T. Risk of infections associated with biological treatment in inflammatory bowel disease. World J Gastroenterol. 2014;20:16014-16019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 74. | Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (1)] |
| 75. | Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF Therapy in Crohn's Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 76. | Boland K, Bedrani L, Turpin W, Kabakchiev B, Stempak J, Borowski K, Nguyen G, Steinhart AH, Smith MI, Croitoru K, Silverberg MS. Persistent Diarrhea in Patients With Crohn's Disease After Mucosal Healing Is Associated With Lower Diversity of the Intestinal Microbiome and Increased Dysbiosis. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 437] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 78. | Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 79. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 894] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 80. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1130] [Article Influence: 161.4] [Reference Citation Analysis (1)] |
| 81. | Wegh CAM, de Roos NM, Hovenier R, Meijerink J, Besseling-van der Vaart I, van Hemert S, Witteman BJM. Intestinal Permeability Measured by Urinary Sucrose Excretion Correlates with Serum Zonulin and Faecal Calprotectin Concentrations in UC Patients in Remission. J Nutr Metab. 2019;2019:2472754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Welcker K, Martin A, Kölle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456-460. [PubMed] |
| 83. | Büning C, Geissler N, Prager M, Sturm A, Baumgart DC, Büttner J, Bühner S, Haas V, Lochs H. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;18:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Fausel R, Afzali A. Biologics in the management of ulcerative colitis - comparative safety and efficacy of TNF-α antagonists. Ther Clin Risk Manag. 2015;11:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | National Institute of Diabetes and Digestive and Kidney Diseases. Definition & Facts for Irritable Bowel Syndrome. Available from: https://www.niddk.nih.gov/health-information/digestive-diseases/irritable-bowel-syndrome/definition-facts?dkrd=hispt0257. |
| 86. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712-721. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (2)] |
| 87. | Paré P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, Kelly S, McBurney CR. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726-35; discussion 1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Mikocka-Walus A, Turnbull D, Moulding N, Wilson I, Andrews JM, Holtmann G. Psychological comorbidity and complexity of gastrointestinal symptoms in clinically diagnosed irritable bowel syndrome patients. J Gastroenterol Hepatol. 2008;23:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 90. | Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, Lindberg G, Karling P, Ohlsson B, Gazouli M, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Camilleri M, Locke GR, Talley NJ, D'Amato M, Ackerman MJ, Farrugia G. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 91. | Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM; Walkerton Health Study Investigators. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 92. | Aziz I, Mumtaz S, Bholah H, Chowdhury FU, Sanders DS, Ford AC. High Prevalence of Idiopathic Bile Acid Diarrhea Among Patients With Diarrhea-Predominant Irritable Bowel Syndrome Based on Rome III Criteria. Clin Gastroenterol Hepatol 2015; 13: 1650-5. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 93. | Sykes MA, Blanchard EB, Lackner J, Keefer L, Krasner S. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. J Behav Med. 2003;26:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105-14125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (3)] |
| 95. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 96. | Lee H, Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Chae SW. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19:244-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 817] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 98. | Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. 2014;49:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 100. | Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, O'Neill J, Carlson P, Lamsam J, Eckert D, Janzow D, Burton D, Ryks M, Rhoten D, Zinsmeister AR. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1262-G1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Shanahan F, Quigley EM. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology. 2014;146:1554-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 102. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 103. | Gecse K, Róka R, Séra T, Rosztóczy A, Annaházi A, Izbéki F, Nagy F, Molnár T, Szepes Z, Pávics L, Bueno L, Wittmann T. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 105. | Mujagic Z, Ludidi S, Keszthelyi D, Hesselink MA, Kruimel JW, Lenaerts K, Hanssen NM, Conchillo JM, Jonkers DM, Masclee AA. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014;40:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Peters SA, Edogawa S, Sundt WJ, Dyer RB, Dalenberg DA, Mazzone A, Singh RJ, Moses N, Smyrk TC, Weber C, Linden DR, MacNaughton WK, Turner JR, Camilleri M, Katzka DA, Farrugia G, Grover M. Constipation-Predominant Irritable Bowel Syndrome Females Have Normal Colonic Barrier and Secretory Function. Am J Gastroenterol. 2017;112:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Zhao DY, Qi QQ, Long X, Li X, Chen FX, Yu YB, Zuo XL. Ultrastructure of intestinal mucosa in diarrhea-predominant irritable bowel syndrome. Physiol Int. 2019;106:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 109. | Bertrand J, Ghouzali I, Guérin C, Bôle-Feysot C, Gouteux M, Déchelotte P, Ducrotté P, Coëffier M. Glutamine Restores Tight Junction Protein Claudin-1 Expression in Colonic Mucosa of Patients With Diarrhea-Predominant Irritable Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2016;40:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 111. | Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 112. | Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28-35; quiz 36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 113. | Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 114. | Aragon G, Graham DB, Borum M, Doman DB. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2010;6:39-44. [PubMed] |
| 115. | Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (3)] |
| 116. | American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 117. | Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 118. | Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, Lacouture ME. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 119. | Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 120. | Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M, Mortier L, Coutzac C, Soularue E, Lanoy E, Kapel N, Planchard D, Chaput N, Robert C, Carbonnel F. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 121. | Abu-Sbeih H, Faleck DM, Ricciuti B, Mendelsohn RB, Naqash AR, Cohen JV, Sellers MC, Balaji A, Ben-Betzalel G, Hajir I, Zhang J, Awad MM, Leonardi GC, Johnson DB, Pinato DJ, Owen DH, Weiss SA, Lamberti G, Lythgoe MP, Manuzzi L, Arnold C, Qiao W, Naidoo J, Markel G, Powell N, Yeung SJ, Sharon E, Dougan M, Wang Y. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J Clin Oncol. 2020;38:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 122. | Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1060] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 123. | Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 965] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 124. | Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 621] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 125. | Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, Wang YP, Chen MH. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 126. | Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110:517-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1147] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 127. | Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 128. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1096] [Article Influence: 68.5] [Reference Citation Analysis (1)] |
| 129. | Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes--a solid or leaky concept? Pediatr Diabetes. 2015;16:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 130. | Tajik N, Frech M, Schulz O, Schälter F, Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Krönke G, Luo Y, Überla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Cañete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11:1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 131. | Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 377] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 132. | Ma TY, Hollander D, Krugliak P, Katz K. PEG 400, a hydrophilic molecular probe for measuring intestinal permeability. Gastroenterology. 1990;98:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand. 2004;182:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 134. | Meddings JB, Sutherland LR, Byles NI, Wallace JL. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology. 1993;104:1619-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 135. | Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratù R. Intestinal permeability in Crohn's disease patients and their first degree relatives. Dig Liver Dis. 2001;33:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Jenkins RT, Jones DB, Goodacre RL, Collins SM, Coates G, Hunt RH, Bienenstock J. Reversibility of increased intestinal permeability to 51Cr-EDTA in patients with gastrointestinal inflammatory diseases. Am J Gastroenterol. 1987;82:1159-1164. [PubMed] |
| 137. | Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |