Published online Dec 28, 2014. doi: 10.4329/wjr.v6.i12.895
Revised: October 16, 2014
Accepted: October 28, 2014
Published online: December 28, 2014
Processing time: 214 Days and 19.3 Hours
This short review examines the most recent functional studies of the topographic organization of the human corpus callosum, the main interhemispheric commissure. After a brief description of its anatomy, development, microstructure, and function, it examines and discusses the latest findings obtained using diffusion tensor imaging (DTI) and tractography (DTT) and functional magnetic resonance imaging (fMRI), three recently developed imaging techniques that have significantly expanded and refined our knowledge of the commissure. While DTI and DTT have been providing insights into its microstructure, integrity and level of myelination, fMRI has been the key technique in documenting the activation of white matter fibers, particularly in the corpus callosum. By combining DTT and fMRI it has been possible to describe the trajectory of the callosal fibers interconnecting the primary olfactory, gustatory, motor, somatic sensory, auditory and visual cortices at sites where the activation elicited by peripheral stimulation was detected by fMRI. These studies have demonstrated the presence of callosal fiber tracts that cross the commissure at the level of the genu, body, and splenium, at sites showing fMRI activation. Altogether such findings lend further support to the notion that the corpus callosum displays a functional topographic organization that can be explored with fMRI.
Core tip: A combined approach using diffusion tensor imaging and tractography, two recently developed imaging techniques, and functional magnetic resonance imaging (fMRI) has enabled detection of fMRI activation evoked by specific sensory or motor tasks in the corpus callosum, and reconstruction of the trajectory of the commissural fibers interconnecting primary cortical areas activated by the same tasks. These findings confirm that the corpus callosum has a functional topographic organization and that fMRI may be used to explore it.
- Citation: Fabri M, Pierpaoli C, Barbaresi P, Polonara G. Functional topography of the corpus callosum investigated by DTI and fMRI. World J Radiol 2014; 6(12): 895-906
- URL: https://www.wjgnet.com/1949-8470/full/v6/i12/895.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i12.895
The principal interhemispheric commissure is the corpus callosum (CC). It arises in the brain of placental mammals[1] as an elongated midline structure composed of 200-800 million horizontal interconnecting homotopical and heterotopical cortical areas[2]. The mature CC contains myelinated (70%) and unmyelinated fibers (30%), glial cells (astrocytes and oligodendrocytes), and neurons[3-7]. The human CC has been divided into five anatomical regions, which include from front to back the genu, the rostrum, the body or trunk-often subdivided into anterior, middle and posterior body-the isthmus, and the splenium (Figure 1). Since there are no clear borders between regions, a variety of methods based principally on geometric criteria have been proposed to define subregions[8-11]. The different callosal regions have different fiber compositions: large diameter fibers have been described in the posterior part of the splenium and in the body[1,12], where interhemispheric sensory fibers cross the commissure and exchange information at high speed, whereas small fibers mainly connecting association cortical areas are found in the rostrum, genu and anterior body[1,12]. Recently, different protein expression profiles have also been described in the three main CC regions, the genu, body and splenium[13]. In particular, the expression of proteins related to glucose metabolism and antioxidant activity seems to be lower in the genu and body compared with the splenium[13].
The anterior half of the human CC (genu, rostrum and body) contains fibers interconnecting frontal association cortical areas. The isthmus mostly contains primary motor, somatosensory, and auditory fibers. In the splenium primary visual and association temporo-occipital and parietal commissural fibers are mixed, forming a single segment with the hippocampal commissure through which parahippocampal fibers cross[14].
Large diameter fibers (3-5 μm) are densest in the isthmus (connecting motor, somatosensory, and auditory cortices) and in the posterior splenium (connecting visual cortices), whereas small fibers (< 0.4 μm) are more numerous in the genu and anterior splenium (connecting high-order prefrontal and temporo-parietal associative areas). The largest fibers in the human CC interconnect the primary auditory cortices[12,14].
Neurons giving rise to callosal fibers lie in cortical layers III, V and VI. The vast majority of these fibers release excitatory amino acids [glutamate (Glu) and/or aspartate] as neurotransmitters[15]; however, a small proportion of callosal neurons in cat and rat have been shown to release the inhibitory neurotransmitter GABA[16-18].
The CC is a recent phylogenetic acquisition of placental mammals, developing by fusion of the interhemispheric midline fibers with specialized midline glial cells guiding callosal fibers to the contralateral side[14]. It originates from the glial sling, above and rostral to the anterior and hippocampal commissures: it thus forms from the fusion of two separate segments. The anterior, sling-derived callosum (containing fibers connecting frontal associative and possibly primary sensory-motor areas of the two hemispheres) and the hippocampal commissure-associated splenium (containing fibers arising in the parieto-temporo-occipital cortex and directed to the opposite hemisphere) probably fuse just anterior to the hippocampal commissure[14].
The different origin of the anterior and posterior CC portions seems to correlate with different functional properties, and the respective resection gives rise to different effects, since patients with surgical resection of the splenium show disconnection syndrome[19] whereas those with resection of the anterior CC do not[20].
The CC grows in size by the increase of the connectivity and the tangential growth of the cortex. In the womb and in the early postnatal period it mainly grows by fiber addition, whereas later increases are due to the development of myelin, which offsets pruning of callosal fibers; fiber myelination becomes significant at about 6 mo of postnatal life in the splenium and at about 8 mo in the genu. Myelination is believed to proceed from posterior to anterior[21,22], reflecting the fact that myelination of primary cortical areas (somatic sensory, motor, auditory, visual) connected through the isthmus and splenium predates the myelination of the body, genu, and rostrum, which are related to the more anterior associative areas.
The function of the CC has been investigated for centuries. The earliest studies date to the 16th century. Believed for many centuries to be the “seat of the soul”[23], it took until the 18th century for Franz Joseph Gall and Johann Spurzheim, dissecting alcohol-fixed brains, to describe bundles of axons passing through the callosal white matter (WM) and connecting the two hemispheres[24]. Its known functions include: interhemispheric exchange of information, integration of inputs reaching one or both hemispheres, facilitation of some cortical activities, and inhibition of cortical functions[25,26]. It has recently been shown that the size of the human CC positively correlates with intelligence (Einstein’s CC was thicker than normal[27]) and that its integrity is essential for cognitive performances; thus CC resection and microstructural or developmental alterations are often associated with cognitive decline.
The earliest hypotheses on the function of the human CC came from studies of split-brain patients, subjects whose CC was partially or completely resected to prevent the diffusion of epileptic seizures[28]. Patients with total or partial resection involving the posterior CC suffered from disconnection syndrome[19,29,30], whereas in those with partial anterior resection the disconnection could be evidenced only by specific tests[20,31].
These investigations were followed and paralleled by animal studies including neuroanatomical tracing, cytological and microstructural analyses, and electrophysiological recordings. Neuropsychological and clinical studies of patients with total or partial surgical resection of the CC performed to treat drug-resistant epilepsy or remove intracallosal cysts or tumors provided further insights into its function.
Ever since electrophysiological recordings demonstrated somatic sensory receptive fields in the anterior cat CC[32,33] and visual inputs to the splenium[34,35], the CC has been hypothesized to be topographically organized. Later electrophysiological[36] and neuroanatomical findings[37,38] obtained after injection of neural tracers or ablation of selected cortical areas in non-human primates; findings from post-mortem investigations[39]; and studies of patients with surgical resection or callosal lesions[28,40-42] provided further support for the notion. This organization appears to give rise to modality-specific regions[43] in which anterior callosal axons transfer motor information between the frontal lobes and somatic sensory, auditory, and visual information is integrated by posterior fibers linking parietal, temporal and occipital lobes and crossing through the posterior midbody, isthmus and splenium, respectively.
Further support for the notion of a topographic organization of the CC came from the study of subjects with callosal resection. Functional magnetic resonance imaging (fMRI) was applied by our group to investigate callosotomy patients[44-46] and demonstrated that touch information transfer between the hemispheres may be accomplished by axons crossing at the level of the posterior CC. A more recent study of non-epileptic patients with resection of different portions of the anterior CC[40] contributed additional evidence by showing that motor coordination transfer occurs at the level of the middle portion of the genu and somesthetic information is through the anterior CC. Examination of further sensory modalities provided evidence that transfer of visual[47,48] and auditory information[49,50] between the hemispheres takes place in the splenium.
The recent MRI-associated techniques, including fMRI, volumetric based morphometry, diffusion tensor imaging (DTI) and diffusion tensor tractography (DTT), are new, powerful methods to investigate the human brain in vivo. Data collected with DTI and fMRI are reviewed below after a brief survey of the bases of these techniques.
DTI is an MRI-based method enabling in vivo quantification of the microscopic diffusion properties of water in tissues[51]. It allows generation of quantitative maps of diffusion indices and through them assessment of brain WM tissue structure and integrity. The underlying principle of DTI is the random motion of water molecules (Brownian motion), which can be characterized by the diffusion coefficient, D, and is influenced by other factors including molecular weight and viscosity. Water diffuses freely in all directions (isotropic diffusion). In gray matter (GM) water diffusion is similarly isotropic, but it is hindered by cellular structures, whereas diffusion in WM is hindered by the presence of highly ordered axonal structures. The latter conditions result in preferential diffusion parallel to WM tracts, i.e., the route of least resistance, rather than perpendicular to them. This motion was noted in early experiments and designated anisotropic diffusion[52,53]. Myelination of the axons has long been held to be the main obstruction to water diffusion in WM, and to be responsible for anisotropy; however later evidence suggested that axon membranes as well as other factors including organization of neurofilaments and microtubules also play a role[54]. Measures of anisotropy include relative anisotropy, volume ratio and the most commonly cited fractional anisotropy (FA). These rotationally invariant indices reflect the degree of anisotropy in the diffusion tensor and are normalized to values between 0 (isotropic) and 1 (highly anisotropic).
In an extensive paper, Yap et al[55] reviewed several investigations documenting WM changes in subjects of different ages using DTI. In particular they showed that maximum FA is reached in the anterior and middle portions of the CC around 20 years of age and in the splenium around 50 years; in older subjects FA decreases and does so more slowly in the splenium[55,56]. FA is usually slightly lower in the anterior and middle portions of the CC (regions 1-4 described respectively as prefrontal, premotor, precentral and postcentral by Pandya and Seltzer[38]), where it ranges from 0.5 to 0.7, and higher in the splenium (posterior parietal and temporo-occipital regions, respectively, regions 5 and 6 of Pandya and Seltzer[38]), where it ranges from 0.6 to 0.8[56-59].
DTI thus enables exploration of the microstructural organization of the CC by measuring FA, which has recently been shown to correlate positively with conduction velocity and may therefore be considered as an index of myelination or axon diameter[60]. Reductions in FA have been implicated in numerous neuropsychiatric and neurological conditions including alcoholism[61], schizophrenia[62], traumatic brain injury[63], multiple sclerosis[64-66], and Wallerian degeneration[67]. It has recently been suggested that acquisition factors such as b-value and voxel size can affect the quantification of DTI parameters (i.e., FA and mean diffusivity, MD)[68]. For this reason extreme caution is required when comparing data obtained using different acquisition factors.
Interestingly, DTI techniques also evidenced plastic changes occurring in fiber bundles in relation to development or training and resulting in an FA increase after training, thus demonstrating that the technique is not solely an anatomical tool[69-71].
A further application of diffusion tensor data is exploration of the distribution of WM fibers in the brain, known as DTT or fiber tracking. In deterministic tractography fibers typically originate from seed points- which are entered automatically or manually to examine a specific area or the whole brain-and propagate along the direction of the principal eigenvector (e1). Additional parameters or constraints include maximum tract curvature and a stopping criterion for the tracking, such as achievement of a minimum FA threshold[51,72]. In probabilistic tractography a multitude of fibers, typically thousands, are generated from each seed point or voxel. Each fiber propagates in an individual manner: DTT takes into account both e1 direction and change. Tractography is used, for example, to highlight fiber tracts in patients requiring brain surgery[73], to investigate WM reductions related to cognitive impairment[74], cerebellar damage[75], specific cortical brain changes[76], longitudinal changes[77] and intrinsic connectivity[78] in multiple sclerosis. DTT also evidenced increased FA in specific fiber bundles after training in given tasks[69-71].
Over the past three decades these new imaging techniques have enabled confirmation or rejection of earlier hypotheses about the functions of the CC and provided new insights. In non-human primates and other mammals they have also allowed to verify and correlate data obtained by classic neuroanatomical techniques with DTI findings, and results of electrophysiological recordings with fMRI activation.
The callosal topography resulting from the application of DTI and DTT techniques has thus been confirmed to be in line with the one described in previous studies. Fibers connecting prefrontal cortical areas have been seen to cross through the anterior part of the CC; those connecting premotor and motor cortical areas crossed at the level of the central callosal body[9,25,79-81]; the fibers connecting parietal cortical areas crossed through the posterior callosal body; and those from occipital areas crossed at the level of the splenium (see also[82,83]). Another hypothesis that has been confirmed is the topographic organization of the CC as emerging from previous neuroanatomical (axonal degeneration and tract-tracing) animal studies and human lesion and post-mortem investigations.
Slight differences have been demonstrated between human and monkey topographic organization in relation to the much greater expansion of the human frontal cortex.
Functional MRI allows to study the intact brain non invasively. It is a functional neuroimaging approach based on MRI technology that measures brain activity by detecting associated changes in blood flow, based on the well-established notion that neuronal activation in an area of the brain is accompanied by a local increase in blood flow. The blood-oxygen-level dependent (BOLD) effect, or response, is a method based on the different ratio of oxygenated to deoxygenated hemoglobin in blood. Given that the two forms of the molecule have different magnetic behaviors, the change of their relative concentration, due to an increase in blood flow evoked by increased neural activity, generates a magnetic-electric signal that is detected by the equipment, highlighting the areas of the brain that are active at any given time.
It has long been believed that the BOLD effect is mainly due to the metabolic activity associated with synaptic rather than spiking activity, and therefore it could be evoked only in GM[84]. However, data from the newer imaging techniques suggest that a hemodynamic response can also be evoked in WM, particularly in the CC. These findings were at first observations sporadically recorded during interhemispheric transfer tasks performed by subjects within the magnet[85-88], or during activities not involving specific interhemispheric transfer tasks, such as voluntary swallowing[89]. Moreover a BOLD signal was elicited in isthmus and splenium (posterior CC) by a task based on the interhemispheric transfer and integration of visuo-motor information, where crossing of the CC is needed for a behavioral response to be elicited (“crossed condition”[88]). The above mentioned functional studies are summarized in Table 1.
| Ref. | Year | Task | CC localization | Technique | Subjects |
| Mosier et al[89] | 2001 | Swallowing | Anterior | fMRI | Healthy controls |
| Tettamanti et al[85] | 2003 | Visuomotor | Anterior | fMRI | Healthy controls |
| Omura et al[86] | 2004 | Visuomotor transfer | Anterior | fMRI | Healthy controls |
| Weber et al[87] | 2005 | Visuomotor | Anterior | fMRI | Healthy controls |
| Mazerolle et al[88] | 2008 | Visual transfer | Posterior | fMRI | Healthy controls |
| Mazerolle et al[126] | 2010 | Visual transfer | Posterior | fMRI and DTI | Healthy controls |
| Fabri et al[94] | 2011 | Tactile, gustatory, visual | Different regions according peripheral stimuli | fMRI | Healthy controls |
| Fabri et al[59] | 2013 | Tactile, gustatory, visual, auditory | Different regions according peripheral stimuli | fMRI and DTI | Healthy control and Callosotomized patients |
| Polonara et al[95] | 2014 | Tactile, gustatory, visual, auditory | Different regions according peripheral stimuli | fMRI and DTI | Callosotomized patients |
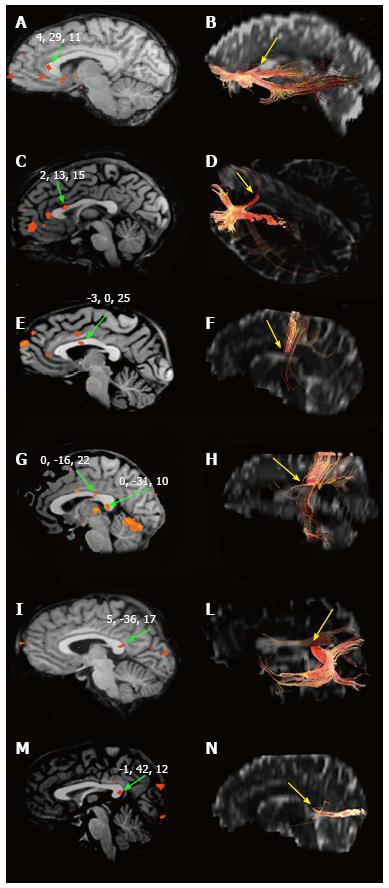
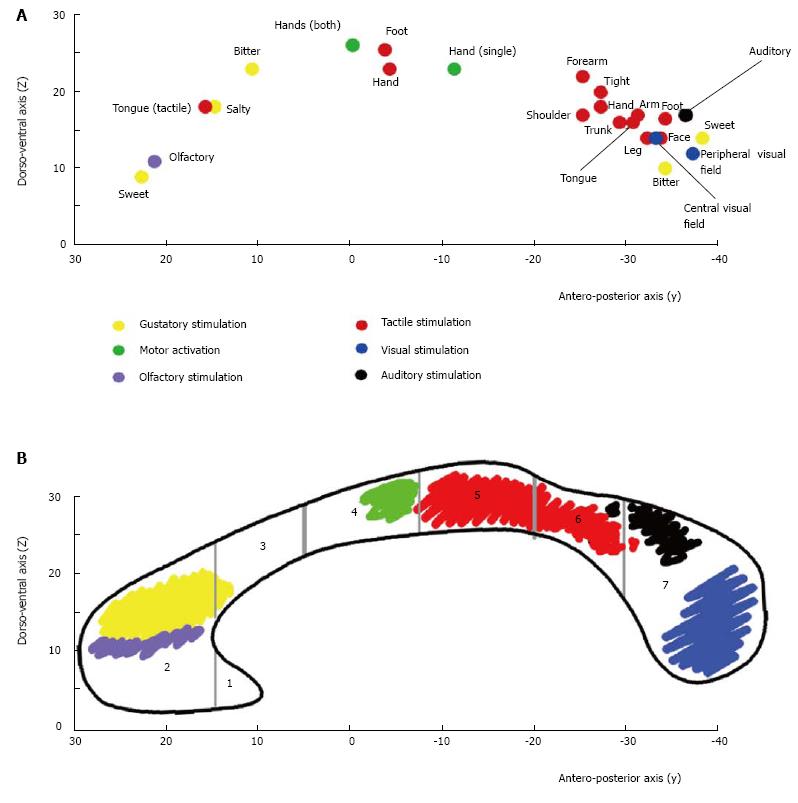
A number of studies have documented that information transfer between premotor and prefrontal areas involves the anterior CC, and transfer between parietal, occipital and temporal regions involves the posterior CC[81,90-92]. A recent systematic study by our group[93,94] examined the BOLD effect evoked in the CC by simple sensory stimuli or by the performance of motor tasks activating the cortical areas which in healthy control subjects harbor the representation of motor activation and of gustatory, olfactory, auditory, visual and tactile sensitivity. The study was directed at establishing whether (1) a BOLD signal was able to be evoked in CC fibers; and (2) the foci related to motor tasks and sensory stimuli agreed with the notion of a topographic organization. The study did detect consistent activation foci in discrete regions of the CC: anterior (olfactory and gustatory stimulation), central (motor tasks), central-posterior (touch stimulation), isthmus (auditory stimulation) and splenium (visual stimulation) (Figure 2). It also confirmed the existence of a topographic organization of the CC from a functional point of view, demonstrating that it may be investigated using fMRI. In recent years the peripheral sensory stimulation protocols applied in the earlier studies[94] were administered to partial callosotomy patients[95]. The test results were assessed to determine whether the extant CC portions displayed a BOLD signal, to provide additional evidence for the concept of a functional map in the CC. In the same study DTI test data were also obtained in callosotomy and control subjects, to determine whether tracts seeded from cortical areas activated by specific sensory stimuli co-localized with CC activation (Figure 3).
The neurovascular interactions inducing hemodynamic changes during increased cortical activity is the basis of functional neuroimaging with PET and fMRI[96-98]. The BOLD signal reflects the hemodynamic responses related to neuronal activity[98,99]. The exact mechanism underlying the BOLD effect is still debated. Hemodynamic changes have been seen to be induced by motor and visuomotor tasks and peripheral stimulation[85-89,100] and, recently, by simple sensory tasks[94]. Energy-dependent processes occur in the WM, too, given that ATP-dependent Na+-K+ ion pumps mediate the conduction of axonal action potentials at the nodes of Ranvier, restoring ion gradients in neuron membranes[99,101]. Actually, the block of voltage-dependent Na+ channels inhibits the responses to forepaw somatosensory stimulation that can be detected by fMRI[102]. Moreover, spiking activity and fMRI activation are also correlated based on recent data[103-105]. Various hypotheses have been advanced to explain the BOLD effect seen in WM: vessel dilation by astrocytes[106,107] aimed at meeting the increased energy demand related to the increased neural activation; an increase in extracellular K+ in relation to heightened brain cell activity; or an increase in cytoplasmic Ca2+[99,106,108]. Astrocytes and capillaries are both found in the CC[109], and since the conduction of action potentials by CC axons requires energy, the mechanism is probably also active in CC fibers. According to LeBihan (2009, personal communication) the heat produced by the augmented axonal metabolism would by itself be able to induce dilation of CC microvessels.
Another hypothesis, recently advanced by Barbaresi et al[3], explains the BOLD effect seen in specific CC regions with the presence of NADPH-d+/NOS-immunopositive intracallosal neurons, whose depolarization may result in increased blood flow. The depolarization may occur in two ways: (1) through activation of specific cortical regions by peripheral stimulation, resulting in depolarization of intracallosal neurons containing nitric oxide (NO), whose dendrites reach the activated overlying cerebral cortex; NO could thus be released from neuronal processes associated with callosal vessels; this mechanism has been hypothesized to occur in the cerebral cortex, since inhibition of the NO-producing enzyme NO synthase attenuates the increase in blood flow associated with neuronal activity[110-112]; and (2) alternatively, increased cortical activity may cause release of more Glu along callosal fibers[113,114] belonging to glutamatergic cortical neurons[15], possibly exciting NO-producing intracallosal neurons[115] through NMDA receptors[116,117]; the interaction of Glu with NMDA receptors could therefore elicit a BOLD response in the CC similar to other central nervous system regions where application of NMDA receptor antagonists attenuates blood flow responses[118-123].
However, a concomitant role of astrocytes in neurovascular coupling[112] in the CC cannot be ruled out. Current findings show that glial cells lack NO-producing enzymes[3]; therefore Glu released from callosal axons could induce release from astrocytes of vasoactive agents other than NO, such as cyclo-oxygenase (COX) products, whose inhibition significantly reduces vasodilation[108,124].
As mentioned above, sensory and motor stimulation evokes activation in various areas of the CC[94]. Two main observations have emerged from this brief review: the first is that activation foci have rarely been detected in the middle-anterior area; the second is that foci have been elicited in the posterior CC, i.e., the splenium, by different sensory stimuli.
Functional activation in the middle-anterior area has sometimes been described in conditions where subjects performed interhemispheric transfer tasks involving crossed and uncrossed conditions[85-87,125,126], which entailed a choice underpinned by a mental operation. Anterior callosal activation has been interpreted as the transfer of a premotor program leading to motor output. Results of recent behavioral and functional research suggest that activation of the anterior midbody is actually involved in the integration of cortical areas recruited in abstract mental operations. Miller et al[127] found that callosotomy patients subjected to resection of the anterior CC were unable to provide moral judgments based on a hypothetical situation; when the same patients were shown a gesture performed by a model standing in front of them and were asked to imitate it, they were unable to do so using an anatomical perspective[128]. When during an fMRI session healthy subjects were asked to imitate mentally a series of intransitive gestures with the limb used by the model in performing them, callosal activation was detected in the anterior midbody[129]. Altogether these data suggest that the anterior callosal midbody is involved in mental operations enabling individuals to relate themselves to other subjects, thus also allowing social interaction. The hypothesis is supported by microstructural DTI data showing that this regions has a reduced FA value in autistic and psychotic patients, indicating an impaired connectivity that in these patients is paralleled by poor or absent social competences.
As mentioned above, activation foci in the posterior region of the CC, the splenium, have sometimes been elicited in some controls and patients by taste and by touch stimulation to the hand, in addition to the specific foci seen in all subjects at more anterior sites. Since these foci do not seem to be accidental, they are likely evoked by peripheral stimulation. The foci elicited by gustatory and touch stimuli to the hand in the splenium might reflect higher-order association area activation: e.g., posterior parietal cortex (touch); temporal cortex (taste and touch), since these cortical regions are interconnected by nerve fibers that cross the splenium[10,11]. Activation of the splenium may explain the good performance in the transfer of touch information obtained by partial callosotomy patients, in whom only this callosal region is extant[31,42,130-134]. Other findings from neuropsychological investigations of callosotomy patients[134,135] point to a role for the splenium in transferring taste information. The recruitment of the splenium in the transfer of information other than visual information could be related to the large role of the visual representation of the external environment characteristic of humans, where different sensory experiences tend to be associated with a visual component. Its flexibility sets the splenium apart from more anterior callosal regions, and parallels other differences stemming from the development[14], fiber composition[12] and chemical specificity of this region[136]. These morpho-functional observations are also in line with the fact that patients where this part of the CC is extant do not exhibit disconnection syndromes[19,28], and also suggest that the splenium might subserve most of the interhemispheric connectivity and the plasticity required for functional recovery after callosotomy or other insults.
The next step in this line of research should be the direct demonstration that functionally activated regions displaying a BOLD response correspond with the site where interhemispheric fibers interconnecting sensory or motor cortical areas involved in processing the peripheral stimuli applied cross through the commissure.
Another important issue to be addressed with the newer techniques, like diffusion fMRI[137], is whether the anterior and posterior portions of the CC have different roles.
This review provides a brief outline of key notions and examines recent DTI studies of the topographic organization of the CC in healthy subjects and in patients with different extents of callosal resection examined by fMRI during administration of peripheral sensory stimuli. These studies have documented a BOLD response in various portions of the commissure; they have demonstrated that it can be induced by peripheral stimuli and motor tasks; and have shown CC activation foci are found at discrete sites in relation to the sensory stimulation applied and the motor tasks performed. The resulting functional topographic map agrees with earlier findings. Additional fMRI and DTI data are clearly needed if we are to gain further insights into the callosal activation map and establish or rule out that functionally activated CC areas displaying a BOLD response correspond with sites where callosal fibers, interconnecting sensory or motor cortical areas involved in processing specific stimuli, cross through the commissure. The organization of the callosal fibers relaying information regarding different sub-modalities or areas of the sensory periphery also deserves further investigation.
The researches by our group reported here have been carried out with the collaboration of the late Professor Tullio Manzoni and Professor Ugo Salvolini, who provided invaluable help and criticisms, of Doctor Giulia Mascioli, who contributed to fMRI data analysis, and of Doctors Aldo Paggi and Nicoletta Foschi, who were responsible for patient care. We gratefully acknowledge their contributions. The authors are also grateful to Dr. Silvia Modena (Word Design; http://www.silviamodena.com) for the language revision, and to the technical staff of the Radiology Institute for assistance in scan sessions and data transfer.
P- Reviewer: Arsalidou M, Sijens PE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res. 2003;36:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Innocenti GM. General organization of callosal connections in the cerebral cortex. Cerebral Cortex. New York: Plenum Press 1986; 291-353. |
| 3. | Barbaresi P, Fabri M, Mensà E. Characterization of NO-producing neurons in the rat corpus callosum. Brain Behav. 2014;4:317-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Rockland KS, Nayyar N. Association of type I neurons positive for NADPH-diaphorase with blood vessels in the adult monkey corpus callosum. Front Neural Circuits. 2012;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Malobabić S, Bogdanović D, Drekić D. On the neurons with dendrites intermingling with the fibers of the human corpus callosum: a Golgi picture. Gegenbaurs Morphol Jahrb. 1984;130:557-564. [PubMed] |
| 6. | Revishchin AV, Okhotin VE, Korochkin LI, Pavlova GV. A new population of calretinin-positive cells, presumptively neurons, with polymorphous spines in the mouse forebrain. Neurosci Behav Physiol. 2010;40:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Riederer BM, Berbel P, Innocenti GM. Neurons in the corpus callosum of the cat during postnatal development. Eur J Neurosci. 2004;19:2039-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 969] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 891] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 10. | Hofer S, Merboldt KD, Tammer R, Frahm J. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cereb Cortex. 2008;18:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Kashem MA, Sarker R, Des Etages H, Machaalani R, King N, McGregor IS, Matsumoto I. Comparative proteomics in the corpus callosal sub-regions of postmortem human brain. Neurochem Int. 2009;55:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010;52:447-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Barbaresi P, Fabri M, Conti F, Manzoni T. D-[3H]aspartate retrograde labelling of callosal and association neurones of somatosensory areas I and II of cats. J Comp Neurol. 1987;263:159-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Gonchar YA, Johnson PB, Weinberg RJ. GABA-immunopositive neurons in rat neocortex with contralateral projections to S-I. Brain Res. 1995;697:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Fabri M, Manzoni T. Glutamic acid decarboxylase immunoreactivity in callosal projecting neurons of cat and rat somatic sensory areas. Neuroscience. 2004;123:557-566. [PubMed] |
| 18. | Higo S, Akashi K, Sakimura K, Tamamaki N. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Berlucchi G. Visual interhemispheric communication and callosal connections of the occipital lobes. Cortex. 2014;56:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Berlucchi G. Frontal callosal disconnection syndromes. Cortex. 2012;48:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 22. | Provenzale JM, Isaacson J, Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. AJR Am J Roentgenol. 2012;198:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Manzoni T. The cerebral ventricles, the animal spirits and the dawn of brain localization of function. Arch Ital Biol. 1998;136:103-152. [PubMed] |
| 24. | Manzoni T. Fibre associative e commisurali. In Corteccia Cerebrale e funzioni cognitive. Ventitré secoli di storia. Cap.5-5. Roma (Italy): Carocci editore 2011; 230-232. |
| 25. | Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132-12138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Koch G, Cercignani M, Bonnì S, Giacobbe V, Bucchi G, Versace V, Caltagirone C, Bozzali M. Asymmetry of parietal interhemispheric connections in humans. J Neurosci. 2011;31:8967-8975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Men W, Falk D, Sun T, Chen W, Li J, Yin D, Zang L, Fan M. The corpus callosum of Albert Einstein’s brain: another clue to his high intelligence? Brain. 2014;137:e268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005;6:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 734] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Gordon HW, Bogen JE, Sperry RW. Absence of deconnexion syndrome in two patients with partial section of the neocommissures. Brain. 1971;94:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 105] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Innocenti GM, Manzoni T, Spidalieri G. Cutaneous receptive fields of single fibers of the corpus callosum. Brain Res. 1972;40:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Innocenti GM, Manzoni T, Spidalieri G. Patterns of the somesthetic messages transferred through the corpus callosum. Exp Brain Res. 1974;19:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Berlucchi G, Gazzaniga MS, Rizzolatti G. Microelectrode analysis of transfer of visual information by the corpus callosum. Arch Ital Biol. 1967;105:583-596. [PubMed] |
| 35. | Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol. 1967;30:1561-1573. [PubMed] |
| 36. | Guillemot JP, Richer L, Prevost L, Ptito M, Lepore F. Receptive field properties of somatosensory callosal fibres in the monkey. Brain Res. 1987;402:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 232] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Pandya DN, Seltzer B. 1986. The topography of commissural fibers. Two Hemispheres-One Brain: Functions of the Corpus Callosum. New York: Alan Liss 1986; 47-73. |
| 39. | de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985;44:578-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 361] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Caillé S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery. 2005;57:50-59; discussion 50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Berlucchi G. Some effects of cortical and callosal damage on conscious and unconscious processing of visual information and other sensory inputs. Prog Brain Res. 2004;144:79-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Fabri M, Del Pesce M, Paggi A, Polonara G, Bartolini M, Salvolini U, Manzoni T. Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Brain Res Cogn Brain Res. 2005;24:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Funnell MG, Corballis PM, Gazzaniga MS. Cortical and subcortical interhemispheric interactions following partial and complete callosotomy. Arch Neurol. 2000;57:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Fabri M, Polonara G, Quattrini A, Salvolini U, Del Pesce M, Manzoni T. Role of the corpus callosum in the somatosensory activation of the ipsilateral cerebral cortex: an fMRI study of callosotomized patients. Eur J Neurosci. 1999;11:3983-3994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T. Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci. 2001;13:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Fabri M, Polonara G, Salvolini U, Manzoni T. Bilateral cortical representation of the trunk midline in human first somatic sensory area. Hum Brain Mapp. 2005;25:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Gazzaniga MS, Freedman H. Observations on visual processes after posterior callosal section. Neurology. 1973;23:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Clarke S, Maeder P, Meuli R, Staub F, Bellmann A, Regli L, de Tribolet N, Assal G. Interhemispheric transfer of visual motion information after a posterior callosal lesion: a neuropsychological and fMRI study. Exp Brain Res. 2000;132:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Sugishita M, Otomo K, Yamazaki K, Shimizu H, Yoshioka M, Shinohara A. Dichotic listening in patients with partial section of the corpus callosum. Brain. 1995;118:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Pollmann S, Maertens M, von Cramon DY, Lepsien J, Hugdahl K. Dichotic listening in patients with splenial and nonsplenial callosal lesions. Neuropsychology. 2002;16:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4249] [Cited by in RCA: 3995] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 52. | Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 750] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 53. | Thomsen C, Henriksen O, Ring P. In vivo measurement of water self diffusion in the human brain by magnetic resonance imaging. Acta Radiol. 1987;28:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3340] [Cited by in RCA: 3410] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 55. | Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm. 2013;120:1369-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev Neuropsychol. 2010;35:233-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Polonara G, Fabri M, Mascioli G, Paggi A, Manzoni T, Salvolini U. Interhemispheric connectivity in patients with callosal resection described and quantified using diffusion tensor imaging. 32nd Eur Soc NeuroRadiol Ann Meeting. Italy: Genoa 2007; 20-23. |
| 58. | Pizzini FB, Polonara G, Mascioli G, Beltramello A, Foroni R, Paggi A, Salvolini U, Tassinari G, Fabri M. Diffusion tensor tracking of callosal fibers several years after callosotomy. Brain Res. 2010;1312:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Fabri M, Polonara G. Functional topography of human corpus callosum: an FMRI mapping study. Neural Plast. 2013;2013:251308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Caminiti R, Carducci F, Piervincenzi C, Battaglia-Mayer A, Confalone G, Visco-Comandini F, Pantano P, Innocenti GM. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci. 2013;33:14501-14511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 61. | Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJ. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res. 2001;106:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Werring DJ, Clark CA, Barker GJ, Miller DH, Parker GJ, Brammer MJ, Bullmore ET, Giampietro VP, Thompson AJ. The structural and functional mechanisms of motor recovery: complementary use of diffusion tensor and functional magnetic resonance imaging in a traumatic injury of the internal capsule. J Neurol Neurosurg Psychiatry. 1998;65:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 346] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 65. | Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic-Savatic M, Krupp LB, Wagshul ME. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59:3713-3722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Hannoun S, Bagory M, Durand-Dubief F, Ibarrola D, Comte JC, Confavreux C, Cotton F, Sappey-Marinier D. Correlation of diffusion and metabolic alterations in different clinical forms of multiple sclerosis. PLoS One. 2012;7:e32525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Igarashi H, Katayama Y, Tsuganezawa T, Yamamuro M, Terashi A, Owan C. Three-dimensional anisotropy contrast (3DAC) magnetic resonance imaging of the human brain: application to assess Wallerian degeneration. Intern Med. 1998;37:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Papinutto ND, Maule F, Jovicich J. Reproducibility and biases in high field brain diffusion MRI: An evaluation of acquisition and analysis variables. Magn Reson Imaging. 2013;31:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Ditye T, Kanai R, Bahrami B, Muggleton NG, Rees G, Walsh V. Rapid changes in brain structure predict improvements induced by perceptual learning. Neuroimage. 2013;81:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. J Neurosci. 2013;33:12844-12850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Tavor I, Hofstetter S, Assaf Y. Micro-structural assessment of short term plasticity dynamics. Neuroimage. 2013;81:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Holodny AI, Schwartz TH, Ollenschleger M, Liu WC, Schulder M. Tumor involvement of the corticospinal tract: diffusion magnetic resonance tractography with intraoperative correlation. J Neurosurg. 2001;95:1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Mesaros S, Rocca MA, Kacar K, Kostic J, Copetti M, Stosic-Opincal T, Preziosa P, Sala S, Riccitelli G, Horsfield MA. Diffusion tensor MRI tractography and cognitive impairment in multiple sclerosis. Neurology. 2012;78:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 75. | Anderson VM, Wheeler-Kingshott CA, Abdel-Aziz K, Miller DH, Toosy A, Thompson AJ, Ciccarelli O. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler. 2011;17:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Gorgoraptis N, Wheeler-Kingshott CA, Jenkins TM, Altmann DR, Miller DH, Thompson AJ, Ciccarelli O. Combining tractography and cortical measures to test system-specific hypotheses in multiple sclerosis. Mult Scler. 2010;16:555-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Harrison DM, Caffo BS, Shiee N, Farrell JA, Bazin PL, Farrell SK, Ratchford JN, Calabresi PA, Reich DS. Longitudinal changes in diffusion tensor-based quantitative MRI in multiple sclerosis. Neurology. 2011;76:179-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 79. | Abe O, Masutani Y, Aoki S, Yamasue H, Yamada H, Kasai K, Mori H, Hayashi N, Masumoto T, Ohtomo K. Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr. 2004;28:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 81. | Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Dougherty RF, Ben-Shachar M, Bammer R, Brewer AA, Wandell BA. Functional organization of human occipital-callosal fiber tracts. Proc Natl Acad Sci USA. 2005;102:7350-7355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4350] [Cited by in RCA: 4164] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 85. | Tettamanti M, Paulesu E, Scifo P, Maravita A, Fazio F, Perani D, Marzi CA. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol. 2002;88:1051-1058. [PubMed] |
| 86. | Omura K, Tsukamoto T, Kotani Y, Ohgami Y, Minami M, Inoue Y. Different mechanisms involved in interhemispheric transfer of visuomotor information. Neuroreport. 2004;15:2707-2711. [PubMed] |
| 87. | Weber B, Treyer V, Oberholzer N, Jaermann T, Boesiger P, Brugger P, Regard M, Buck A, Savazzi S, Marzi CA. Attention and interhemispheric transfer: a behavioral and fMRI study. J Cogn Neurosci. 2005;17:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 88. | Mazerolle EL, D’Arcy RC, Beyea SD. Detecting functional magnetic resonance imaging activation in white matter: interhemispheric transfer across the corpus callosum. BMC Neurosci. 2008;9:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 90. | Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 455] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 91. | Stancák A, Lücking CH, Kristeva-Feige R. Lateralization of movement-related potentials and the size of corpus callosum. Neuroreport. 2000;11:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28:3227-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Salvolini U, Polonara G, Mascioli G, Fabri M, Manzoni T. [Functional topography of the human corpus callosum]. Bull Acad Natl Med. 2010;194:617-631; discussion 631-632. [PubMed] |
| 94. | Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T. Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res. 2011;1370:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Polonara G, Mascioli G, Foschi N, Salvolini U, Pierpaoli C, Manzoni T, Fabri M, Barbaresi P. Further evidence for the topography and connectivity of the corpus callosum: An fMRI study of patients with partial callosal resection. J Neuroimaging. 2014;1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, Medina-Bolívar OC, Hernández-Acosta NC, González-Gómez M, Meyer G. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:7. [PubMed] [DOI] [Full Text] |
| 97. | Iadecola C. Intrinsic signals and functional brain mapping: caution, blood vessels at work. Cereb Cortex. 2002;12:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1614] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 99. | Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 100. | D’Arcy RC, Hamilton A, Jarmasz M, Sullivan S, Stroink G. Exploratory data analysis reveals visuovisual interhemispheric transfer in functional magnetic resonance imaging. Magn Reson Med. 2006;55:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Magistretti PJ. Brain energy metabolism. In Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press 2003; 339-360. |
| 102. | Kida I, Hyder F, Behar KL. Inhibition of voltage-dependent sodium channels suppresses the functional magnetic resonance imaging respo-nse to forepaw somatosensory activation in the rodent. J Cereb Blood Flow Metab. 2001;21:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765-10770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 104. | Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44:2691-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Nir Y, Dinstein I, Malach R, Heeger DJ. BOLD and spiking activity. Nat Neurosci. 2008;11:523-524; author reply 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Rossi DJ. Another BOLD role for astrocytes: coupling blood flow to neural activity. Nat Neurosci. 2006;9:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Jakovcevic D, Harder DR. Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol. 2007;79:75-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 108. | Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 845] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 109. | Rabi SJ, Madhavi C, Antonisamy B, Koshi R. Quantitative analysis of the human corpus callosum under light microscopy. Eur J Anat. 2007;11:95-100. |
| 110. | Iadecola C, Beitz AJ, Renno W, Xu X, Mayer B, Zhang F. Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Res. 1993;606:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Estrada C, DeFelipe J. Nitric oxide-producing neurons in the neocortex: morphological and functional relationship with intraparenchymal microvasculature. Cereb Cortex. 1998;8:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2007] [Cited by in RCA: 1768] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 113. | Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 114. | Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 115. | Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 839] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 116. | Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1830] [Cited by in RCA: 1838] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 117. | Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783-2802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 567] [Cited by in RCA: 609] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 118. | Tiede R, Krautwald K, Fincke A, Angenstein F. NMDA-dependent mechanisms only affect the BOLD response in the rat dentate gyrus by modifying local signal processing. J Cereb Blood Flow Metab. 2012;32:570-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Bührle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409-8416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Nielsen AN, Fabricius M, Lauritzen M. Scanning laser-Doppler flowmetry of rat cerebral circulation during cortical spreading depression. J Vasc Res. 2001;37:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007;27:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Iadecola C, Li J, Xu S, Yang G. Neural mechanisms of blood flow regulation during synaptic activity in cerebellar cortex. J Neurophysiol. 1996;75:940-950. [PubMed] |
| 123. | Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1048] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 125. | Gawryluk JR, Brewer KD, Beyea SD, D’Arcy RC. Optimizing the detection of white matter fMRI using asymmetric spin echo spiral. Neuroimage. 2009;45:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Mazerolle EL, Beyea SD, Gawryluk JR, Brewer KD, Bowen CV, D’Arcy RC. Confirming white matter fMRI activation in the corpus callosum: co-localization with DTI tractography. Neuroimage. 2010;50:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 127. | Miller MB, Sinnott-Armstrong W, Young L, King D, Paggi A, Fabri M, Polonara G, Gazzaniga MS. Abnormal moral reasoning in complete and partial callosotomy patients. Neuropsychologia. 2010;48:2215-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Pierpaoli C, Ferrante L, Berlucchi G, Ortenzi A, Manzoni T, Fabri M. Imitation strategies in callosotomized patients. 8th National Congress IBRO. Italy: Florence 2011; 14-18. |
| 129. | Pierpaoli C, Polonara G, Foschi N, Salvolini U, Berlucchi G, Fabri M. Cortical activation during imitative behaviour in control subjects and callosotomized patients. 9th FENS Forum of Neuroscience. Italy: Milan 2014; 5-9. |
| 130. | Volpe BT, Sidtis JJ, Holtzman JD, Wilson DH, Gazzaniga MS. Cortical mechanisms involved in praxis: observations following partial and complete section of the corpus callosum in man. Neurology. 1982;32:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Bentin S, Sahar A, Moscovitch M. Intermanual information transfer in patients with lesions in the trunk of the corpus callosum. Neuropsychologia. 1984;22:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Geffen G, Nilsson J, Quinn K, Teng EL. The effect of lesions of the corpus callosum on finger localization. Neuropsychologia. 1985;23:497-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 133. | Risse GL, Gates J, Lund G, Maxwell R, Rubens A. Interhemispheric transfer in patients with incomplete section of the corpus callosum. Anatomic verification with magnetic resonance imaging. Arch Neurol. 1989;46:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Levin HS, Mattson AJ, Levander M, Lindquist CE, Simard JM, Guinto FC, Lilly MA, Eisenberg HM. Effects of transcallosal surgery on interhemispheric transfer of information. Surg Neurol. 1993;40:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Aglioti S, Tassinari G, Corballis MC, Berlucchi G. Incomplete gustatory lateralization as shown by analysis of taste discrimination after callosotomy. J Cogn Neurosci. 2000;12:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Aglioti SM, Tassinari G, Fabri M, Del Pesce M, Quattrini A, Manzoni T, Berlucchi G. Taste laterality in the split brain. Eur J Neurosci. 2001;13:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Le Bihan D. Diffusion, confusion and functional MRI. Neuroimage. 2012;62:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 138. | Gawryluk JR, Mazerolle EL, D’Arcy RC. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci. 2014;8:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |