Published online Apr 28, 2017. doi: 10.4329/wjr.v9.i4.199
Peer-review started: August 29, 2016
First decision: December 13, 2016
Revised: January 11, 2017
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: April 28, 2017
Processing time: 244 Days and 19.7 Hours
To evaluate the correlation between degree of kinetic growth (kGR) of the liver following portal vein embolization (PVE) liver and the enhancement of the during the hepatobiliary phase of contrast administration and to evaluate if the enhancement can be used to predict response to PVE prior to the procedure.
Seventeen patients were consented for the prospective study. All patients had an MR of the abdomen with Gd-EOB-DTPA. Fourteen patients underwent PVE. The correlation between the kGR of the liver and the degree of enhancement was evaluated with linear regression (strong assumptions) and Spearman’s correlation test (rank based, no assumptions). The correlation was examined for the whole liver, segments I, VIII, VII, VI, V, IV, right liver and left liver.
There was no correlation between the degree of enhancement during the hepatobiliary phase and kGR for any segment, lobe of the liver or whole liver (P = 0.19 to 0.91 by Spearman’s correlation test).
The relative enhancement of the liver during the hepatobiliary phase with Gd-EOB-DTPA cannot be used to predict the liver response to PVE.
Core tip: Our hypothesis was that the degree of enhancement of the liver during the hepatobiliary phase will correlate with the degree of liver response to portal vein embolization. This will be able to be used as a screen method for patients scheduled for portal vein embolization (PVE). The use of Gd-EOB-DTPA in the assessment of liver function has been correlated with clinical assessment of liver function classification. We evaluated the correlation between degree of kinetic growth (kGR) of the liver following PVE liver and the enhancement of the during the hepatobiliary phase of contrast administration. There was no correlation between the degree of enhancement during the hepatobiliary phase and kGR.
- Citation: Szklaruk J, Luersen G, Ma J, Wei W, Underwood M. Gd-EOB-DTPA based magnetic resonance imaging for predicting liver response to portal vein embolization. World J Radiol 2017; 9(4): 199-205
- URL: https://www.wjgnet.com/1949-8470/full/v9/i4/199.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i4.199
Portal vein embolization (PVE) is performed to redirect portal flow to the liver remnant in order to increase liver volume. PVE is increasingly used to induce hypertrophy of the anticipated liver remnant in the management of patients with liver metastases undergoing liver resection. The rationale for PVE is to reduce suboptimal post-resection liver size and resulting morbidities[1-5].
The minimum reported safe functioning liver remnant (FLR) is 20% of total liver volume (TLV) in patients with normal liver and 40% of TLV in compromised liver such as cirrhotic patients[1,2,6]. The evaluation of FLR following PVE is recommended at 21 d following the procedure[2,7]. At this time, a FLR of less than 20% or a degree of hypertrophy of less than 5% predicted the likelihood of hepatic resection dysfunction. These patients with suboptimal FLR are reported to have major liver-centered complications, including hepatic dysfunction, and insufficiency following surgery[2,7].
In addition to the FLR, kinetic growth rate (kGR) has been reported to be a better predictor of postoperative morbidity and mortality after liver resection for small FLR than conventional measured volume parameters[7,8]. The kGR calculates the change in FLR as function of time. A kGR of < 2% per week correlates with poor rates of hepatic insufficiency and liver-related 90-d mortality[7,9-11]. At this time there is no predictor of liver hypertrophy response to PVE prior to the procedure. This results in unnecessary PVE in the patient population that do not response to treatment. These unnecessary PVE have inherent morbidities[1,3,6].
Gadoxetic acid disodium (Gd-EOB-DTPA) is a hepatobiliary contrast agent. The enhancement of the liver with Gd-EOB-DTPA depends on liver function[9-13].
The purpose of this project is to evaluate the response to PVE (based on kGR calculations) and the degree of hepatic function (based on the enhancement of the liver with Gd-EOB-DTPA). Our hypothesis is that the degree of enhancement of the liver following the intravenous administration of Gd-EOB-DTPA at the hepatobiliary phase will correlate and predict the kinetic growth rate of the liver following portal vein embolization. This prediction in kGR will allow the selection of patients who will respond to PVE. This will then limit a number of unnecessary PVE procedures for patients that predictably will not respond to treatment.
This is a prospective IRB approved project. The inclusion criteria were all patients who were scheduled for a PVE. Patients who consented to this project were offered an MR examination of the liver with Gd-EOB-DTPA. This MR was performed before the PVE procedure.
All patients had an MR examination of the liver with Gd-EOB-DTPA (Table 1). All MR exams were performed in the same scanner at 1.5T (GE Wisconsin, United States). The examination consisted of T1 (in/out-of-phase at 5/0 mm), T2 (Fast Spine Echo at 5/0 mm), DWI at b = 50, 400, 800 mm2/s, and pre- and post- pre-contrast and post-Gd-EOB-DTPA injected at 1 cc/s. 3D spoiled gradient echo Liver Acquisition Volume Acquisition (LAVA, GEMS, Milwaukee Wisconsin). The LAVA images were obtained during the late arterial phase, portal venous phase, delayed phase, excretory phase, 10 min and 20 min post-Gd-EOB-DTPA. For an internal standard all images were acquired with a test tube (1 cm × 10 cm) of Gd-EOB-DTPA diluted with water placed on the side of the patient. This was used to standardize signal intensity between the different phases of contrast administration.
| T1 (OOP) 2D FSPGR | T1 (IP) 2D FSPGR | T2 (FS) | Pre- and Post-Gd 3D FSPGR | DWI EPI (B- 0, 400, 800) | |
| # ECHOES/SHOTS | 2 | 2 | 1 | 1 | 1 |
| TE1/TE2 (ms) | 2.2-2.4 | 4.2-4.8 | 85 | min | min (about 50-60) |
| TR/#R-R (ms) | 120 | 120 | |||
| FLIP ANGLE | 85 | 85 | 15 | ||
| ETL | 16 | ||||
| FOV (cm) | 48 | 48 | 48 | 48 | 48 |
| SCAN THK (mm) | 6 | 6 | 6 | 5-6 (-2.5) | 6 |
| FREQ × PHASE | 256 × 160 | 256 × 160 | 256 × 160 | 256 × 128 | 100 × 160 |
| NEX | 1 | 1 | 4 | 1 | 1, 4, 6 |
| PHASE FOV | 0.9-1.0 | 0.9-1.0 | 0.75-1.0 | 0.75-1.0 | 0.75-1.0 |
One radiologist with over 20 years of experience in body MR placed multiple regions of interests in the liver. The diameter of the ROI in the liver measure ranged from 1 to 2 cm. The ROI in the liver were placed outside major vessels, bile ducts, or liver masses. A ROI was also placed in the external test tube. Multiple ROIs were placed in each patient. One ROI was placed for each liver segment evaluated (IV, V, VI, VII, VIII) and one for segments II/III. The multiple ROIs were placed to evaluate the correlation of segmental enhancement of the liver with kGR.
The percentage of enhancement (%E) was calculated by subtracting the signal intensity (SI) during the hepatobiliary phase (HBP) from the SI during the pre- contrast phase corrected by the signal intensity of the external test tube (t): The %E was calculated for segments VIII, VII, VI, V, IV, left liver average, right liver average and whole liver average. % E (segment-x) = [SIhbp)/ SIt) (segment-x) - (SIpre/SIt) (segment-x)]/(SIpre/SIt) (segment-x) x 100.
The kGR was calculated by evaluating the degree of hypertrophy (DH) divided by the time period (in days) from the PVE to the post-PVE scan: kGR = DH/Time Period (days)[7]. DH was calculated by comparing the FLR post-PVE minus FLR pre-PVE: DH = % FLRpost-PVE - % FLRpre-PVE[7]. The functional liver reserve for time period (i) was calculated: FLRi = (FLRi/sTLV)[7]. The standardized total liver volume (sTLV) was calculated: sTLV = 794.41 + 1267.28 x body surface area (m2)[2,7]. One radiologist with over 20 years of experience in body imaging demarcated the segments. The segmental and total liver volumes were calculated from the axial MR/CT images with standard software: GE Advantage Workstation AW4.1_06 Volume Viewer Voxtool 3.0.64z (General Electric, Wisconsin, United States)[2,7].
Summary statistics of enhancement and kGR were provided in mean, SD, and range by site. Association between kGR and enhancement during the hepatobiliary phase were estimated using linear regression (linearity and normality assumptions) and Spearman’s correlation test (rank based, no assumptions). All tests were two-sided and P values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9 (SAS Institute, Cary, NC). Biomedical statistical review was performed by one of the authors, Mr. Wei W, who is a biomedical statistician.
Seventeen patients were consented for this prospective project: 10 males and 17 females. Age range was 21-65 years old. The primary diagnosis was colorectal cancer in all patients. Three patients did not undergo a portal vein embolization and were therefore excluded.
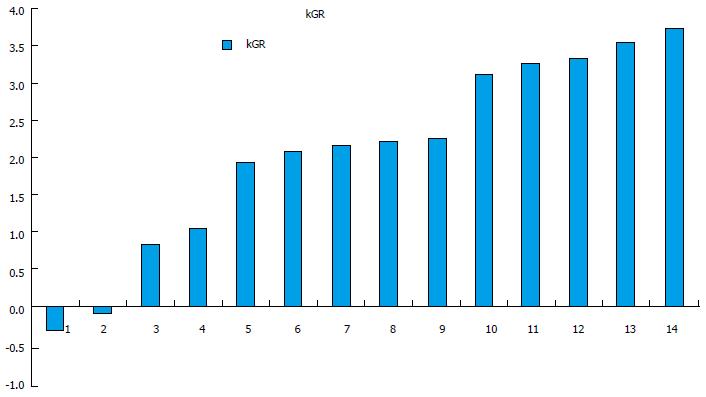
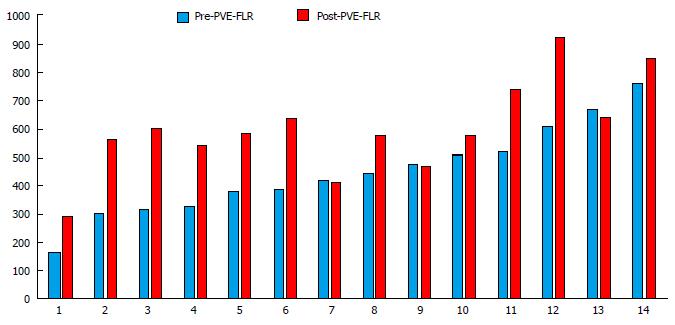
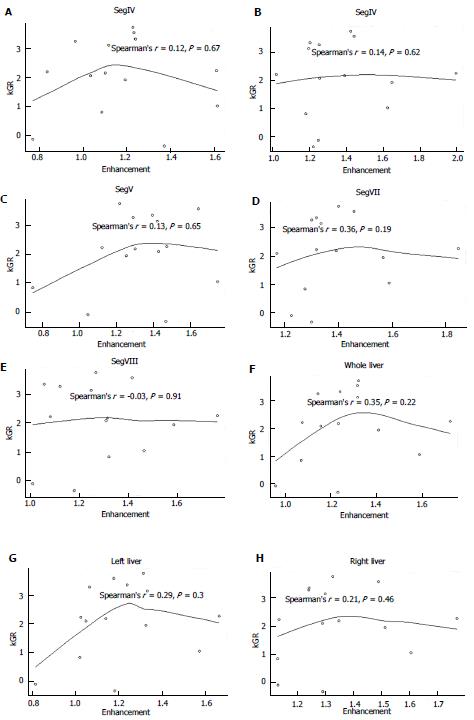
The % E for each segment, lobe and whole liver is shown in Table 2. The %E ranged from 82% to 199%. For all patients, the kGR ranged from -0.34 to 3.73 (Figure 1). The average kGR was 1.97%. Nine patients were above the 2% cut-off for decreased morbidity[7]. The FLR pre-PVE and post-PVE is shown in Figure 2. The relationship between the kGR and the degree of enhancement for various segments, lobes and whole liver are shown in Figure 3 and Table 3. Based on linear regression (strong assumptions) and Spearman’s correlation test (rank based, no assumptions), there was no significant correlation between enhancement and KGR.
| Average left liver | Average right liver | Average whole liver | SEG IV | SEG VIII | SEG VII | SEG V | SEG VI |
| 118.27 | 129.14 | 123.1 | 137.3 | 118.06 | 129.77 | 146.59 | 122.15 |
| 106.69 | 124.13 | 114.44 | 96.6 | 112.28 | 129.85 | 129.01 | 125.38 |
| 131.41 | 132.71 | 132.15 | 122.9 | 126.81 | 139.86 | 121.72 | 142.46 |
| 133.22 | 130 | 131.79 | 111.85 | 124.87 | 133.4 | 142.06 | 119.66 |
| 166.19 | 176.9 | 172.31 | 161.17 | 176.29 | 184.49 | 147.19 | 199.65 |
| 81.83 | 113.24 | 95.79 | 77.14 | 101.13 | 122.32 | 104.6 | 124.92 |
| 102.63 | 113.57 | 107.5 | 83.77 | 108.26 | 131.64 | 112.28 | 102.11 |
| 157.2 | 160.56 | 158.88 | 161.62 | 146.42 | 158.69 | 174.57 | 162.57 |
| 114.15 | 134.94 | 123.39 | 110.28 | 131.55 | 138.96 | 130.07 | 139.16 |
| 104.98 | 129.04 | 115.68 | 103.53 | 130.94 | 116.86 | 142.72 | 125.66 |
| 117.83 | 148.94 | 131.66 | 123.38 | 141.55 | 145.57 | 164.26 | 144.37 |
| 132.54 | 151.34 | 140.9 | 119.4 | 158.55 | 156.5 | 125.41 | 164.92 |
| 102.2 | 113.12 | 107.06 | 108.72 | 132.15 | 127.38 | 74.83 | 118.13 |
| 123.9 | 124.27 | 124.07 | 124.09 | 105.84 | 131.57 | 139.36 | 120.32 |
| Site | Estimated Slope | 95% LCL | 95% UCL | P value |
| Left liver | 1.43 | -2.14 | 5.01 | 0.4 |
| Right liver | 1.08 | -3.24 | 5.4 | 0.6 |
| SegIV | 0.04 | -3.3 | 3.38 | 0.98 |
| SegV | 1.23 | -1.98 | 4.43 | 0.42 |
| SegVI | 0.35 | -2.92 | 3.62 | 0.82 |
| SegVII | 1.05 | -3.59 | 5.69 | 0.63 |
| SegVIII | 0.49 | -3.41 | 4.4 | 0.79 |
| Whole liver | 1.37 | -2.57 | 5.32 | 0.46 |
Our hypothesis was that the degree of enhancement of the liver during the hepatobiliary phase will correlate with the degree of liver response to portal vein embolization. Our results, unfortunately, did not support our hypothesis.
A possibility that our hypothesis was not demonstrated is that the patient population was not representative of the published data on portal vein embolization. However, the average kGR of 1.97% in our study compares favorably with the reported average kGR in the initial publications on PVE of 2.4[7]. In addition, the cut-off of 2% in kGR is reported as the threshold for complications and hepatic failure[7]. In our patient population 9 patients were above the threshold and 6 patients were below the threshold. This is a relative low number of patients but this was distributed between the < 2% or > 2% kGR group. The range of kGR in our study population of -0.33%-3.73% was narrower than that on the prior reports of (0.2-9.4%)[7]. The average DH of our patient population was 9.60%. This also compares favorably with the DH of 10.1% on the original report[7]. The range of DH on our patient population of -1.3%-17.8% was narrower than on prior results (0.1%-39.9%)[7,14]. In summary, our patient population appears to represent the two groups of responders and non-responders to PVE.
The enhancement of the liver with Gd-EOB-DTPA depends on the expression of various transporters[11,15]. This includes organic anion transport factor 8 and organic transporter TP[11,15-17]. The enhancement also depends on the expression of multidrug resistance protein 2[16,17]. The use of Gd-EOB-DTPA to assess liver function has been reported following portal vein embolization[18,19].
The use of Gd-EOB-DTPA in the assessment of liver function has been correlated with clinical assessment of liver function such as in the evaluation of the degree of cirrhosis and in the stratification with the Child-Pugh classification[10,20,21]. The lack of correlation between the degree of liver enhancement and response to PVE seems to indicate that the response of hypertrophy of the liver to PVE is not only based on liver function but also on other factors such as clonal activity of the hepatocytes. This clonal activity does not affect liver enhancement with Gd-EOB-DTPA.
In conclusion, it is unfortunate that the enhancement of the liver during the hepatobiliary phase did not predict response to treatment with PVE. This would have resulted in a robust screening process for patients schedule for a PVE. Our result should alert other groups to seek alternative screening test to PVE that include evaluation of clonal activity rather than functional liver activity. Although Gd-EOB-DTPA has increasingly shown to be a very powerful tool for the evaluation of liver disease, the enhancement of this agent during the hepatobiliary phase does not predict the degree of liver hypertrophy following PVE.
Ms. Palencia Lewis in the preparation of the documents.
Hepatic resection is commonly used to cure metastatic disease to the liver. The success of the resection depends on the functional liver reserve post-hepatectomy. To decreased morbidity portal vein embolization is commonly used. Not all patients respond to portal vein embolization (PVE) and PVE has inherent risk factors. The authors were looking for a method to predict response to PVE. Gd-EOB-DTPA is a hepatobiliary agent for MR. The enhancement at 20 min post-Gd has been associated with liver function. The authors wanted to explore if Gd-EOB-DTPA enhanced MR could provide information to predict which patients will response to PVE. This may be then used as a screening tool for patients undergoing PVE.
This is a novel project and the area there are no publications on prediction of response to PVE with MR.
The results of this project did not prove the hypothesis. The enhancement on the hepatobiliary phase of Gd-EOB-DTPA enhanced MR cannot predict liver response to PVE. The breakthrough is that very likely the response of the liver to PVE is likely a clonal response rather than a liver function response.
The results did not prove the hypothesis. This suggests that new methodology should be considered to evaluate predictors of response to PVE. This new methodology may include evaluation of clonal activity rather than liver function.
PVE: Procedure performed to induce regrowth on one side of the liver in advance of a planned hepatic resection on the other side. This is frequently used in hepatomas and colorectal metastases; Kinetic growth rate (kGR): Defined as the degree of liver hypertrophy at initial volume assessment divided by number of weeks elapsed after PVE; Gd-EOB-DTPA: Is the only approved liver specific MR contrast agent. Enhancement at the hepatobiliary phase, at 20 min, correlates with the degree of liver function. The enhancement is also a function of expression of OTPB and multidrug resistance proteins.
The authors evaluated the response to PVE (based on kGR calculations) and the degree of hepatic function (based on the enhancement of the liver with Gd-EOB-DTPA). Their hypothesis is that the degree of enhancement of the liver following the intravenous administration of Gd-EOB-DTPA at the hepatobiliary phase will correlate and predict the kinetic growth rate of the liver following portal vein embolization. They demonstrated that although Gd-EOB-DTPA has increasingly shown to be a very powerful tool for the evaluation of liver disease, the enhancement of this agent during the hepatobiliary phase does not predict the degree of liver hypertrophy following PVE.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Akamatsu N, Li YZ, Tarazov PG S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Ganeshan DM, Szklaruk J. Portal vein embolization: cross-sectional imaging of normal features and complications. AJR Am J Roentgenol. 2012;199:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Laméris JS, Gouma DJ. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Madoff DC, Gaba RC, Weber CN, Clark TW, Saad WE. Portal Venous Interventions: State of the Art. Radiology. 2016;278:333-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Avritscher R, Duke E, Madoff DC. Portal vein embolization: rationale, outcomes, controversies and future directions. Expert Rev Gastroenterol Hepatol. 2010;4:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Croome KP, Hernandez-Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford). 2015;17:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Jhaveri K, Cleary S, Audet P, Balaa F, Bhayana D, Burak K, Chang S, Dixon E, Haider M, Molinari M. Consensus statements from a multidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid). AJR Am J Roentgenol. 2015;204:498-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Kim HY, Choi JY, Park CH, Song MJ, Song DS, Kim CW, Bae SH, Yoon SK, Lee YJ, Rha SE. Clinical factors predictive of insufficient liver enhancement on the hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging in patients with liver cirrhosis. J Gastroenterol. 2013;48:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Nilsson H, Blomqvist L, Douglas L, Nordell A, Janczewska I, Näslund E, Jonas E. Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br J Radiol. 2013;86:20120653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Geisel D, Lüdemann L, Keuchel T, Malinowski M, Seehofer D, Stockmann M, Hamm B, Gebauer B, Denecke T. Increase in left liver lobe function after preoperative right portal vein embolisation assessed with gadolinium-EOB-DTPA MRI. Eur Radiol. 2013;23:2555-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Saito K, Ledsam J, Sourbron S, Otaka J, Araki Y, Akata S, Tokuuye K. Assessing liver function using dynamic Gd-EOB-DTPA-enhanced MRI with a standard 5-phase imaging protocol. J Magn Reson Imaging. 2013;37:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Zeile M, Bakal A, Volkmer JE, Stavrou GA, Dautel P, Hoeltje J, Stang A, Oldhafer KJ, Brüning R. Identification of cofactors influencing hypertrophy of the future liver remnant after portal vein embolization-the effect of collaterals on embolized liver volume. Br J Radiol. 2016;89:20160306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, Taura K, Yasuchika K, Nitta T, Ikai I. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 16. | Kimura Y, Sato S, Hitomi E, Ohyama M, Adachi K, Inagaki Y, Yamakawa Y, Hirano A, Kawai H, Tsuchida K. Coexpression of organic anion-transporting polypeptides 1B3 and multidrug-resistant proteins 2 increases the enhancement effect of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid on hepatocellular carcinoma in magnetic resonance imaging. Hepatol Res. 2014;44:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Tsuda N, Harada K, Matsui O. Effect of change in transporter expression on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging during hepatocarcinogenesis in rats. J Gastroenterol Hepatol. 2011;26:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Akiba A, Murata S, Mine T, Onozawa S, Sekine T, Amano Y, Kawano Y, Uchida E, Kumita S. Volume change and liver parenchymal signal intensity in Gd-EOB-DTPA-enhanced magnetic resonance imaging after portal vein embolization prior to hepatectomy. Biomed Res Int. 2014;2014:684754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Geisel D, Raabe P, Lüdemann L, Malinowski M, Stockmann M, Seehofer D, Pratschke J, Hamm B, Denecke T. Gd-EOB-DTPA-enhanced MRI for monitoring future liver remnant function after portal vein embolization and extended hemihepatectomy: A prospective trial. Eur Radiol. 2016; Dec 13; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Kamimura K, Fukukura Y, Yoneyama T, Takumi K, Tateyama A, Umanodan A, Shindo T, Kumagae Y, Ueno S, Koriyama C. Quantitative evaluation of liver function with T1 relaxation time index on Gd-EOB-DTPA-enhanced MRI: comparison with signal intensity-based indices. J Magn Reson Imaging. 2014;40:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Lee S, Choi D, Jeong WK. Hepatic enhancement of Gd-EOB-DTPA-enhanced 3 Tesla MR imaging: Assessing severity of liver cirrhosis. J Magn Reson Imaging. 2016;44:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |