Published online Sep 28, 2015. doi: 10.4329/wjr.v7.i9.286
Peer-review started: March 4, 2015
First decision: April 24, 2015
Revised: May 12, 2015
Accepted: July 18, 2015
Article in press: July 27, 2015
Published online: September 28, 2015
Processing time: 224 Days and 23.8 Hours
AIM: To evaluate the computed tomography (CT) features of intraperitoneal tuberculous abscess (IPTA).
METHODS: Eight patients with IPTA confirmed by pathology were analyzed retrospectively. The clinical symptoms, medical images, and surgical findings were evaluated. Involvement of the intestine, peritoneum, viscera, and lymph nodes was also assessed.
RESULTS: All 8 patients had a history of abdominal discomfort for 1 to 6 mo. Physical examination revealed a palpable abdominal mass in 6 patients. Three patients had no evidence of pulmonary tuberculosis (TB). All IPTAs (11 abscesses) were seen as a multiseptated, peripherally enhanced, hypodense mass with enlarged, rim-enhanced lymph nodes. The largest abscess diameter ranged from 4.5 cm to 12.2 cm. CT showed 2 types of IPTA: Lymph node fusion and encapsulation. Of the 8 patients, one had liver tuberculosis and one had splenic and ovarian tuberculosis. Two cases showed involvement of the terminal ileum and ileocecal junction. Ascites were found in 4 cases. Three patients had peritonitis and mesenteritis. Three patients showed involvement of the omentum. Three patients had histological evidence of caseating granuloma, and 5 had histological evidence of acid-fast bacilli.
CONCLUSION: CT is crucial in the detection and characterization of IPTA. Certain CT findings are necessary for correct diagnosis.
Core tip: Intra-peritoneal tuberculous abscess (IPTA) is a rare and serious form of extra-pulmonary tuberculosis. Early and accurate diagnosis of tuberculous abscess is important for treatment. This retrospective study was to evaluate the computed tomography (CT) features in 8 patients with pathologically confirmed IPTA. CT is crucial in the detection and characterization of IPTA. Although the qualitative diagnosis of IPTA requires positive pathologic findings, certain CT features (such as a multiseptated, peripherally enhanced, hypodense mass with rim-enhanced lymph nodes, peritoneum/mesentery/omentum changes) are necessary for correct diagnosis.
- Citation: Dong P, Chen JJ, Wang XZ, Wang YQ. Intraperitoneal tuberculous abscess: Computed tomography features. World J Radiol 2015; 7(9): 286-293
- URL: https://www.wjgnet.com/1949-8470/full/v7/i9/286.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i9.286
Infection with Mycobacterium tuberculosis (TB) is more common in developing countries than in developed countries[1]. In developed countries, TB remains a health care challenge due to human immunodeficiency virus (HIV) infection and immigration from endemic areas[2-5].
Although the most commonly involved organ in TB is the lung, approximately 12.5% of cases are extra-pulmonary, and the abdomen is involved in 11%-16% of patients with extra-pulmonary TB[6]. The more commonly involved sites of abdominal TB are the intestine, peritoneum and lymph nodes[6-8].
Accurate diagnosis of tuberculous peritonitis is crucial because delayed treatment may lead to high risk of death[9]. An intra-peritoneal tuberculous abscess (IPTA) is a rare and serious form of extra-pulmonary TB[6,8,10,11]. Early and accurate diagnosis of tuberculous abscess is important for treatment[12,13]. Only a few non-HIV patients with IPTA have been reported previously[6,14]. IPTA in non-HIV patients is a diagnostic challenge as the clinical symptoms and imaging findings can be mistaken for other peritoneal diseases. If computed tomography (CT) findings were helpful in the differentiation between IPTA and other peritoneal diseases, this would reduce or avoid unnecessary invasive diagnostic procedures such as laparoscopy.
The aim of this retrospective study was to evaluate the CT features in 8 patients with pathologically confirmed IPTA.
The clinical symptoms, CT findings and pathological findings of 8 patients with IPTA identified by pathology, including 6 females and 2 males (age range: 22-58 years) were collected from 2001 to 2013. None of the 8 patients had evidence of HIV infection.
Five patients were examined using a 16-detector CT scanner (Siemens Sensation, Germany). Three patients were examined using a 64-slice CT scanner (Brilliance 64, Philips Medical Systems, the Netherlands). CT parameters were as follows: 140 KV, 220-600 mAs, slice thickness 5 mm, and multi-planar reconstruction (MPR) slice width 1 mm. The CT scan was performed following intravenous administration of contrast material (80-100 mL, Ultravist 300 mgI/mL, Bayer Schering Pharma AG, Berlin, Germany) at an injection rate of 2.0-3.0 mL/s in all patients. Oral contrast material (1.2% Angiografin) was administrated to all patients.
The CT images were retrospectively reviewed by 2 experienced radiologists with consensus. The CT signs included the abscess itself and its relationship with the adjacent structures. The involvement of lymph nodes, peritoneum, mesentery and other organs were also evaluated.
Laparotomy was performed with abscess removal in 2 cases. Laparoscopy was performed in 2 cases and aspiration biopsy was performed in 4 cases. The pathological diagnosis was established on the basis of the following criteria: Histological evidence of caseating granuloma or histological evidence of acid-fast bacilli.
The clinical symptoms of all patients are shown in Table 1. Eight patients reported a history of abdominal discomfort for 1 to 6 mo. One patient had persistent right upper abdominal pain for 10 d. Physical examination revealed a palpable abdominal mass in 6 patients. Three patients had no history of lung TB.
| Case | Age (yr) | Sex | Clinical symptoms | Pulmonary TB | Surgery |
| 1 | 58 | Female | Weight loss, low grade fever and night sweats for 5 mo Persistent right upper quadrant pain for 10 d, accompanied by loss of appetite. Right lower quadrant pain and fullness for 1 mo with a clinically palpable abdominal mass | Hematogenous pulmonary TB | Laparoscopy |
| 2 | 35 | Male | Weight loss, low grade fever, night sweats and obscure abdominal pain for 5 mo with a clinically palpable abdominal mass | - | Laparotomy |
| 3 | 24 | Male | Obscure abdominal pain and low grade fever for 2 mo | - | Laparotomy |
| 4 | 40 | Female | Weight loss, low grade fever, night sweats and obscure abdominal pain for 4 mo, with a clinically palpable abdominal mass | Obsolete pulmonary TB | Laparoscopy |
| 5 | 27 | Female | Obscure abdominal pain and low grade fever for 2 mo with a clinically palpable abdominal mass | - | Biopsy |
| 6 | 22 | Female | Obscure abdominal pain and low grade fever for 2 mo with a clinically palpable abdominal mass | Obsolete pulmonary TB | Biopsy |
| 7 | 37 | Female | Weight loss, low grade fever, night sweats, and obscure abdominal pain for 5 mo with a clinically palpable abdominal mass | Obsolete pulmonary TB | Biopsy |
| 8 | 34 | Female | Weight loss, low grade fever, night sweats, and obscure abdominal pain for 5 mo | Obsolete pulmonary TB | Biopsy |
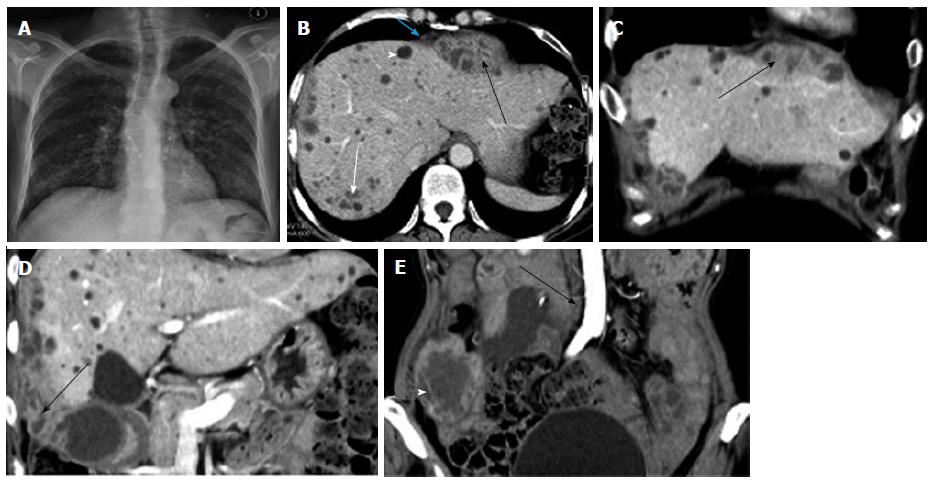
All IPTAs in this study were seen as a multiseptated, peripherally enhanced, hypodense mass with a regular or irregular shape. The location, size, shape, and margin of the abscesses are shown in Table 2. The maximum abscess diameter ranged from 4.5 to 12.2 cm (Figures 1-4).
| Case | Location, size (cm), shape and margin of the abscess | Location of the enlarged lymph nodeand its size (cm) | Number of rim-enhanced lymph nodes |
| 1 | (1) Subphrenic space, 3 × 3.5 × 7.2, irregular shape, the margin was poorly defined (2) Perihepatic space, 5 × 8.9 × 12.2, irregular shape, the margin was poorly defined with adhesion to the gastric wall and gallbladder (3) Lower abdominal cavity, 4.5 × 5.8 × 7.1, irregular shape, the margin was well defined | Pericardial region and para-aortic region; the largest lymph node was < 1 | Small number |
| 2 | Middle-lower abdominal cavity, 6.5 × 6.8 × 7.6, irregular shape, the margin was well defined encasing the mesenteric vessels | Mesenteric root and beside the abscess; the largest lymph node was < 1 | Small number |
| 3 | Peripancreatic region, 3.7 × 4.1 × 4.5, regular shape, the regional margin of the abscess was poorly defined | Peripancreatic and portacaval space; the largest lymph node was 2.7 × 1.8 | Multiple |
| 4 | (1) Lower abdominal cavity, 5.1 × 6.0 × 6.2, irregular shape, the regional margin was poorly defined (2) Lower abdominal cavity, 2.1 × 3.8 × 5.5, irregular shape, the margin was well defined | Lymph nodes clustered in the mesenteric root; the largest lymph node was < 1 | Small number |
| 5 | Peripancreatic region, 3.1 × 3.8 × 5.1, regular shape, the regional margin was poorly defined | Peripancreatic region and the hepatogastric ligament; the largest lymph node was 2.5 × 1.7 | Multiple |
| 6 | Beside the jejunum, 2.5 × 4.2 × 5.0, regular shape, the regional margin was poorly defined encasing the mesenteric vessels | Peripancreatic region and the hepatogastric ligament; the largest lymph node was 2.3 × 1.5 | Multiple |
| 7 | Beside the ileum, 3.2 × 4.0 × 5.1, regular shape; the regional margin was poorly defined encasing the mesenteric vessels | Para-aortic region and mesentery; the largest lymph node was 0.8 × 1.3 | Small number |
| 8 | Peripancreatic region, 3.2 × 3.9 × 5.2, regular shape, the regional margin was poorly defined | Hepatoduodenal ligament, peripancreatic region and mesentery; the largest lymph node was 1.7 × 1.9 | Multiple |
The enlarged lymph nodes had homogeneous enhancement or rim enhancement. The CT findings of the lymph nodes are shown in Table 2. The CT findings of the peritoneal and visceral lesions are shown in Table 3.
| Case | Peritoneum | Ascites | Other TB sites |
| 1 | Irregular or nodular thickening of the diaphragmatic and perihepatic peritoneum with homogeneous enhancement; nodular omentum | Small amount | Liver TB; adjacent cecal wall is thickened |
| 2 | Mesenteric thickening with increasing density of the adjacent mesentery; crowded mesenteric vascular bundles and thickened fiber strands in the mesentery | - | - |
| 3 | Increasing density in the hepatoduodenal ligament | - | - |
| 4 | Mesenteric thickening with increasing density of the adjacent mesentery. Crowded mesenteric vascular bundles and thickened fiber strands in the mesentery | Small amount | Wall of the terminal ileum and ileocecal junction was thickened |
| 5 | Increasing density in the hepatoduodenal ligament | - | - |
| 6 | Mesenteric thickening with increasing density of the adjacent mesentery | - | Splenic and ovarian involvement |
| 7 | Mesenteric thickening with increasing density of the adjacent mesentery and caked omentum. Smooth uniform thickened peritoneum with homogeneous enhancement | Large number | - |
| 8 | Mesenteric thickening with increasing density of the adjacent mesentery; multiple nodular shadows in the mesentery; nodular omentum; irregular or nodular thickening of the peritoneum with homogeneous enhancement | Large number | Wall of the terminal ileum was thickened |
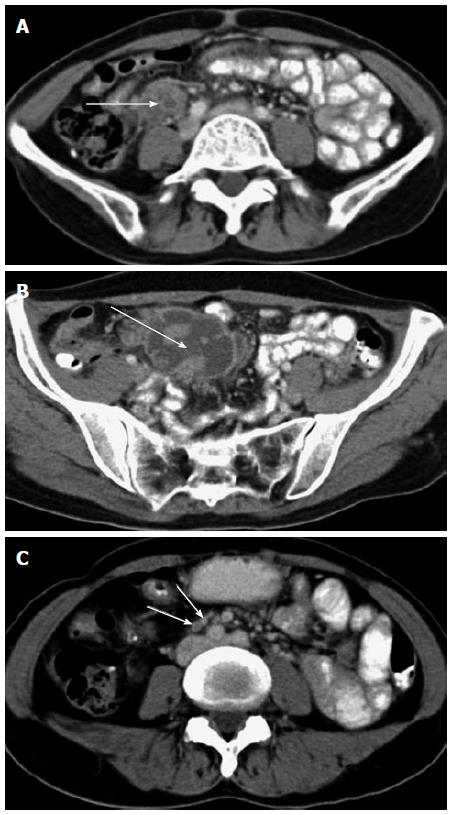
In these 8 patients, a total of 11 abscesses were detected. One patient had 3 abscesses, and the margin of all 3 abscesses was poorly defined. The other 7 patients had 8 abscesses, and their margins were well defined or the regional margin was poorly defined.
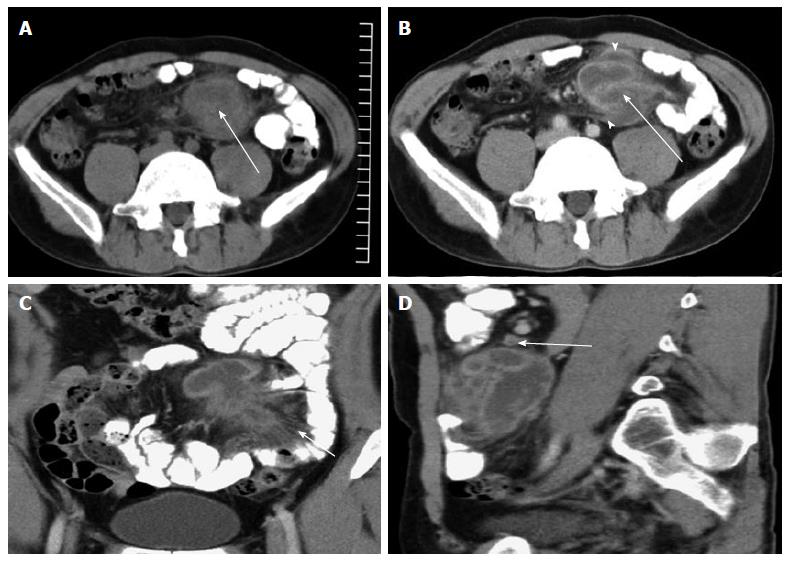
Three cases with abscesses located in the peripancreatic area and one case with an abscess located in the small bowel mesentery had multiple enlarged lymph nodes with rim enhancement and homogeneous enhancement (Figures 1-4).
Four cases with abscesses located in the peritoneal cavity had multiple enlarged lymph nodes, most of which showed homogeneous enhancement and a small number of lymph nodes showed rim enhancement (Figures 1-4).
Of the 8 cases, one was confirmed to have liver TB, and one had splenic and ovarian TB. Two cases showed involvement of the terminal ileum and ileocecal junction. Two of the 8 cases had a small amount of ascites and another 2 cases had a large number of ascites.
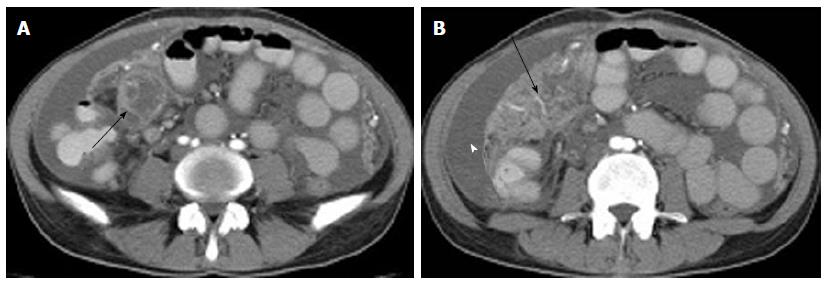
Three cases with tuberculous peritonitis showed a smooth uniform thickened peritoneum (one case) or nodular/irregular thickened peritoneum (2 cases). Five cases showed increasing density of the adjacent mesentery and mesenteric thickening, 2 of which had crowded mesenteric vascular bundles and thickened fiber strands. Two cases showed abscess adhesion to the hepatoduodenal ligament. One case showed a caked appearance of the omentum, and 2 cases showed a nodular omentum (Figure 5).
Of the 8 cases, 3 had histological evidence of caseating granuloma, and 5 had histological evidence of acid-fast bacilli.
Accurate diagnosis of tuberculous peritonitis is important for clinical treatment[9]. IPTA (a rare and serious form of tuberculous peritonitis) is a diagnostic challenge for the radiologist as its clinical symptoms and imaging findings may easily be mistaken for other lesions[6,8,10,11].
Abdominal TB is more common in patients aged 25-45 years, and has a slight female predominance[15]. In this study, the IPTA patients were aged 22 to 58 years with a female predominance.
Possible routes of abdominal TB include reactivation of a silent tuberculous lesion, infection spreading via swallowed sputum, hematogenous dissemination, and contiguity from adjacent tuberculous lesions[1]. Choi et al[16] reported that the absence of fever or laboratory data and negative cultures for TB cannot reliably rule out involvement of the peritoneum in TB. In the present study, all 8 patients had a history of abdominal discomfort and fever which supported the diagnosis of TB infection.
In cases with abdominal TB, the chest X-ray films of 10%-68.5% of cases showed evidence of healed or active pulmonary TB[15]. For patients with isolated pancreatic TB, the chest X-ray film may be negative or show evidence of obsolete TB in areas where TB infection is endemic[13]. In our study, the chest X-ray films of 37.5% of cases were negative, the chest X-ray films of 50.0% of cases showed evidence of obsolete pulmonary TB, and only 12.5% of patients showed evidence of active pulmonary TB on chest X-ray films. Thus, the lack of evidence of active pulmonary TB on chest X-ray film does not rule out the diagnosis of IPTA.
Nos et al[11] described an isolated mesenteric tuberculous abscess in a patient with HIV infection as a huge hypodense mass with a thick capsule and multiple septations. There was no associated abdominal lymph node enlargement. In our study, IPTA in non-HIV patients was seen as a multiseptated, peripherally enhanced, hypodense mass, with multiple enlarged lymph nodes. Combining the CT findings of IPTA, the location of the abscesses, and the CT findings of the lymph nodes, the IPTAs in this study were of 2 types: (1) lymph node fusion; and (2) encapsulation.
Lymph node fusion type IPTA: The more common sites of abdominal TB were the intestine, peritoneum, and lymph nodes[6-8]. Lymphadenopathy resulting from abdominal TB is usually associated with gastrointestinal TB and is less common in peritoneal or solid organ lesions. In some patients with abdominal TB, lymphadenopathy is the only positive imaging finding, especially in the periportal region[17]. Lymphadenopathy induced by abdominal TB commonly involves the mesenteric root, and celiac and peripancreatic lymph nodes. This is due to drainage of the ingested TB by the lymphatics in the small bowel, right hemicolon and ileocecal region[17].
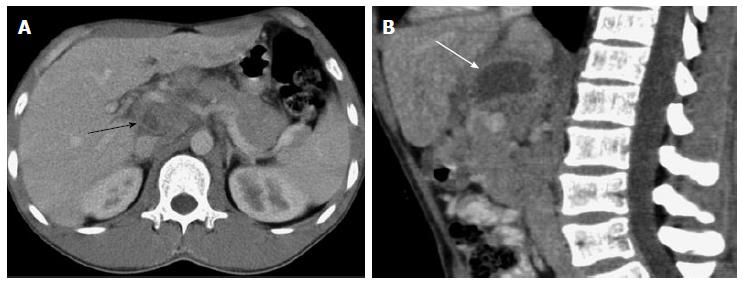
In this study, 3 cases showed an isolated abscess located in the peripancreatic area, with multiple enlarged lymph nodes (the diameter of the largest lymph node was > 1.9 cm). The maximum abscess diameter ranged from 4.5 cm to 5.2 cm. The regional margin of the abscesses was poorly defined. Multiple enlarged lymph nodes showed rim enhancement. The abscess and enlarged lymph nodes were the only signs of the disease in 2 cases. One of the 3 cases also showed involvement of the omentum, parietal peritoneum, mesentery, and terminal ileum. Another case showed an isolated abscess located in the jejunal mesentery with multiple, rim-enhanced, enlarged lymph nodes (the diameter of the largest lymph node was 2.5 cm). The abscess measured 2.5 cm × 4.2 cm × 5.0 cm in size, and the regional margin of the mass was poorly defined. Of these 4 cases, 2 showed obsolete pulmonary tuberculosis and 2 had no evidence of pulmonary TB.
Pereira et al[17] reported that the caseation and liquefaction substances in the center of the enlarged lymph node with TB infection result in the rim enhancement on contrast-enhanced CT images. It was reported that the enlarged lymph nodes with TB infection measured less than 4.0 cm in diameter because of pathologic self-limiting growth[18]. In these 4 cases, the abscesses located in the mesenteric root, celiac, porta hepatis, and peripancreatic region with multiple, rim-enhanced, enlarged lymph nodes had unclear boundaries, and the abscess measured less than 5.2 cm in diameter. Thus, a tuberculous abscess may be the result of the fusion of rim-enhanced, enlarged lymph nodes.
A multiseptated, peripherally enhanced, hypodense mass with a regular shape and multiple, rim-enhanced, enlarged lymph nodes beside the mass were typical features of the lymph node fusion type IPTA.
Encapsulation type IPTA: Tuberculous peritonitis includes the “wet” type, “dry” or “plastic” type, and “fibrotic-fixed” type[19]. The “dry” or “plastic” type is rare and characterized by caseous nodules, a fibrous peritoneal reaction and dense adhesions[17].
In this study, 3 cases with abscesses located in the peritoneal cavity and one case with an abscess located in the small bowel mesentery showed multiple enlarged lymph nodes, most of which showed homogeneous enhancement, while a small number showed rim enhancement.
Of these 4 cases, one had active pulmonary tuberculosis, 2 had obsolete pulmonary tuberculosis, and one had no past history of pulmonary tuberculosis. In addition, 2 of these 4 cases had an isolated abscess and 2 had multiple abscesses with or without an irregular shape. The maximum abscess diameter was 5.1 cm to 12.2 cm. A few rim-enhanced, enlarged lymph nodes (the diameter of the largest lymph node was < 1.5 cm) were detected.
Because of the small number of rim-enhanced lymph nodes in these 4 patients and the location of the abscesses (not located in the lymph node gathering area), formation of the IPTA was not caused by rim-enhanced lymph node fusion.
Intraoperative findings in 2 cases showed that the sigmoid colon and jejunum constituted the side walls of the abscess; the front wall was the small bowel mesentery, and the posterior wall was the parietal peritoneum. Based on these findings, this type of IPTA was formed by the bowel loops, peritoneum, mesentery, or organs encapsulating the intraperitoneal caseous substances.
A multiseptated, peripherally enhanced, hypodense mass with a small number of rim-enhanced, enlarged lymph nodes were typical features of the encapsulation type. The appearance of the rim-enhanced lymph nodes was the key feature in the diagnosis.
In our study, involvement of the omentum was shown as a nodular/caked appearance, and peritoneal TB was seen as a uniform or irregular thickening of the peritoneum, which was similar to reports in the literature[9,20]. Involvement of the mesentery in this study was seen as an increased density of the mesentery and mesenteric thickening, a nodular mesentery appearance, mesenteric vascular bundles, and thickened fiber strands. These findings were consistent with reports in the literature[9,20].
Heterogeneously enhanced, homogeneously enhanced, and poorly enhanced lymph nodes may occur in patients with TB infection[21]. Lymph nodes with central low attenuation and rim enhancement in patients with TB infection can be confused with necrotic metastatic lymphadenopathy[22,23]. In patients with a known primary malignancy, enlarged abdominal lymph nodes are more likely to be nodal metastases[2]. Patients with non-Hodgkin’s lymphoma who have undergone therapy may show rim-enhanced lymph nodes in the mesentery on CT images, simulating tuberculous lymphadenopathy involving the mesentery and other diseases[24-26]. In patients with a known clinical history of non-Hodgkin’s lymphoma, a differential diagnosis is likely to be obtained.
CT findings indicating intra-peritoneal abscess caused by Crohn’s disease show involvement of the left colon and homogenously enhanced lymph nodes[14,27,28]. Intra-abdominal lymphadenopathy of more than 1 cm was observed more frequently in intestinal TB and abdominal TB lymphadenopathy[29,30].
There are some potential limitations in our study. First, this was a retrospective study and some cases did not have 3-dimensional reconstruction images. Second, the patient sample was not large enough, which limited evaluation of statistical significance.
In conclusion, CT plays an important role in the detection and characterization of IPTA. Understanding the wide spectrum of CT appearances in IPTA should alert the radiologist to consider its diagnosis, especially among high-risk patients. Although the qualitative diagnosis of IPTA requires positive pathologic findings, certain CT features (such as a multiseptated, peripherally enhanced, hypodense mass with rim-enhanced lymph nodes, peritoneum/mesentery/omentum changes) are necessary for diagnosis.
Intra-peritoneal tuberculous abscess (IPTA) is a rare and serious form of extra-pulmonary tuberculosis. Early and accurate diagnosis of the tuberculous abscess is important for treatment. IPTA in non-human immunodeficiency virus patients is a diagnostic challenge as the clinical symptoms and imaging findings are easily mistaken for other peritoneal diseases. If computed tomography (CT) findings were helpful for the differentiation between IPTA and other peritoneal diseases, this would reduce or avoid unnecessary invasive diagnostic procedures.
CT plays an important role in the diagnosis of abdominal abscesses. This study reports on the CT features in 8 patients with IPTA.
IPTA represents a diagnostic dilemma. The authors reviewed 8 patients with this condition and reviewed the related literature to identify CT features for diagnosis.
The identification of relatively specific CT findings of IPTA may help to reduce or avoid unnecessary invasive diagnostic procedures.
The aim pursued by the authors in this work is quite interesting. The computed tomography findings could be very helpful for the differentiation between the intra-peritoneal tuberculous abscess and other peritoneal diseases, because with this technique it would reduce or avoid unnecessary invasive diagnostic procedures. This would need a good reference for the different lesion forms that can be observed. In this sense, the authors do an excellent description of the 8 cases studied (especially those with three-dimensional reconstruction images).
P- Reviewer: Garcia-Jimenez WL S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Flemming GM, Oberschmid B, Siebolts U, Hirsch W, Schuster V. Abdominal tuberculosis in children and adolescents: to this day a diagnostic challenge. Klin Padiatr. 2013;225:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA, Chang SD. CT appearances of abdominal tuberculosis. Clin Radiol. 2012;67:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Kapoor VK. Abdominal tuberculosis. Postgrad Med J. 1998;74:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998;93:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | LoBue PA, Enarson DA, Thoen TC. Tuberculosis in humans and its epidemiology, diagnosis and treatment in the United States. Int J Tuberc Lung Dis. 2010;14:1226-1232. [PubMed] |
| 6. | Khan R, Abid S, Jafri W, Abbas Z, Hameed K, Ahmad Z. Diagnostic dilemma of abdominal tuberculosis in non-HIV patients: an ongoing challenge for physicians. World J Gastroenterol. 2006;12:6371-6375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Maniar JK, Kamath RR, Mandalia S, Shah K, Maniar A. HIV and tuberculosis: partners in crime. Indian J Dermatol Venereol Leprol. 2006;72:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Lazarus AA, Thilagar B. Abdominal tuberculosis. Dis Mon. 2007;53:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Charoensak A, Nantavithya P, Apisarnthanarak P. Abdominal CT findings to distinguish between tuberculous peritonitis and peritoneal carcinomatosis. J Med Assoc Thai. 2012;95:1449-1456. [PubMed] |
| 10. | Lupatkin H, Bräu N, Flomenberg P, Simberkoff MS. Tuberculous abscesses in patients with AIDS. Clin Infect Dis. 1992;14:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Nos P, Ricart C, García E, Moles JR, Lacruz J, Berenguer J. Isolated mesenteric tuberculosis as the first manifestation of AIDS. Rev Esp Enferm Dig. 1992;82:59-60. [PubMed] |
| 12. | Meesiri S. Pancreatic tuberculosis with acquired immunodeficiency syndrome: a case report and systematic review. World J Gastroenterol. 2012;18:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Asim S, Manjari L, Kenneth MS, Alexander CR, Christopher K, Khek-Yu H. Pancreatic tuberculous abscess diagnosed by endoscopic ultrasound-guided fine needle aspiration. J Pak Med Assoc. 2010;60:499-501. [PubMed] |
| 14. | Zhao XS, Wang ZT, Wu ZY, Yin QH, Zhong J, Miao F, Yan FH. Differentiation of Crohn’s disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014;20:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Akgun Y. Intestinal and peritoneal tuberculosis: changing trends over 10 years and a review of 80 patients. Can J Surg. 2005;48:131-136. [PubMed] |
| 16. | Choi CH, Kim CJ, Lee YY, Kim JS, Song T, Park HS, Kim MK, Kim TJ, Lee JW, Lee JH. Peritoneal tuberculosis: a retrospective review of 20 cases and comparison with primary peritoneal carcinoma. Int J Gynecol Cancer. 2010;20:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Pereira JM, Madureira AJ, Vieira A, Ramos I. Abdominal tuberculosis: imaging features. Eur J Radiol. 2005;55:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Yang ZG, Min PQ, Sone S, He ZY, Liao ZY, Zhou XP, Yang GQ, Silverman PM. Tuberculosis versus lymphomas in the abdominal lymph nodes: evaluation with contrast-enhanced CT. AJR Am J Roentgenol. 1999;172:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Thoeni RF, Margulis AR. Gastrointestinal tuberculosis. Semin Roentgenol. 1979;14:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Na-ChiangMai W, Pojchamarnwiputh S, Lertprasertsuke N, Chitapanarux T. CT findings of tuberculous peritonitis. Singapore Med J. 2008;49:488-491. [PubMed] |
| 21. | Pombo F, Rodríguez E, Mato J, Pérez-Fontán J, Rivera E, Valvuena L. Patterns of contrast enhancement of tuberculous lymph nodes demonstrated by computed tomography. Clin Radiol. 1992;46:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Puri R, Vilmann P, Sud R, Kumar M, Taneja S, Verma K, Kaushik N. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Puri R, Mangla R, Eloubeidi M, Vilmann P, Thandassery R, Sud R. Diagnostic yield of EUS-guided FNA and cytology in suspected tubercular intra-abdominal lymphadenopathy. Gastrointest Endosc. 2012;75:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Dong P, Wang B, Sun QY, Cui H. Tuberculosis versus non-Hodgkin’s lymphomas involving small bowel mesentery: evaluation with contrast-enhanced computed tomography. World J Gastroenterol. 2008;14:3914-3918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Taffel MT, Khati NJ, Hai N, Yaghmai V, Nikolaidis P. De-misty-fying the mesentery: an algorithmic approach to neoplastic and non-neoplastic mesenteric abnormalities. Abdom Imaging. 2014;39:892-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Corwin MT, Smith AJ, Karam AR, Sheiman RG. Incidentally detected misty mesentery on CT: risk of malignancy correlates with mesenteric lymph node size. J Comput Assist Tomogr. 2012;36:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Sinan T, Sheikh M, Ramadan S, Sahwney S, Behbehani A. CT features in abdominal tuberculosis: 20 years experience. BMC Med Imaging. 2002;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Boudiaf M, Zidi SH, Soyer P, Lavergne-Slove A, Kardache M, Logeay O, Rymer R. Tuberculous colitis mimicking Crohn’s disease: utility of computed tomography in the differentiation. Eur Radiol. 1998;8:1221-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kim SH, Kim JW, Jeong JB, Lee KL, Kim BG, Choi YH. Differential diagnosis of Crohn’s disease and intestinal tuberculosis in patients with spontaneous small-bowel perforation. Dig Surg. 2014;31:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Zhang M, Li M, Xu GP, Liu HJ. Neoplasm-like abdominal nonhematogenous disseminated tuberculous lymphadenopathy: CT evaluation of 12 cases and literature review. World J Gastroenterol. 2011;17:4038-4043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |