Published online Jan 28, 2025. doi: 10.4329/wjr.v17.i1.99207
Revised: December 8, 2024
Accepted: December 25, 2024
Published online: January 28, 2025
Processing time: 188 Days and 13.4 Hours
Whole-body magnetic resonance imaging (wbMRI) allows general assessment of systemic cancers including lymphomas without radiation burden.
To evaluate the diagnostic performance of wbMRI in the staging of diffuse large B-cell lymphoma (DLBCL), determine the value of individual MRI sequences, and assess patients’ concerns with wbMRI.
In this single-center prospective study, adult patients newly diagnosed with systemic DLBCL underwent wbMRI on a 3T scanner [diffusion weighted images with background suppression (DWIBS), T2, short tau inversion recovery (STIR), contrast-enhanced T1] and fluorodeoxyglucose (18F-FDG) positron emission tomo
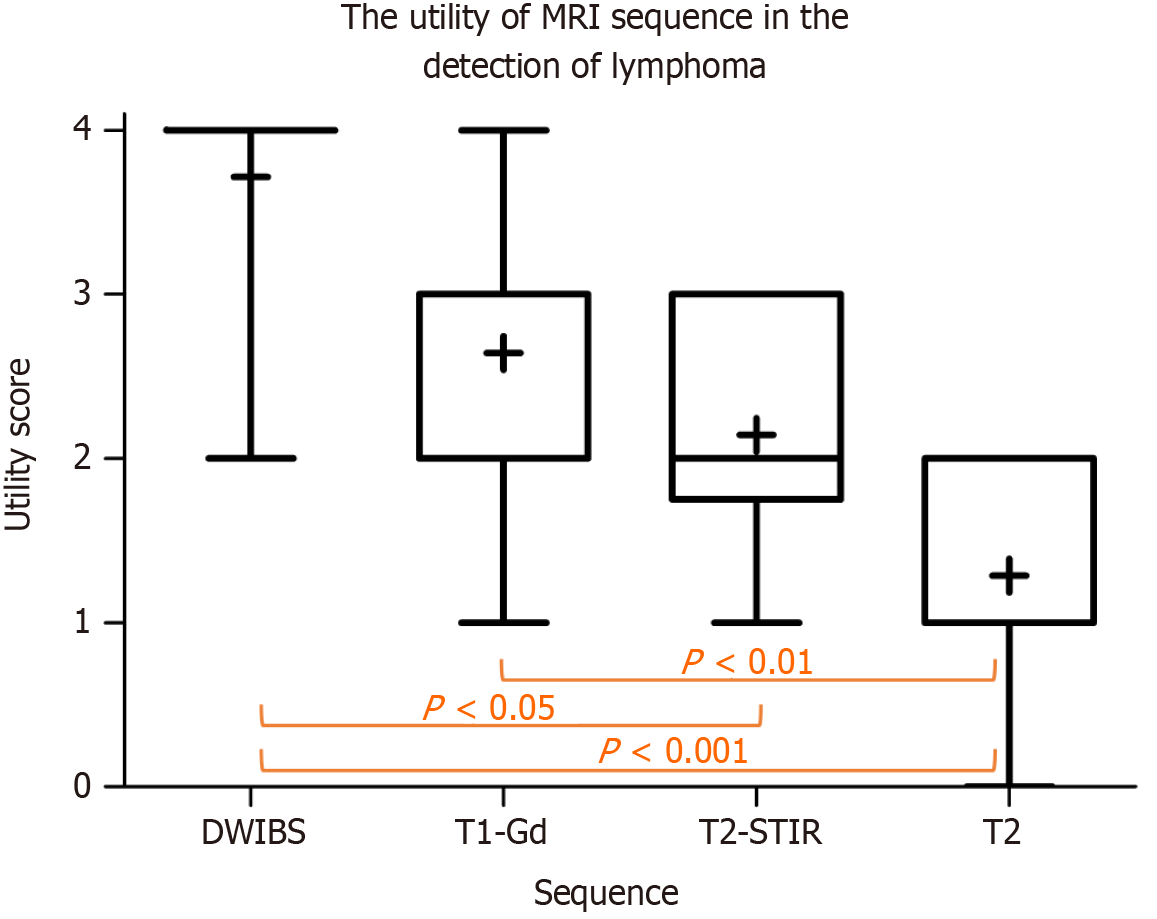
Of 60 eligible patients, 14 (23%) were enrolled and completed the study. The sensitivity of wbMRI in the nodal involvement (182 nodal sites) was 0.84, with 0.99 specificity, positive predictive value of 0.96, negative predictive value of 0.97, and 0.97 accuracy. PET/CT and wbMRI were concordant both in extranodal involvement (13 instances) and staging (κ = 1.0). The mean scores of the utility of MRI sequences were 3.71 ± 0.73 for DWIBS, 2.64 ± 0.84 for T1, 2.14 ± 0.77 for STIR, and 1.29 ± 0.73 for T2 (P < 0.0001). Patients were mostly concerned about the enclosed environment and duration of the MRI examination (27% of patients).
The wbMRI exhibited excellent sensitivity and specificity in staging DLBCL. DWIBS and contrast-enhanced T1 were rated as the most useful sequences. Patients were less willing to undergo wbMRI as a second examination parallel to PET/CT, especially owing to the long duration and the enclosed environment.
Core Tip: Although the agreement in nodal and extranodal involvement by diffuse large-cell B lymphoma is excellent, patients are less willing to undergo whole-body magnetic resonance imaging (wbMRI) as a second examination parallel to positron emission tomography/computed tomography. wbMRI is less well accepted, and patients are concerned about the enclosed environment and duration of the MRI examination. The measurements of lymph node dimensions and quantification of restricted diffusion are not better guides of nodal involvement than visual assessment by an experienced radiologist. Diffusion weighted images with background suppression and contrast-enhanced T1 were the most useful sequences in wbMRI, allowing the imaging protocol to be shortened.
- Citation: Lambert L, Wagnerova M, Vodicka P, Benesova K, Zogala D, Trneny M, Burgetova A. Whole-body magnetic resonance imaging provides accurate staging of diffuse large B-cell lymphoma, but is less preferred by patients. World J Radiol 2025; 17(1): 99207
- URL: https://www.wjgnet.com/1949-8470/full/v17/i1/99207.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i1.99207
Positron emission tomography/computed tomography (PET/CT) is a standard tool for diffuse large B-cell lymphoma (DLBCL) staging and response evaluation[1]. Whole-body magnetic resonance imaging (wbMRI) is not inferior in the staging of aggressive lymphomas to fluorodeoxyglucose (18F-FDG) PET/CT. However, it has not been extensively adopted despite the absence of radiation burden from repeated examinations[2,3]. The disadvantage of wbMRI is that treatment response can only be assessed based on morphology and not functional imaging as in PET[4-6]. DLBCL is a curable disease and has an approximately 80% probability of complete metabolic remission, with the currently used front-line chemoimmunotherapy, and an overall 60%-70% chance of long-term cure[7-9]. The optimal wbMRI imaging protocol for staging of lymphomas has not been established. Nevertheless, the protocol always includes diffusion-weighted imaging (DWI) with background suppression and one or more morphological sequences [T2 short tau inversion recovery (STIR), T2 or T1], infrequently with the administration of a contrast agent[2,10]. Based on our experience with wbMRI in patients with ovarian cancer[11] and a previous meta-analysis[2], we decided to compare wbMRI and PET/CT, the standard of reference in staging patients with DLBCL.
The aim of this study was to evaluate the diagnostic performance of wbMRI in assessing nodal and extranodal involvement and staging of DLBCL compared with PET/CT, determine the diagnostic value of MR sequences used, and assess patients’ perception of wbMRI examination.
This single-center prospective study was performed in accordance with the Declaration of Helsinki (rev. 2013) and was approved by the Ethics Committee of the General University Hospital in Prague. All participants signed an informed consent form.
Between January 2021 and January 2022, consecutive patients with biopsy-proven systemic DLBCL, who met the following inclusion criteria were enrolled in this study: (1) Age ≥ 18 years; (2) Performance status according to the Eastern Cooperative Oncology Group 0-3; (3) Satisfactory clinical condition to participate in the study; (4) Adequate hematologic and renal functions; (5) No contraindications to MRI; (6) Recent or planned PET/CT at the institution; and (7) Informed consent to participate. The exclusion criteria were as follows: (1) History of indolent lymphoma; (2) Anti-lymphoma treatment initiated before PET/CT or wbMRI; and (3) Central nervous system lymphoma. The patients were recruited in the prospective project of the Czech Lymphoma Study Group (NiHiL, NCT03199066), and their baseline characteristics were reviewed in detail.
wbMRI was performed on a 3T MRI scanner (Ingenia Elition, Philips, Best, Netherlands) using phased array coils from the orbital roof to the proximal thighs. The patients were placed in the supine position, with arms along the body. DWI with background suppression (DWIBS) and contrast-enhanced (0.2 mL/kg body weight at 2 mL/second, gadoterate meglumine, Clariscan, GE Healthcare, Oslo, Norway) T1-weighted DIXON sequences were acquired in the axial plane in four stations. T2-weighted sequences with and without STIR were acquired in the coronal plane in three stations. T1-weighted four dimensions free-breathing dynamic contrast enhancement sequence after injection of the contrast material was positioned on the epigastrium (Table 1). The approximate scanning time was 49 minutes (including planning scan, pre-scans, table positioning and breathing synchronisation).
| Sequence | Orientation | Resp | Slice thickness (mm) | Slice gap (mm) | Slices | TE (millisecond) | TR (millisecond) | Time (minute: second) | Stations |
| DWIBS | Axial | No | 6.0 | 1.0 | 43 | 58 | 8400 | 3:13 | 4 |
| T2 | Coronal | No | 5.0 | 1.0 | 54 | 80 | 1300 | 1:08 | 3 |
| T2 STIR | Coronal | No | 5.0 | 1.0 | 54 | 70 | 14100 | 0:57 | 3 |
| T1 DCE | Axial | 4D-FB | 4.0 | -2.0 | 185 | 1.3 | 3.3 | 5:50 | 1 |
| T1 DIXON | Axial | BH | 4.0 | -2.0 | 137 | 1.3 | 3.5 | 0:15 | 4 |
The PET/CT examinations were performed using discovery 690 (GE Healthcare, Milwaukee, WI, United States), an accredited system[12]. A standard imaging protocol with 4.5 MBq/kg 18F-FDG dose and 60 to 90 minutes accumulation time was used without any specific adaptations. The patients were required to fast for at least 6 hours before the procedure. Blood glucose levels were measured with a portable glucometer before every examination, and the required glycemia was < 10 mmol/L. The patients were examined in the supine position, with arms stretched overhead.
PET scans were acquired from the base of the skull to the mid-thigh, with 3 minute per bed position. The PET images were reconstructed using the ordered subset expectation maximization algorithm with the following parameters: 256 × 256 pixels matrix, 3 iterations, 32 subsets and 6.0 mm postfilter. CT data were used for attenuation correction of PET images. CT acquisition was performed prior to PET after injecting 100 mL of iodinated contrast agent (Iomeon 400, Bracco Ltd, United Kingdom) at a flow rate of 2.5 mL/second (unless contraindicated), with the following parameters: Peak tube voltage: 120 kV; Tube current: 30 to 210 mA; Pitch: 1.0; Rotation time: 0.7 seconds.
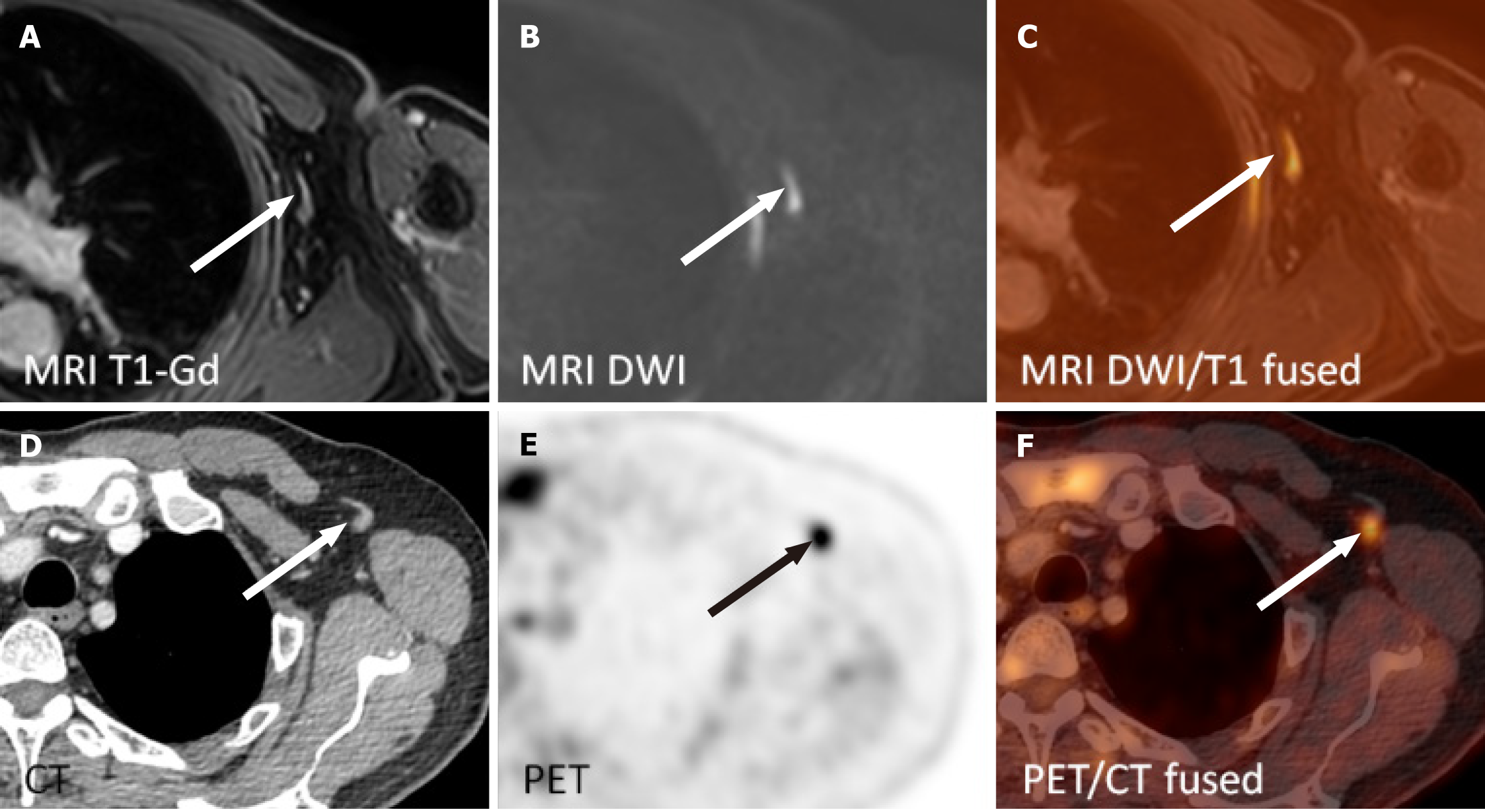
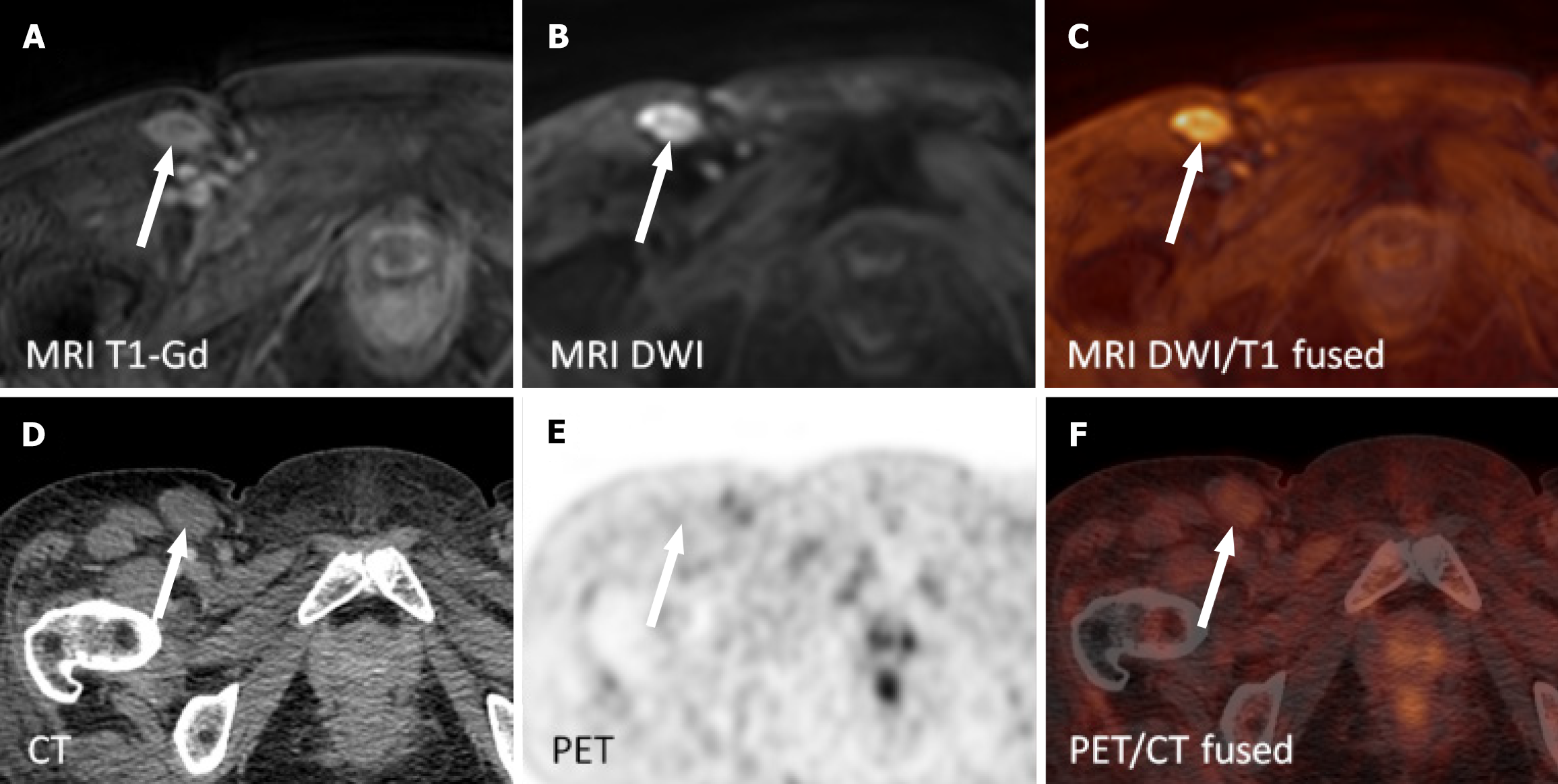
The wbMRI examinations were independently evaluated by two radiologists (Lambert L and Wagnerova M) with 13 and 10 years of experience in MRI. Both researchers assessed the involvement of the lymphoma on wbMRI in 13 nodal regions (including the spleen), in the liver and bone marrow on a four-point scale (PS): 0: Absent; 1: Rather absent; 2: Rather present; 3: Present. Any disagreement was resolved by consensus. One radiologist measured the short- and long-axis diameters of the largest lymph node in each region and quantified the presence of fatty hilum as a benign sign (4PS: 0: Absent; 1: Visible; 2: Prominent; 3: Prevailing) on T1 sequence and signal intensity on DWIBS (4PS: 0: Similar to muscle; 1: < spinal cord or brain white matter; 2: Similar to the spinal cord; 3: > spinal cord). Other sites of suspected extranodal involvement were similarly reported.
Both radiologists rated the utility of wbMRI sequences detecting lymphoma involvement on a 5PS: 0: Not useful; 1: Slightly useful; 2: Fairly useful; 3: Useful; 4: Very useful (critical sequence). The overall image quality of wbMRI was rated on a 4PS (0: Non-diagnostic; 1: Severely impaired; 2: Sub-optimal; 3: Optimal).
PET/CT studies, which served as the reference standard, were evaluated after a wash-out period of 2 months after assessing wbMRI to avoid recall bias. One radiologist (Wagnerova M) evaluated the involvement of lymphatic and extralymphatic regions on PET/CT. Areas with focal or diffuse FDG uptake greater than of the liver in non-physiologic locations (correlation with CT) were considered positive[4,13]. The Cotswold modification of the Ann Arbor Classification was used for both wbMRI- and PET/CT-based staging[14].
All patients who underwent wbMRI received a questionnaire regarding the MRI scan after completing the therapy (> 6 months after wbMRI), which included the following questions: (1) How many times have you undergone an MRI examination in the past (i.e. prior to inclusion in this study)? (2) Did you receive a clear explanation of the procedure before the examination (yes/no)? (3) What concerns did you have before the examination? (4) What was the most inconvenient aspect of the wbMRI examination? and (5) Would you undergo an MRI examination again in the future, if needed (yes/no)?
Statistical evaluation was performed using Prism (GraphPad, La Jolla, CA, United States). Continuous and ordinal data were compared using the Mann-Whitney U test and expressed as median interquartile range (IQR) or average ± SD according to their distribution (D’Agostino and Pearson omnibus normality test). The area under the curve (AUC) was calculated for lymph node diameters. Agreement between wbMRI and PET/CT in staging was expressed as kappa (κ). Friedman test with post-hoc Dunn’s test was used to compare the utility scores of wbMRI sequences. A P value of < 0.05 was considered significant.
Between January 2021 and January 2022, 78 patients were diagnosed with systemic DLBCL (Figure 1). Of these, 60 were eligible for this study, and 14 (23%) patients consented to undergo wbMRI as a second examination and were enrolled. Table 2 summarizes patient characteristics.
| Variable | Value |
| n | 14 |
| Age (years) | 62.7 ± 12.1 |
| Male gender | 7 (50) |
| Clinical stage III–IV | 11 (79) |
| Extranodal involvement of ≥ 2 sites | 10 (71) |
| PS ECOG ≥ 2 | 4 (29) |
| Elevated serum lactate dehydrogenase | 7 (50) |
| International prognostic index 3-5 | 9 (64) |
| Time between PET/CT and wbMRI (days) | 3 (IQR: 1-4) |
The patients underwent wbMRI and PET/CT with a median of 3 days apart (IQR: 1-4 days, range: 0-59 days). The sensitivity of wbMRI in determining the nodal involvement in 182 nodal sites was 0.84, with 0.99 specificity, 0.96 positive predictive value, 0.97 negative predictive value, and an accuracy of 0.97 (Table 3, Figure 2 and 3). Extranodal involvement was apparent in all 13 instances (Table 3) confirmed via PET/CT. There was no difference in the disease stage assessed via wbMRI and PET/CT (κ = 1.0). Disagreement between the readers in evaluating wbMRI in two instances (left axillary nodes and the spleen) was resolved by consensus.
| wbMRI, n | PET/CT, n | TP, n | FP, n | TN, n | FN, n | Senz. | Spec. | PPV | NPV | Acc | |
| Nodal | |||||||||||
| Waldeyer ring | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Cervical and supraclavicular | 3 | 3 | 3 | 0 | 11 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Axillary (R) | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Axillary (L) | 1 | 2 | 1 | 0 | 12 | 1 | 0.50 | 1.00 | 1.00 | 0.92 | 0.93 |
| Mediastinal and hilar | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Epigastric | 3 | 3 | 3 | 0 | 11 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mesenteric | 4 | 4 | 4 | 0 | 10 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Retroperitoneal | 4 | 5 | 4 | 0 | 9 | 1 | 0.80 | 1.00 | 1.00 | 0.90 | 0.93 |
| Iliac and pelvic (R) | 3 | 4 | 3 | 0 | 10 | 1 | 0.75 | 1.00 | 1.00 | 0.91 | 0.93 |
| Iliac and pelvic (L) | 3 | 4 | 3 | 0 | 10 | 1 | 0.75 | 1.00 | 1.00 | 0.91 | 0.93 |
| Inguinal (R) | 2 | 2 | 1 | 1 | 11 | 1 | 0.50 | 0.92 | 0.50 | 0.92 | 0.86 |
| Inguinal (L) | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Spleen | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Lymphatic overall | 27 | 32 | 27 | 1 | 149 | 5 | 0.84 | 0.99 | 0.96 | 0.97 | 0.97 |
| Extranodal1 | |||||||||||
| Bone | 5 | 5 | 5 | 0 | 9 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Bone marrow | 4 | 4 | 4 | 0 | 10 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soft tissue | 4 | 4 | 4 | 0 | 10 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Lung | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Pleura | 1 | 1 | 1 | 0 | 13 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
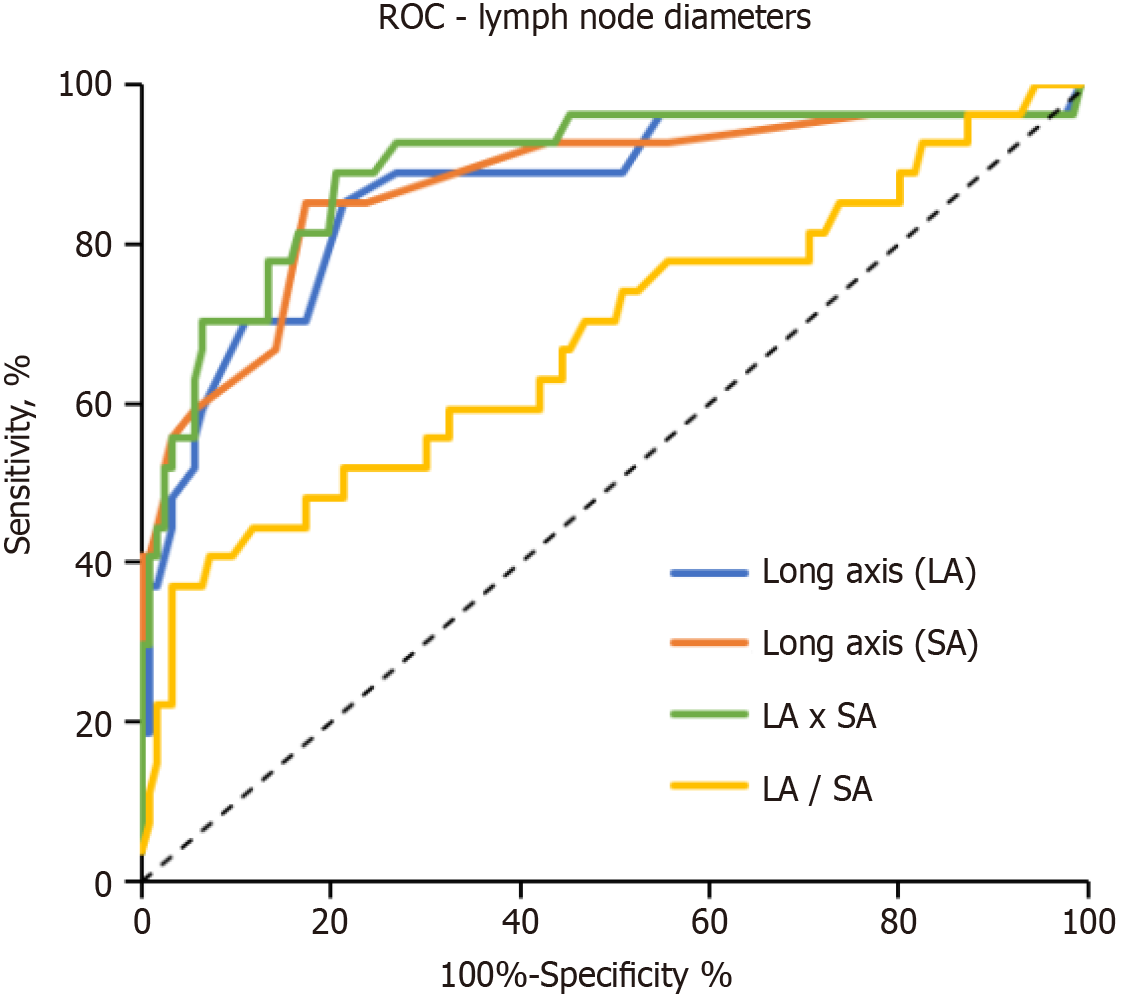
The AUC for evaluating nodal involvement (lymph nodes) was 0.87 for long-axis diameter, 0.87 for short-axis diameter, 0.89 for the product of long- and short-axis diameters, 0.68 for the ratio of short- and long-axis diameters, 0.69 for the presence of fatty hilum, and 0.71 for signal intensity on DWIBS (Table 4 and Figure 4).
| Negative LN regions (n = 127) median (IQR) | Positive LN regions (n = 27) median (IQR) | P value | AUC (95%CI) | P value | |
| Long axis (mm) | 17 (12-21) | 28 (22-36) | < 0.0001 | 0.87 (0.80 to 0.95) | < 0.0001 |
| Short axis (mm) | 7 (6-8) | 14 (10-25) | < 0.0001 | 0.87 (0.79 to 0.96) | < 0.0001 |
| LA × SA (mm × mm) | 120 (72-161) | 420 (210-936) | < 0.0001 | 0.89 (0.81 to 0.97) | < 0.0001 |
| LA: SA | 2.3 (1.8-2.8) | 1.8 (1.5-2.3) | 0.0039 | 0.68 (0.56 to 0.81) | 0.0032 |
| Fatty hilum (0-3)1 | 1 (0.0-2.3) | 0 (0-1) | 0.0015 | 0.69 (0.58 to 0.79) | 0.0023 |
| DWIBS (0-3) | 2 (2-3) | 3 (3-3) | < 0.0001 | 0.71 (0.62 to 0.80) | 0.00059 |
The image quality was rated sub-optimal but still diagnostic in four patients owing to the presence of motion artifacts on T1 and DWIBS (n = 1) and low signal-to-noise ratio on DWIBS (n = 3). The mean scores for the utility of MR sequences in detecting lymphoma involvement were 3.71 ± 0.73 for DWIBS, 2.64 ± 0.84 for T1, 2.14 ± 0.77 for T2-STIR, and 1.29 ± 0.73 for T2 (P < 0.0001, Figure 5).
Of the 14 patients, 11 (79%) completed the questionnaire. The median number of previous MRI examinations was two (IQR: 1-4). Ten (91%) patients declared that they had received a clear explanation of the examination. The common concerns before the wbMRI examination included an enclosed environment in three (27%) patients, followed by fear of an unknown examination, fear of pain, and nausea in one patient (9%). The most inconvenient aspect of the examination was the duration of the procedure and the enclosed environment, in three (27%) cases each and noise in two (18%) cases. Most patients (8; 73%) agreed that they would undergo wbMRI in the future if needed; however, the vast majority still preferred the PET/CT scan (7; 64%).
In this study, wbMRI’s sensitivity for nodal involvement across 13 nodal regions was 0.84 with a 0.99 specificity. wbMRI detected all instances of extranodal involvement. The staging agreement between wbMRI and PET/CT was perfect. Neither the size nor DWI score performed better than the radiologist’s judgment assessing of nodal involvement.
An optimistic view of previous studies summarized in a recent metanalysis[2] showed that wbMRI’s diagnostic performance was comparable to that of PET/CT in staging aggressive lymphomas. Furthermore, our experience with a prospective cohort of patients with DLBCL supports this conclusion.
In our study, four wbMRI sequences and contrast material administration were included. This protocol is more extensive than that previously reported, with a scan time of nearly one hour, which increases the cost of the examination and also patient discomfort[2,15,16].
DWIBS, contrast-enhanced T1 in the axial plane and their fusions were the most valuable sequences used for primary evaluation. These two sequences in four stations require < 30 minutes of scan time, which is still more time-consuming than PET/CT[2,17]. Technological advancements in reconstructing MRI images using deep learning may bridge this gap[18,19]. However, most researchers prefer a combination of DWIBS and T2-STIR, probably because T1 sequences can be acquired without contrast administration[2,16].
Our patients were less willing to undergo a second duplicate examination despite the procedure not involving radiation exposure. The patients were clearly explained that the second examination was not necessary; although it may add clarity to PET/CT findings, it is not a standard practice. This information was probably one of the reasons why several patients refused it. Other reasons were predominantly related to the duration of the procedure and the enclosed environment. Patients have to lie motionless, covered with two phased-array coils in an unfriendly environment, where their core absorbs the thermal energy and their periphery is cooled using fans. PET/CT is preferred over wbMRI by most patients.
Although technological advancements in MRI have attempted to improve patient comfort (less confined bore, audiovisual guidance, and faster imaging), they have not provided measurable relief for patients[20]. The anxiety related to the MRI examination could be attributed to the previous experience with the procedure, with the perceived uncontrollability and uncertainty, rather than the physical characteristics of the examination, being the causative factors[21]. In a similar setting, where an additional wbMRI was offered for research purposes to patients with cancer (Streamline C trial), only 299 of the 1020 screened patients finally underwent the wbMRI pathway[22]. Effective communication between the referring physician and the MRI team is perhaps the most important factor in decreasing the no-show rates and increasing the completion rates of MRI examinations[23,24]. Moreover, presenting patients with audiovisual guidance will likely alleviate their anxiety and enhance participation rates[25].
wbMRI may be preferred to PET/CT for staging systemic extracranial DLBCL lymphomas in patients who fear ionizing radiation burden, particularly during repeated examinations or in children who are at a greater risk of stochastic effects of radiation[15]. For interim or end-of-treatment restaging, wbMRI provides only morphological information. However, morphological complete remission may not follow metabolic remission on PET in one-quarter of the patients[7,15,26]. Therefore, wbMRI may be a viable alternative for lymphomas with low and variable glucose uptake[15,27].
Hybrid imaging with PET/MR combines the benefits of both 18F-FDG-PET and wbMRI and reduces the radiation burden, which is highly desirable, especially in the pediatric population[28]. A head-to-head comparison of PET/CT and PET/MRI showed high concordance both in staging and treatment response assessment[29-31]. The disadvantage of MRI lies in detecting pulmonary and mediastinal lymph node involvements[31].
The following limitations of this study have been identified. First, the number of patients included in the study was low because of their unwillingness to undergo a second examination when the primary method for staging was PET/CT, and to avoid treatment delays by the attending physicians. Second, a more extensive and longer imaging protocol was used for wbMRI than that in previous studies, which may be less practical owing to the longer scan time and contrast agent administration. Third, the treatment response was not evaluated using wbMRI, which is an essential part of managing patients with lymphomas. Last, patients who refused to participate in the study did not receive the question
In conclusion, wbMRI had excellent sensitivity and specificity in staging the disease and exhibited perfect agreement with PET/CT in staging patients with systemic extracranial DLBCL. An experienced radiologist evaluated lymph node involvement better than measurement of the lymph node diameters or semi-quantitative assessment of diffusion restriction. Of the four whole-body sequences, DWIBS and contrast-enhanced T1 were rated as the most useful for staging. Patients were less willing to undergo wbMRI as a second examination parallel to PET/CT, mainly because of the long duration and the enclosed environment.
| 1. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 3860] [Article Influence: 350.9] [Reference Citation Analysis (0)] |
| 2. | Lambert L, Burgetova A, Trneny M, Bircakova B, Molinsky J, Benesova K, Zogala D, Michalek P. The diagnostic performance of whole-body MRI in the staging of lymphomas in adult patients compared to PET/CT and enhanced reference standard-systematic review and meta-analysis. Quant Imaging Med Surg. 2022;12:1558-1570. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Yoo KH. Staging and response assessment of lymphoma: a brief review of the Lugano classification and the role of FDG-PET/CT. Blood Res. 2022;57:75-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Barrington SF, Mikhaeel NG. PET Scans for Staging and Restaging in Diffuse Large B-Cell and Follicular Lymphomas. Curr Hematol Malig Rep. 2016;11:185-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Kostakoglu L, Martelli M, Sehn LH, Belada D, Carella AM, Chua N, Gonzalez-Barca E, Hong X, Pinto A, Shi Y, Tatsumi Y, Knapp A, Mattiello F, Nielsen T, Sahin D, Sellam G, Oestergaard MZ, Vitolo U, Trněný M. End-of-treatment PET/CT predicts PFS and OS in DLBCL after first-line treatment: results from GOYA. Blood Adv. 2021;5:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Hagtvedt T, Seierstad T, Lund KV, Løndalen AM, Bogsrud TV, Smith HJ, Geier OM, Holte H, Aaløkken TM. Diffusion-weighted MRI compared to FDG PET/CT for assessment of early treatment response in lymphoma. Acta Radiol. 2015;56:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, Herbaux C, Burke JM, Matasar M, Rai S, Izutsu K, Mehta-Shah N, Oberic L, Chauchet A, Jurczak W, Song Y, Greil R, Mykhalska L, Bergua-Burgués JM, Cheung MC, Pinto A, Shin HJ, Hapgood G, Munhoz E, Abrisqueta P, Gau JP, Hirata J, Jiang Y, Yan M, Lee C, Flowers CR, Salles G. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;386:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 464] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 8. | Vitolo U, Trněný M, Belada D, Burke JM, Carella AM, Chua N, Abrisqueta P, Demeter J, Flinn I, Hong X, Kim WS, Pinto A, Shi YK, Tatsumi Y, Oestergaard MZ, Wenger M, Fingerle-Rowson G, Catalani O, Nielsen T, Martelli M, Sehn LH. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2017;35:3529-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 313] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 9. | Davies AJ, Barrans S, Stanton L, Caddy J, Wilding S, Saunders G, Mamot C, Novak U, McMillan A, Fields P, Collins GP, Stephens R, Cucco F, Sha C, van Hoppe M, Tooze R, Davies JR, Griffiths G, Schuh A, Burton C, Westhead DR, Du MQ, Johnson PWM. Differential Efficacy From the Addition of Bortezomib to R-CHOP in Diffuse Large B-Cell Lymphoma According to the Molecular Subgroup in the REMoDL-B Study With a 5-Year Follow-Up. J Clin Oncol. 2023;41:2718-2723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Abdulqadhr G, Molin D, Aström G, Suurküla M, Johansson L, Hagberg H, Ahlström H. Whole-body diffusion-weighted imaging compared with FDG-PET/CT in staging of lymphoma patients. Acta Radiol. 2011;52:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Fischerova D, Pinto P, Burgetova A, Masek M, Slama J, Kocian R, Frühauf F, Zikan M, Dusek L, Dundr P, Cibula D. Preoperative staging of ovarian cancer: comparison between ultrasound, CT and whole-body diffusion-weighted MRI (ISAAC study). Ultrasound Obstet Gynecol. 2022;59:248-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Kaalep A, Sera T, Oyen W, Krause BJ, Chiti A, Liu Y, Boellaard R. EANM/EARL FDG-PET/CT accreditation - summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. 2018;45:412-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Hofman MS, Smeeton NC, Rankin SC, Nunan T, O'Doherty MJ. Observer variation in interpreting 18F-FDG PET/CT findings for lymphoma staging. J Nucl Med. 2009;50:1594-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | El-Galaly TC, Gormsen LC, Hutchings M. PET/CT for Staging; Past, Present, and Future. Semin Nucl Med. 2018;48:4-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Littooij AS, Kwee TC, de Keizer B, Bruin MC, Coma A, Beek FJ, Fijnheer R, Nievelstein RA. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: A prospective multicenter study. J Magn Reson Imaging. 2015;42:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Wang D, Huo Y, Chen S, Wang H, Ding Y, Zhu X, Ma C. Whole-body MRI versus (18)F-FDG PET/CT for pretherapeutic assessment and staging of lymphoma: a meta-analysis. Onco Targets Ther. 2018;11:3597-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Abdel-aziz SMA, Badrelden SKM, Salama AMM. Utility of PET/CT for therapy response assessment in non-Hodgkin's lymphoma. QJM. 2021;114. [DOI] [Full Text] |
| 18. | Aamir F, Aslam I, Arshad M, Omer H. Accelerated Diffusion-Weighted MR Image Reconstruction Using Deep Neural Networks. J Digit Imaging. 2023;36:276-288. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Jurka M, Macova I, Wagnerova M, Capoun O, Jakubicek R, Ourednicek P, Lambert L, Burgetova A. Deep-learning-based reconstruction of T2-weighted magnetic resonance imaging of the prostate accelerated by compressed sensing provides improved image quality at half the acquisition time. Quant Imaging Med Surg. 2024;14:3534-3543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Madl JEM, Nieto Alvarez I, Amft O, Rohleder N, Becker L. The Psychological, Physiological, and Behavioral Responses of Patients to Magnetic Resonance Imaging (MRI): A Systematic Review and Meta-Analysis. J Magn Reson Imaging. 2024;59:675-687. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Madl J, Janka R, Bay S, Rohleder N. MRI as a Stressor: The Psychological and Physiological Response of Patients to MRI, Influencing Factors, and Consequences. J Am Coll Radiol. 2022;19:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Taylor SA, Mallett S, Beare S, Bhatnagar G, Blunt D, Boavida P, Bridgewater J, Clarke CS, Duggan M, Ellis S, Glynne-Jones R, Goh V, Groves AM, Hameeduddin A, Janes SM, Johnston EW, Koh DM, Miles A, Morris S, Morton A, Navani N, O'Donohue J, Oliver A, Padhani AR, Pardoe H, Patel U, Punwani S, Quinn L, Rafiee H, Reczko K, Rockall AG, Shahabuddin K, Sidhu HS, Teague J, Thaha MA, Train M, van Ree K, Wijeyekoon S, Halligan S; Streamline investigators. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed colorectal cancer: the prospective Streamline C trial. Lancet Gastroenterol Hepatol. 2019;4:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Manuweera T, Karunakaran K, Baechler C, Rosales J, Kleckner AS, Rosenblatt P, Ciner A, Kleckner IR. Barriers and Facilitators for Participation in Brain Magnetic Resonance Imaging (MRI) Scans in Cancer Research: A Feasibility and Acceptability Analysis. Res Sq. 2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Norbash A, Yucel K, Yuh W, Doros G, Ajam A, Lang E, Pauker S, Mayr N. Effect of team training on improving MRI study completion rates and no-show rates. J Magn Reson Imaging. 2016;44:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Madl J, Janka R, Bay S, Sturmbauer SC, Rohleder N. Effects of video-based patient preparation for MRI on clinical processes and patient experience. Eur J Radiol. 2023;158:110621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Fermé C, Tilly H. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1138] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 27. | Hong GS, Chae EJ, Ryu JS, Chae SY, Lee HS, Yoon DH, Suh C. Assessment of naive indolent lymphoma using whole-body diffusion-weighted imaging and T2-weighted MRI: results of a prospective study in 30 patients. Cancer Imaging. 2021;21:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kurch L, Kluge R, Sabri O, Fischer L, Wendt S, Graf Einsiedel H, Starke S, Kühl JS, Christiansen H, Hirsch FW, Sorge I, Roth C. Whole-body [(18)F]-FDG-PET/MRI for staging of pediatric non-Hodgkin lymphoma: first results from a single-center evaluation. EJNMMI Res. 2021;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Husby T, Johansen H, Bogsrud T, Hustad KV, Evensen BV, Boellard R, Giskeødegård GF, Fagerli UM, Eikenes L. A comparison of FDG PET/MR and PET/CT for staging, response assessment, and prognostic imaging biomarkers in lymphoma. Ann Hematol. 2022;101:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Picardi M, Cavaliere C, Della Pepa R, Nicolai E, Soricelli A, Giordano C, Pugliese N, Rascato MG, Cappuccio I, Campagna G, Cerchione C, Vigliar E, Troncone G, Mascolo M, Franzese M, Castaldo R, Salvatore M, Pane F. PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial. Ann Hematol. 2021;100:1525-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Mistry V, Scott JR, Wang TY, Mollee P, Miles KA, Law WP, Hapgood G. Diagnostic performance of prospective same-day 18F-FDG PET/MRI and 18F-FDG PET/CT in the staging and response assessment of lymphoma. Cancer Imaging. 2023;23:11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |