INTRODUCTION
Pancreatic cancer remains one of the most deadly cancers, ranked as the fourth most common cause of cancer related death in the United States per the American Cancer Society for 2009[1]. One of the reasons is because the pancreas is located deep in the abdomen, in close proximity to vital vascular structures that are early involved in the course of this disease. The median time between onset of usually nonspecific symptoms and presentation is 6 mo[2]. Only a small fraction of patients at presentation have resectable disease because of either already present metastases or locally advanced disease involving neighboring vasculature, and associated perineural invasion, to such an extent that surgical excision is no longer possible[3,4]. For all of these reasons, five year survival is only 5%[5].
Imaging plays a central role in the management of this disease. Imaging facilitates establishing a diagnosis, determining staging information, monitoring treatment response, and detecting tumor recurrence following surgery. Multiple modalities are involved, including computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography with computed tomography (PET/CT), and endoscopic ultrasound (EUS). These modalities have all evolved over the past many years and so have their roles with regard to pancreatic cancer.
This article will review the use of multiple imaging modalities in many of these facets, and will show illustrative examples from a broad range of modalities. It will attempt to emphasize the unique contributions of each modality and where their strengths can best be used.
DIAGNOSIS
Usually patients with pancreatic cancer present because of symptoms of abdominal pain, accompanied by jaundice and/or unexplained weight loss[6]. For this reason, patients will often undergo transabdominal ultrasound as their initial examination in order to evaluate for possible cholecystitis and/or obstructing calculi within the cystic duct, common hepatic duct or common bile duct. In our experience, while ultrasound is excellent for evaluation of gallbladder pathology, it is still limited for evaluation of the pancreas despite multiple recent advances. This is likely because of limitations of obscuring bowel gas, and dependence on the experience of the sonographer, among other factors. In pancreatic cancer, if the common bile duct is obstructed, ultrasound will likely demonstrate that ductal obstruction is within the region of the pancreatic head. In many cases in the past, patients have then undergone endoscopic retrograde cholangio-pancreatography (ERCP) to diagnose and treat biliary obstruction. Unfortunately, post-ERCP changes, as well as imaging artifacts secondary to biliary stents, significantly degrade the imaging results of subsequent cross-sectional imaging, whether by CT, MRI or EUS. As will be discussed subsequently, cross-sectional imaging has assumed a central role in the diagnosis and management of pancreatic cancer. For this reason, an important change at our institution has been to do cross-sectional imaging, typically by CT or MRI, before any intervention (ERCP, stent placement, biopsy, etc.). At our institution, EUS can be done at the same setting as ERCP, and is done first to avoid the problems of imaging after stent placement.
At many institutions, multidetector computed tomography (MDCT) has become the primary modality for evaluating suspected pancreatic cancer. This technology has been rapidly evolving with the development of ever faster and thinner section imaging with the evolution of MDCT. As advocated by the National Comprehensive Cancer Network guidelines, this is typically through multiphase imaging technique, with pre-contrast imaging and imaging of the abdomen obtained during the peak pancreatic parenchymal phase and peak liver portal venous enhancement phase[7]. The earlier phase best depicts the primary tumor (Figure 1) while the latter phase best demonstrates tumor involvement of venous structures and liver metastases[8-10]. Use of saline flush in addition to power injection may additionally improve enhancement of the pancreatic parenchyma and adjacent vasculature[11]. One of the newest developments, dual energy or multispectral CT, may be useful in the detection of subtle differences in enhancement with iodinated contrast but currently no studies have been published regarding its utility in pancreatic cancer.
Figure 1 Axial images from dual phase pancreas protocol contrast enhanced computed tomography examination in patient with history of pancreatic cancer shows (A) better demarcation of tumor (solid arrows) on the pancreatic parenchymal phase than on the (B) portal venous phase.
There is marked narrowing of the splenoportal confluence (dashed arrows) secondary to encasement.
Thin section imaging is important for not only showing the primary tumor but also for showing its relationship to vasculature not only in the original axial plane of acquisition but also in detailed multiplanar reconstructions (Figure 2). At our institution, dual phase studies are reconstructed into two data sets. Thicker 2.5 mm are used for primary interpretation, and thinner 0.625-1.25 mm axial sections are used for problem solving and for multiplanar reconstructions.
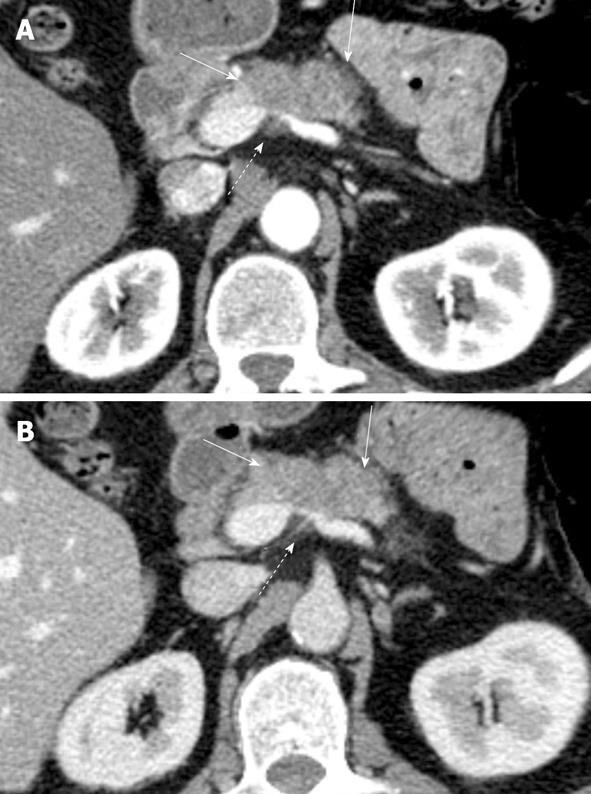
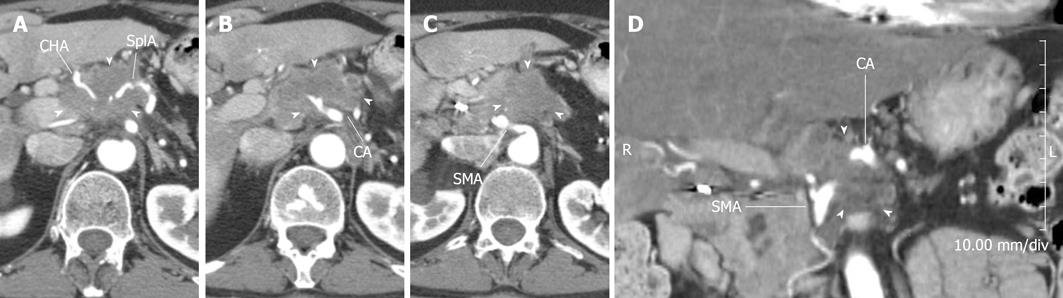
Figure 2 Patient with locally advanced pancreatic cancer.
A-C: Pancreatic parenchymal phase images show tumor (arrowheads) (A) encasing the common hepatic artery (CHA) and splenic artery (SA) (B) encasing the celiac axis (CA) and (C) abutting the superior mesenteric artery (SMA); D: Coronal reformation helps demonstrate the encasement of the celiac artery and abutment of the superior mesenteric artery. R: Right; L: Left.
Pancreatic cancer usually manifests on CT as an ill-defined, solid mass, slightly hypodense to normal pancreatic parenchyma. Overall sensitivity of MDCT for pancreatic cancer is 86%-97% for tumors of any size, but sensitivity is probably near 77% for small (< 2 cm) lesions[12-16]. It is important to pay close attention to secondary signs when the primary tumor is difficult to visualize, which is particularly the case for small lesions. These signs include focal pancreatic enlargement, extension of tumor beyond the pancreas, “upstream” pancreatic atrophy (secondary to ductal obstruction) and, most importantly, dilatation and/or cut off of the pancreatic duct and/or common bile duct. Gangi et al[17] reported in a study of 28 patients with pancreatic cancer who had CT imaging prior to histologic diagnosis that findings suspicious for pancreatic cancer were present in 50% of cases obtained 2-6 mo and 6-18 mo prior to the diagnosis of pancreatic cancer.
Such signs are also of particular utility in the 5.4% of pancreatic cancers that are truly isoattenuating on both phases of a dual phase pancreatic protocol[18]. In such circumstances, other modalities such as EUS can be considered. A study of isoattenuating tumors showed that MRI and PET/CT had sensitivities of 79.2% and 73.7% in such cases and can also be considered for followup when CT is equivocal[18].
Another recent development has been the use of a variety of types of reformations to enhance the conspicuity of tumor and its relationship to local structures. Prokesch et al[19] showed that use of curved multiplanar reconstructions drawn along the common bile duct, pancreatic duct, and/or mesenteric vessels may help improve sensitivity for tumor detection and the speed of interpretation over axial images alone. Salles et al[20] noted that minimum intensity projection images, besides showing ductal structures, may help improve the conspicuity of the primary tumor within the pancreas (Figure 3).
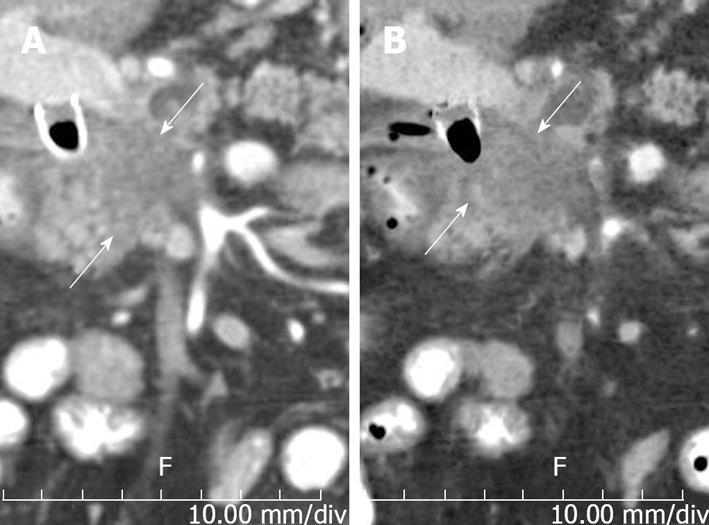
Figure 3 Utility of reconstructions.
A: Coronal reconstruction demonstrating craniocaudal extent of tumor (arrows); B: Coronal Minip (minimum intensity projection), same obliquity and orientation as a emphasizes low density structures, making tumor more conspicuous.
There have been multiple recent developments in MRI including parallel imaging, imaging at field strengths greater than 1.5 T, liver specific contrast agents (for detection of liver metastases), and broader implementation of diffusion weighted imaging. At our institution, a typical imaging protocol includes multiple axial acquisitions including fat suppressed T2 weighted imaging of the abdomen, thin-section T2 weighted imaging of the pancreatic region, in-and-out of phase T1 weighted imaging, fat suppressed dynamically obtained 3-D T1 weighted imaging (20 s, 60 s, 120 s, and 180 s after the start of contrast injection), fat suppressed thin-section fast imaging employing steady-state acquisition (GE Medical Systems, Milwaukee, WI), and axial diffusion weighted imaging.
Pancreatic cancer typically has the appearance on MRI of an ill defined mass, as on CT. MRI offers the advantage of multiple sequences with inherently greater soft tissue contrast without enhancement than unenhanced CT. Tumor typically appears hypointense on fat suppressed T1, and pancreatic parenchymal phase dynamically enhanced fat suppressed T1-weighted sequences. Pancreatic cancer has a variable appearance on T2 weighted images. Pancreatic cancer also has a variable appearance on diffusion weighted images (Figure 4). In a recent study of 80 patients, 38 appeared hyperintense, 12 isointense, and four hypointense[21]. Early reports from the literature are beginning to investigate the utility of diffusion weighted imaging for diagnosing pancreatic cancer, and for differentiating chronic pancreatitis from tumor[22-25].
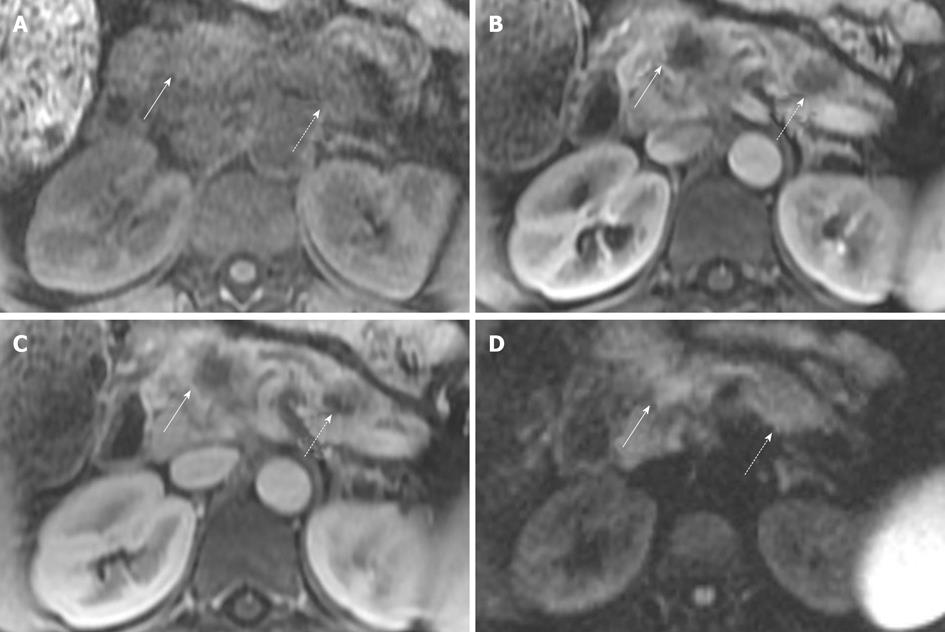
Figure 4 Magnetic resonance imaging of patient with two sites of pancreatic cancer.
A: T1 fat suppressed images do not show the tumor well in this patient, but are often helpful. Solid arrow indicates the pancreatic neck tumor, dashed arrow indicates the pancreatic tail tumor; B, C: However, both sites are well seen, in the neck (solid arrows) and tail (dashed arrows) on the pancreatic parenchymal phase (B) and the portal venous phase (C); D: In this case sites of tumor are not well seen on diffusion weighted imaging. Solid arrow indicates the pancreatic neck tumor, dashed arrow indicates the pancreatic tail tumor. Conspicuity of the primary lesion can be very variable on diffusion weighted imaging.
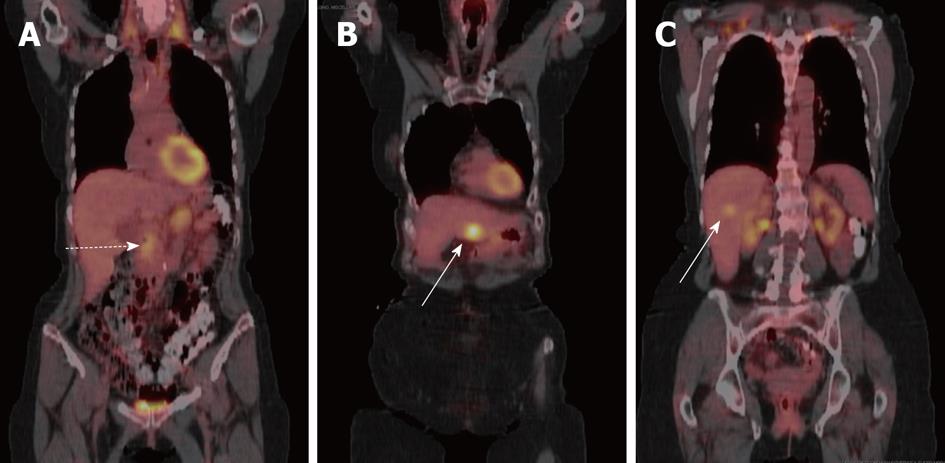
At most institutions, PET/CT, as currently performed without intravenous contrast, has a limited role in the diagnosis and staging of pancreatic cancer (Figure 5). A recent study of 103 patients with pancreatic cancer showed a similar detection rate between contrast-enhanced MDCT and PET/CT (89% vs 91%)[26].
Figure 5 Coronal fused positron emission tomography with computed tomography images of patient with pancreatic cancer and liver metastases.
A: Primary tumor shows mild-moderate uptake (dashed arrow); B, C: While liver metastases in (B) and (C) show variable uptake (solid arrows).
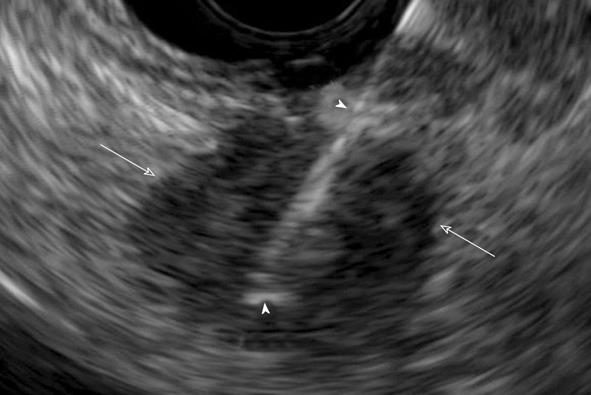
EUS has developed a strong role in the detection and confirmation of pancreatic cancer. EUS has the advantage that tumor can be visualized in real time without the requirement of a contrast agent (Figure 6). A study from our institution showed EUS-fine needle aspiration (FNA) to have higher sensitivity (99%) than MDCT (89%-93%)[16]. The technique is of particular utility for small lesions < 2 cm in size, with the sensitivity of EUS being 96%, and 70%-93% for MDCT[16]. However, the technique is invasive, and is operator dependent. The presence of a biliary stent can greatly lower the ability of EUS-FNA to exclude tumor[16]. For this reason at our institution, cross-sectional imaging (MDCT or MRI), is performed first, followed by EUS-FNA. As noted previously, at our institution ERCP and EUS are done at the same setting, with EUS done first.
Figure 6 Endoscopic ultrasound guided biopsy of pancreatic cancer manifesting as a hypoechoic mass (arrows).
Biopsy needle is visualized during the procedure (arrowheads).
STAGING
In 2002, The American Joint Committee on Cancer Staging, changed the T staging criteria to focus on tumor involvement of arteries and removed reference to venous involvement[2]. This is likely secondary to advances made in the use of venous bypass grafts that allowed for successful en bloc resection of tumor involving veins[27]. Additionally, perineural invasion, a histopathologic poor prognostic factor, parallels the course of arteries, likely increasing the importance of arterial involvement[4]. As a result, the challenge of imaging has become to better identify the extent of tumor involvement of vasculature, particularly the celiac axis, the superior mesenteric artery and the common hepatic artery.
Vascular involvement
The determination of the extent of vascular involvement is typically made by identifying the extent that tumor involves the cross-sectional circumference of a vessel. This can be done by describing with regard to the circular cross-section of a vessel the degrees of circumferential involvement as described by Lu et al[28]. Since that time, the terms “abutment” and “encasement,” have also been utilized, abutment referring to 180° of involvement or less of a vessel’s circumference and encasement referring to greater than 180° of vascular circumferential involvement (Figure 2)[29,30]. These terms (degrees of circumferential involvement, abutment/encasement, as well as contiguous/discontiguous involvement) are also utilized in the Radiological Society of North America’s suggested reporting template for primary pancreatic masses[31]. The utility of these terms includes the ability to differentiate clearly resectable tumor, from “borderline” resectable tumor, from clearly unresectable tumor[30]. The assessment of vascular involvement is typically made at most institutions on either CT or MRI. A prospective blinded study comparing EUS, MRI and CT showed accuracies of 62%, 60% and 74% respectively for locoregional extent of tumor[32]. A recent study of 116 patients comparing MRI with MR angiography against MDCT showed similar sensitivities between the modalities (approximately 90%) for determining resectability[33].
The use of reformations have also been shown to have a role in assessing vascular involvement. Horton et al[34-37] noted anecdotally that MIP images improve visualization of SMV branches and distal arterial branches, volume rendered images help depict arterial variants and the relationship of tumor to vessels, while coronal oblique volume rendered views show well tumor involvement along the length of the portal venous system.
Nodal involvement
Nodal involvement by tumor, whether the total number of nodes involved, or the lymph node ratio (number of positive nodes out of all nodes examined), is a significant prognostic factor[38-40]. Unfortunately, all of the modalities are limited in regard to the evaluation of nodal disease. For cross-sectional modalities, such as CT and MRI, the criterion for identifying adenopathy has typically been size. Unfortunately, as shown by Valls et al[41] in their study of patients with pancreatic cancer undergoing preoperative assessment with CT, size is a nonspecific criterion. They showed that only 3 of 18 patients (16.7%) who had pathologically proved nodal involvement were detected when a size criterion of > 1.5 cm was utilized. Other criteria, such as poorly defined nodal boundaries, conversion of nodes to a rounded shape, and conversion to a hypodense appearance are more specific, but are less sensitive. MRI, when using fat suppressed T2 sequences, can be helpful in differentiating nodes from adjacent liver in the region of the porta hepatis[42]. PET/CT has been reported to have sensitivities of 46%-71% and specificities of 63%-100% for nodal involvement[43-45]. EUS-FNA offers the benefit of histopathologic proof, but the number of sites that can be evaluated in this manner is limited.
Distant metastases
Pancreatic cancer typically metastasizes to the liver, peritoneum, and lungs, with metastases to osseous structures being less common, and in our experience, typically later in the course of the disease.
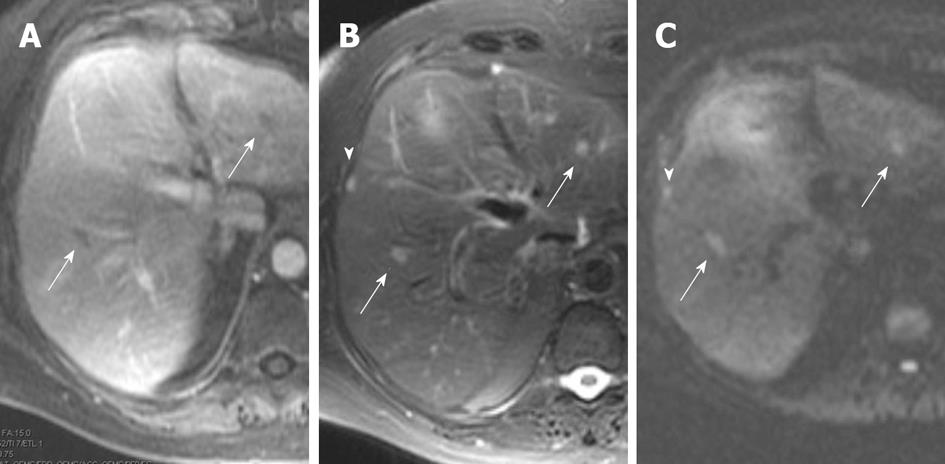
In most institutions, the assessment for liver metastases is usually made by CT or MRI. The sensitivity of intravenous contrast enhanced CT has been reported to be approximately 75%-87%[46-48]. Conventional gadolinium enhanced dynamic MRI has been considered to be similar or slightly better than CT. In a study directly comparing MRI and CT in 58 patients, the accuracy of MRI was 93.5% vs 87.2% for CT[47]. It is notable that liver metastases can have a variable appearance on MRI. Danet et al[49] studied a small group of 16 patients with liver metastases with biopsy confirmed pancreatic adenocarcinoma and/or biopsy proven liver metastases, with dynamic gadolinium enhanced imaging and T2 weighted imaging. Of these patients, 75% of the patients had lesions that were 1.5 cm in size, 62% had hypovascular lesions, 38% had reportedly hypervascular lesions, and 62% showed perilesional enhancement, with 38% having this form of enhancement in a ring pattern. Newer liver specific agents such as gadobenate dimeglumine (Gd-BOPTA) can be used for dynamic imaging, allowing for arterial and portal venous phase imaging, and can also be used for delayed phase imaging because of contrast retention by hepatocytes which may improve sensitivity for liver metastases. While, to our knowledge, specific assessment for pancreatic cancer liver metastases has not be examined, the overall mean accuracy for liver metastases using Gd-BOPTA in one study was approximately 95.5% compared to superparamagnetic iron oxide-enhanced imaging with a mean accuracy of 97.2%[50]. Diffusion weighted imaging has the advantage that no intravenous contrast needs to be administered (Figure 7). In a study of 31 patients, MDCT was compared against diffusion weighted imaging using b values of 0, 300 and 600 s/mm2. The sensitivities and specificities for liver metastases that had been correlated with intraoperative surgical and ultrasound findings were 53.3% and 77.8% for MDCT, and 86.7% and 97.5% for diffusion weighted imaging, which was noted to be statistically significant[51].
Figure 7 Magnetic resonance imaging images from patient with liver metastases from biopsy proven pancreatic cancer.
A: Portal venous phase image, shows two hypointense foci (arrows) within the liver; B: T2 fat suppressed images also show the two lesions and show to better effect the subcapsular metastasis (arrowhead), arrows are metastases in liver parenchyma; C: Diffusion weighted image (b value of 500 s/mm2) shows all three lesions well, without confounding T2 bright signal in biliary tree or vessels seen on T2 weighted images. Arrows are metastases in liver parenchyma, arrowhead is the subcapsular liver metastasis.
All modalities have limited effectiveness in detecting the typically very small peritoneal implants of pancreatic cancer. Laparoscopy has been utilized to improve detection of peritoneal implants. This technique is invasive, and therefore can’t be used to monitor response to therapy. A meta-analysis in 2001 showed that laparoscopy may only alter management in 4%-15% of patients after optimized, thin section, CT[52]. Some studies suggest that PET/CT may offer benefits over contrast enhanced CT for the detection of distant metastases (Figure 5) as it provides a whole body scan, and when combined with IV contrast, may provide a comprehensive assessment for diagnosis and staging[53-55].