Published online Mar 28, 2012. doi: 10.4329/wjr.v4.i3.109
Revised: December 8, 2011
Accepted: December 15, 2011
Published online: March 28, 2012
AIM: To evaluate the feasibility of intravenous contrast-enhanced C-arm computed tomography (CT) for assessing ablative areas and margins of liver tumors.
METHODS: Twelve patients (5 men, 7 women; mean age, 69.5 years) who had liver tumors (8 hepatocellular carcinomas, 4 metastatic liver tumors; mean size, 16.3 mm; size range, 8-20 mm) and who underwent percutaneous radiofrequency ablations (RFAs) with a flat-detector C-arm system were retrospectively reviewed. Intravenously enhanced C-arm CT and multidetector computed tomography (MDCT) images were obtained at the end of the RFA sessions and 3-7 d after RFA to evaluate the ablative areas and margins. The ablated areas and margins were measured using axial plane images acquired by both imaging techniques, with prior contrast-enhanced MDCT images as the reference. The sensitivity, specificity, and positive and negative predictive values of C-arm CT for detecting insufficient ablative margins (< 5 mm) were calculated. Statistical differences in the ablative areas and margins evaluated with both imaging techniques were compared using a paired t-test.
RESULTS: All RFA procedures were technically successful. Of 48 total ablative margins, 19 (39.6%) and 20 (41.6%) margins were found to be insufficient with C-arm CT and MDCT, respectively. Moreover, there were no significant differences between these 2 imaging techniques in the detection of these insufficient ablative margins. The sensitivity, specificity, and positive and negative predictive values for detecting insufficient margins by C-arm CT were 90.0%, 96.4%, 94.7% and 93.1%, respectively. The mean estimated ablative areas calculated from C-arm CT (462.5 ± 202.1 mm2) and from MDCT (441.2 ± 212.5 mm2) were not significantly different. The mean ablative margins evaluated by C-arm CT (6.4 ± 2.2 mm) and by MDCT (6.0 ± 2.4 mm) were also not significantly different.
CONCLUSION: The efficacy of intravenous contrast-enhanced C-arm CT in assessing the ablative areas and margins after RFA of liver tumors is nearly equivalent to that of MDCT.
- Citation: Iwazawa J, Ohue S, Hashimoto N, Mitani T. Ablation margin assessment of liver tumors with intravenous contrast-enhanced C-arm computed tomography. World J Radiol 2012; 4(3): 109-114
- URL: https://www.wjgnet.com/1949-8470/full/v4/i3/109.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i3.109
Radiofrequency ablation (RFA) is an established local therapy for managing malignant liver tumors because of its applicability to minimally invasive treatment. A recent randomized controlled trial demonstrated that RFA for small hepatocellular carcinoma confers a survival benefit comparable to that of surgical resection[1]. In addition, RFA offers patients the potential benefits of safety, reasonable cost, and reduced hospitalization, while resulting in postoperative outcomes comparable to those of surgery[2]. To achieve curative ablation, complete ablation of the tumor with ablative margins of at least 5 mm is required[3]. Failure to obtain these sufficient safety margins is one of the significant prognostic factors affecting local tumor progression[4]. The ablative areas and margins are usually evaluated after RFA therapy with postprocedural contrast-enhanced multidetector computed tomography (MDCT) or magnetic resonance (MR) imaging[3,5]. However, an immediate assessment of therapeutic efficacy using CT or MR imaging can be difficult to accomplish unless the procedure can be performed on the available CT or MR imaging equipment. Otherwise, the patient must be transferred to the nearest imaging scanner, which requires significant preparation time and carries an increased risk of contamination of both the patient and the RFA equipment.
The flat-detector C-arm angiographic system is a relatively new imaging system that generates both conventional angiographic images and multisectional soft tissue images similar to those of CT[6,7]. Moreover, recent developments in C-arm cone-beam CT technology have enabled the prompt acquisition of CT-like images without the need for patient transfer and with a CT dose index lower than that of conventional CT[8,9]. Furthermore, a wider free space than that for a conventional CT scanner is available in the C-arm equipment, and thus, easier and safer RFA procedures would be expected. C-arm CT images are acquired by rotating a flat detector around the patient, and these images provide useful information about tumor location and configuration[10]. The images can also be used to determine the best navigation route for the needle[11] to perform RFA of liver tumors. Due to the low-contrast nature of C-arm CT[12], assessment of the therapeutic efficacy of RFA requires contrast-enhanced C-arm CT images that are commonly acquired by injecting contrast material through a catheter previously placed in the hepatic artery[11]. The intravenous administration of contrast material is a convenient and timesaving technique that is less invasive for the patients. However, the application of intravenously enhanced C-arm CT for assessing the therapeutic efficacy of RFA has not yet been documented.
In this preliminary study, we evaluated the feasibility of intravenously enhanced C-arm CT for the immediate assessment of ablative areas and margins after RFA of malignant liver tumors.
We retrospectively reviewed 12 patients (5 men, 7 women; mean age, 69.5 years) who had solitary liver tumors (8 hepatocellular carcinomas and 4 metastatic tumors; size range, 8-20 mm; mean size, 16.3 mm) and who underwent intravenous contrast-enhanced C-arm CT and MDCT after RFA between December 2009 and April 2011. All patients underwent contrast-enhanced MDCT within 56 d (mean, 35.6 d) prior to the RFA session. The diagnosis of the tumor was confirmed from previous imaging findings as well as from elevated levels of serum tumor markers. For evaluating ablative areas and margins, intravenously enhanced C-arm CT and MDCT images were obtained at the end of the RFA sessions and 3-7 d after the sessions. Ablative areas and margins measured with both imaging techniques were compared, with preprocedural MDCT images as the reference.
This study proceeded in accordance with the guidelines of our institutional review board, and written informed consent was obtained from the patients.
All RFA procedures were performed with a flat-detector C-arm angiographic system (Innova 3100; GE Healthcare, Waukesha, WI, United States). A 17-gauge radiofrequency electrode (Cool-tip radiofrequency electrode; Radionics, Burlington, MA, United States) with an exposed needle tip of 2 or 3 cm was used depending on the size of the tumor. The needle was inserted using ultrasound (US) guidance in all cases. When the target tumor was poorly defined by the US, C-arm CT was used to assist the needle placement. The ablation was terminated when tissue resistance reached the maximum value or when the ablative duration exceeded 6 min. After the ablation was completed, the temperature of the ablated tissue was measured. When the tissue temperature had not exceeded 60 °C, additional ablation was performed after needle replacement until the tissue temperature exceeded 60 °C[13].
Intravenous contrast-enhanced C-arm CT images were obtained 2 min after injecting a 90-mL bolus of iopamidol (300 mgI/mL; flow rate, 1 mL/s) through the antecubital vein by using a power injector. The image acquisition parameters were as follows: total scanning angle, 200°; acquisition time, 10 s; matrix size, 1500 × 1500; isotropic voxel size, 0.2 mm; and effective field of view (FOV), 18 cm2. Raw data sets were transferred to an external workstation (Advantage Workstation 4.2; GE Healthcare), where the images were reconstructed with a slice thickness of 3-5 mm on multiple planes. Images were reconstructed within approximately 2.25 min of the end of each scan.
Triphasic contrast-enhanced MDCT images were obtained using a 16-MDCT scanner (Somatom Sensation, Siemens Medical Solutions, Forchheim, Germany) with the following scanning parameters: 120 kV; 182 mA; beam collimation, 0.75 mm; helical pitch, 1.15 mm; and rotation table speed, 0.5 s. Helical acquisition for arterial, portal, and venous phase imaging was initiated at 20, 60 and 160 s, respectively, after a threshold level of 100 Hounsfield units (HU) was reached in the abdominal aorta. Images were reconstructed in 5-mm thick transverse sections.
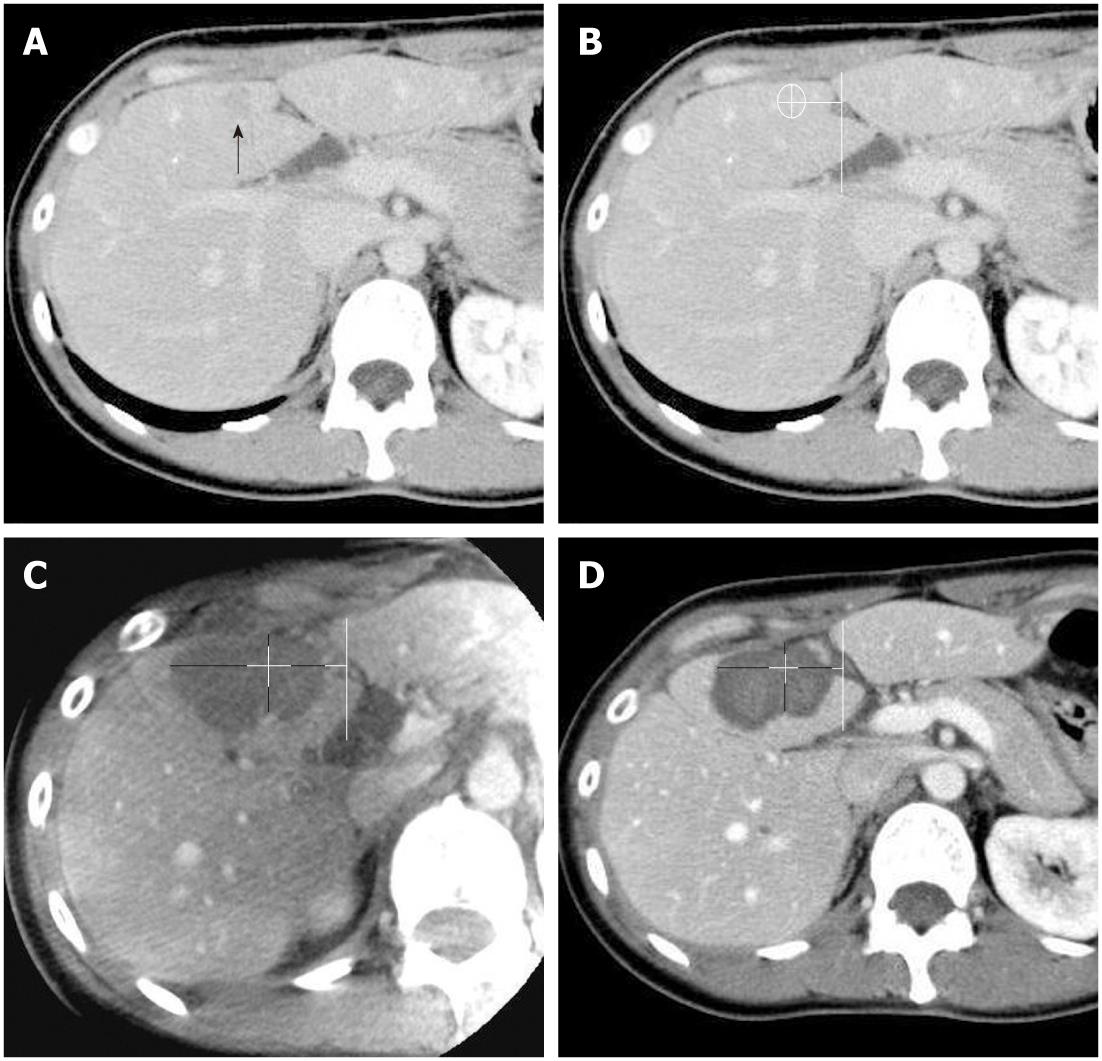
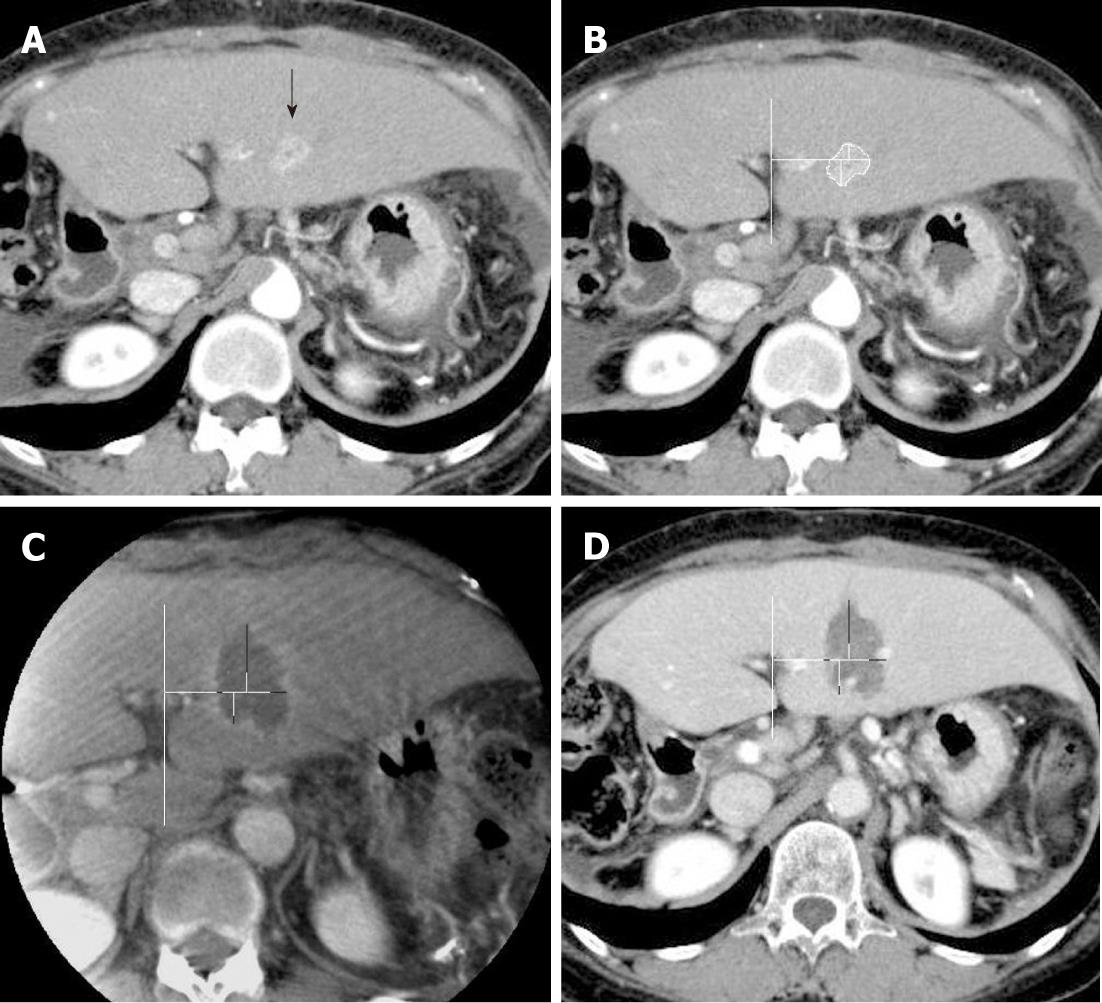
Both contrast-enhanced C-arm CT images and portal-phase MDCT images were viewed on a commercial workstation (eFilm Workstation; Infocom, Tokyo, Japan) by a single observer who had more than 10 years of experience in hepatic vascular intervention and liver imaging. Firstly, the diameters of the ablated regions were measured in 2 orthogonal long and short axes by using axial plane images of each imaging modality. By using an eclipse model, the estimated ablated area was calculated as πab, where a and b are one-half of the long and short axes, respectively. Secondly, the ablative margins of each region were measured in 4 orthogonal directions on the axial plane image by measuring the distance from the nearest hepatic or portal vein, with preprocedural MDCT images as the reference (Figures 1 and 2). The distance measurements were performed manually on the workstation.
Differences in the detection of insufficient ablative margins (< 5 mm), mean estimated ablative areas, and mean ablative margins in C-arm CT and MDCT images were statistically compared using the paired t-test. The sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for detecting insufficient ablation margins by C-arm CT were calculated, using MDCT images as the reference.
All RFA procedures were technically successful without major complications in all 12 patients. Two of the twelve patients received C-arm CT assistance during the RF needle placement because the target lesion was located outside the US scan range. Of the 12 patients, 6 received a single ablation, whereas the remaining 6 underwent an additional ablation after adjusting the needle position based on US images. The mean ablation time for single lesions was 8.9 min (range, 6-12 min). The mean therapeutic time from needle insertion to completion of the final image acquisition of C-arm CT was approximately 40 min. For all 12 patients, intravenous contrast-enhanced C-arm CT images were successfully obtained after the procedure. There were no significant differences in detection for the long and short axes of the ablated tissues, mean estimated ablative area, mean ablative margin, and an insufficient safety margin with C-arm CT and with MDCT (Table 1). The sensitivity, specificity, PPV, and NPV for detecting insufficient safety margins with the C-arm CT were 90.0%, 96.4%, 94.7% and 93.1%, respectively. One false positive and 2 false negative findings were recorded on C-arm CT images. The false positive result was attributed to the oblique acquisition of the C-arm CT images, and the 2 false negatives were caused by size reduction of the ablated tissues. Local tumor recurrence was observed in 2 of the 12 study lesions during the follow-up observation periods (mean, 10.1 mo; range 5-18 mo). These 2 recurrent lesions arose from the locations that were equally judged as insufficient ablative margins using both C-arm CT and MDCT.
| C-arm computed tomography | Multidetector computed tomography | P value | |
| Mean long axis (mm) | 28.1 ± 8.1 | 27.3 ± 8.1 | 0.134 |
| Mean short axis (mm) | 19.9 ± 4.8 | 19.3 ± 5.1 | 0.161 |
| Mean estimated ablative area (mm2) | 462.5 ± 202.1 | 441.2 ± 212.5 | 0.206 |
| Mean ablative margin (mm) | 6.4 ± 2.2 | 6.0 ± 2.4 | 0.159 |
| Insufficient safety margin detection (%) | 39.6 (19/48) | 41.6 (20/48) | 0.569 |
In this study, we found that C-arm CT is nearly equivalent to MDCT in terms of detecting ablative areas and insufficient safety margins directly after RFA procedures. This result could be applied to determine the therapeutic endpoint of RFA and map further treatment strategies. When C-arm CT detects an insufficient ablation margin or a residual lesion, targeted additional ablation would then be promptly applied under C-arm CT assistance. Conversely, when sufficient ablative margins are confirmed on C-arm CT images, additional therapeutic assessments with other imaging modalities may not be required. Another advantage of C-arm CT is its multiplicity in usage. As C-arm CT has comparable ability to MDCT for the detection of hypervascular liver tumors[10,14], operators can obtain pretreatment information for treatment planning, including skin entry point, needle path, and target lesion location, size, and configuration from C-arm CT images immediately prior to the therapy. C-arm CT is also useful during therapy for enabling 3D fluoroscopic guidance for insertion of the radiofrequency electrode to the target tumor, which provides operators with a safer and easier approach to the target[11]. Furthermore, as suggested by our results, the treatment efficacy of RFA can be evaluated with C-arm CT acquired immediately after the therapy. Thus, C-arm CT has various potential applications throughout the RFA procedures.
Although there were no significant differences in detection for the long and short axes of the ablated tissues, the mean estimated ablative area, and mean ablative margin with C-arm CT and with MDCT, all of the parameters were slightly overestimated by C-arm CT relative to MDCT. This result might be attributable to differences in the time points at which the image acquisitions occurred. During the ablation process, the tissue is destroyed by coagulative necrosis. The resultant ablated region then gradually decreases in size through tissue shrinkage[15]. Therefore, a more accurate comparison would have been expected if C-arm CT and MDCT images were obtained on the same day.
Kim et al[16] reported that a 5-mm safety margin was achieved in only 2.7% of patients, and vessel-induced indentation of the ablation zone contributed to the thinnest ablative margins observed in 47.3% in RFA of patients with hepatocellular carcinoma whose tumor diameters were 2-5 cm. In this study, a 5-mm safety margin was achieved in 60.4% (29/48) of patients with C-arm CT and 58.3% (28/48) of patients with MDCT. Tumor size in this study (mean, 1.6 cm; range 0.8-2.0 cm) was relatively small, which might have affected this higher rate of achieving sufficient ablative margins in our study. Vessel-related indentation for insufficient ablative margin was observed in 37.5% (18/48) of subjects for both C-arm CT and MDCT in this study, as shown in Figure 2.
C-arm CT intrinsically has potential disadvantages in the assessment of ablative areas and the safety margins in terms of contrast resolution, image quality, and FOV. Although C-arm CT has a comparable spatial resolution to MDCT[17], its contrast resolutions are expected to be only 50 HU for abdominal application[6]. The usage of contrast material for C-arm CT acquisition may overcome this limitation; however, imaging information for liver tumors using intravenously enhanced C-arm CT has not yet been quantified. C-arm CT has modality-specific artifacts that occasionally can negatively affect the image quality. For example, transient movement during image acquisition spoils the entire image quality in C-arm CT, whereas only the affected slice image is spoiled in MDCT. Recently, respiratory motion artifacts in C-arm CT have been successfully corrected in a phantom model[18]. Thus, patients who have difficulty holding their breath or have tumors in the left hepatic lobe susceptible to the heartbeat could be accurately examined by C-arm CT in the near future. In the current study, although some movement artifacts affected image quality, the detection of ablative areas and margins remained acceptable. The effective FOV available with the flat-detector C-arm system used in this study was only 18 mm2. This might be acceptable for planning and assessing the RFA for a single lesion; however, it becomes problematic when visualization of 2 or more hepatic lesions located throughout the entire liver is required. However, these shortcomings can be overcome using a wider detector with an increased scanning range.
This study has several limitations. Firstly, we compared single-phase contrast-enhanced C-arm CT with triple-phase contrast-enhanced MDCT. Due to the relatively long reconstruction time for the current version of C-arm CT, acquiring triphasic contrast-enhanced C-arm CT images was unachievable. We therefore compared contrast-enhanced C-arm CT images with the portal-phase images of MDCT. Secondly, the ablated area and margins were measured manually on the workstation by a single observer. The measurements may have contained certain errors. Thirdly, the amount of iopamidol administered for a single acquisition of C-arm CT was 90 mL in this study. Repeated acquisition of contrast-enhanced C-arm CT is limited due to increased risk of renal disturbance. Fourthly, we evaluated ablative areas and margins only on an axial plane image. In clinical settings, 3-dimensional images are required for assessing the safety margins in every direction.
In this preliminary study, the efficacy of intravenous contrast-enhanced C-arm CT for assessing ablative areas and safety margins immediately after the RFA of liver tumors is nearly equivalent to that of MDCT performed 3-7 d after RFA.
Radiofrequency ablation is an established local therapy for managing malignant liver tumors. Immediate assessment of ablative margins is necessary for prompt decisions regarding further treatment. The ablative margins are usually evaluated using postprocedural contrast-enhanced computed tomography (CT). However, immediate assessment of ablative margins can often be difficult, unless the procedure can be performed using the available CT imaging equipment.
Recent developments in C-arm cone-beam CT technology have enabled the prompt acquisition of soft-tissue images without the need for patient transfer, and the CT dose index for C-arm CT is expected to be lower than that for conventional CT. C-arm equipment also includes a wider working space than that available in conventional CT scanners. However, the adequacy of the quality of images obtained using C-arm CT during liver tumor imaging is still controversial because of the low contrast obtained in C-arm CT.
The paper found that intravenous contrast-enhanced C-arm CT is nearly equivalent to multidetector CT in terms of detecting ablative areas and insufficient safety margins directly after radiofrequency ablation of liver tumors.
Prompt and sufficient image acquisition during C-arm CT performed for assessing the therapeutic efficacy of liver tumor ablation during the procedure provides useful information regarding further treatment strategies. When an insufficient ablative margin is detected in C-arm CT, targeted additional ablation would then be promptly performed. Conversely, when sufficient ablative margins are confirmed, no additional therapeutic assessments with other imaging modalities may be required. These advantages of C-arm CT may improve the overall therapeutic efficacy of liver tumor ablation.
The term “C-arm CT” refers to the images provided by the C-arm flat-panel cone-beam system. The digital flat-panel detector rotates around the patient to generate volumetric images similar to those obtained with conventional CT. The C-arm system also provides projection radiography, fluoroscopy, and digital subtraction angiography in a single-patient setup in the interventional suite. Such facilities allow the operators to perform intraprocedural imaging without the need for patient transfer.
The paper addresses an important issue related to safety margin evaluation in liver radiofrequency ablation and compares C-arm CT and multidetector CT imaging in this regard. The paper is well written and well presented and correctly reports study limitations and future perspectives.
Peer reviewers: Sergio Casciaro, PhD, Institute of Clinical Physiology, National Research Council, Campus Universitario Ecotekne, Via Monteroni, 73100 Lecce, Italy; Kazushi Numata, MD, PhD, Associate Professor, Gastroenterological Center, Yokohama City University Medical Center, 4-57 Urafune-cho, Minami-ku, Yokohama, Kanagawa 232-0024, Japan
S- Editor Cheng JX L- Editor Logan S E- Editor Xiong L
| 1. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010;2:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Mori K, Fukuda K, Asaoka H, Ueda T, Kunimatsu A, Okamoto Y, Nasu K, Fukunaga K, Morishita Y, Minami M. Radiofrequency ablation of the liver: determination of ablative margin at MR imaging with impaired clearance of ferucarbotran--feasibility study. Radiology. 2009;251:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, Han JK, Choi BI. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011;196:W565-W572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol. 2008;19:799-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol. 2008;19:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Kim S, Yoshizumi TT, Toncheva G, Yoo S, Yin FF. Comparison of radiation doses between cone beam CT and multi detector CT: TLD measurements. Radiat Prot Dosimetry. 2008;132:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hirota S, Nakao N, Yamamoto S, Kobayashi K, Maeda H, Ishikura R, Miura K, Sakamoto K, Ueda K, Baba R. Cone-beam CT with flat-panel-detector digital angiography system: early experience in abdominal interventional procedures. Cardiovasc Intervent Radiol. 2006;29:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Iwazawa J, Ohue S, Hashimoto N, Abe H, Hamuro M, Mitani T. Detection of hepatocellular carcinoma: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol. 2010;195:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, Tanaka K. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol. 2010;194:W452-W454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Linsenmaier U, Rock C, Euler E, Wirth S, Brandl R, Kotsianos D, Mutschler W, Pfeifer KJ. Three-dimensional CT with a modified C-arm image intensifier: feasibility. Radiology. 2002;224:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 185] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Meyer BC, Frericks BB, Voges M, Borchert M, Martus P, Justiz J, Wolf KJ, Wacker FK. Visualization of hypervascular liver lesions During TACE: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol. 2008;190:W263-W269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 237] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (& gt; 2 and & lt; 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Watanabe H, Honda E, Tetsumura A, Kurabayashi T. A comparative study for spatial resolution and subjective image characteristics of a multi-slice CT and a cone-beam CT for dental use. Eur J Radiol. 2011;77:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Zhang Q, Hu YC, Liu F, Goodman K, Rosenzweig KE, Mageras GS. Correction of motion artifacts in cone-beam CT using a patient-specific respiratory motion model. Med Phys. 2010;37:2901-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |