Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.429
Revised: August 29, 2024
Accepted: September 6, 2024
Published online: September 28, 2024
Processing time: 76 Days and 19.2 Hours
Global and regional cerebral blood flow (CBF) changes in patients with unilateral internal carotid artery occlusion (ICAO) are unclear when the dual post-labeling delays (PLD) arterial spin labeling (ASL) magnetic resonance imaging (MRI) te
To assess global and regional CBF changes in patients with unilateral ICAO with the ASL-MRI perfusion technique.
Twenty hospitalized patients with ICAO and sex- and age-matched controls were included in the study. Regional CBF was measured by Dr. Brain's ASL software. The present study evaluated differences in global, middle cerebral artery (MCA) territory, anterior cerebral artery territory, and Alberta Stroke Program Early Co
When comparing CBF between ICAO patients and controls, the global CBF in ICAO patients was lower at both PLD 1.5 s and PLD 2.5 s; the CBF on the occ
Unilateral ICAO results in hypoperfusion in the global and MCA territories, especially in the ASPECTS area. Dual PLD settings prove more suitable for accurate CBF quantification in ICAO.
Core Tip: In this study, the dual post-labeling delays (PLD) arterial spin labeling (ASL) technique was used for cerebral blood flow (CBF) imaging in unilateral internal carotid artery occlusion (ICAO) patients. Intelligent ASL analysis software was used for rapid quantification of regional CBF, including the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) regions. A comparison with the controls suggests that unilateral ICAO resulted in hypoperfusion in the global and middle cerebral artery territory, affecting most of the ASPECTS area on the occluded side and a small part of the ASPECTS area on the nonoccluded side. The dual PLD settings are more suitable for accurate CBF quantification in ICAO.
- Citation: Zhang GR, Zhang YY, Liang WB, Ding D. Cerebral perfusion in patients with unilateral internal carotid artery occlusion by dual post-labeling delays arterial spin labeling imaging. World J Radiol 2024; 16(9): 429-438
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/429.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.429
Internal carotid artery occlusion (ICAO) is less common but still significant for the etiology of transient ischemic attack (TIA) and cerebral infarction[1]. Atherosclerosis is the major contributing etiology[2]. ICAO diminishes the perfusion pressure on the occluded side, potentially leading to blood redistribution from the contralateral ICA or posterior circulation, along with collateral blood flow towards the affected hemisphere[3]. ICAO patients exhibit ischemic sy
Previous studies have predominantly used single post-labeling delay (PLD) or manual region-of-interest selection for CBF measurement, but these approaches are time-consuming and labor-intensive. Leveraging advances in artificial intelligence, Dr. Brain's software enables the automatic calculation of global and anatomical regional CBF. This facilitates easier adoption of advanced magnetic resonance imaging (MRI) technology for patient diagnosis and monitoring.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) serves as a straightforward and reliable qualitative scoring system widely used to assess early ischemic cerebral infarction severity. It plays a critical role in decision-making in endovascular treatment (EVT)[5-8], assisting in treatment prediction and prognosis. However, a no
This retrospective study aims to analyze global and regional CBF changes in unilateral atherosclerotic ICAO patients with the aforementioned tools.
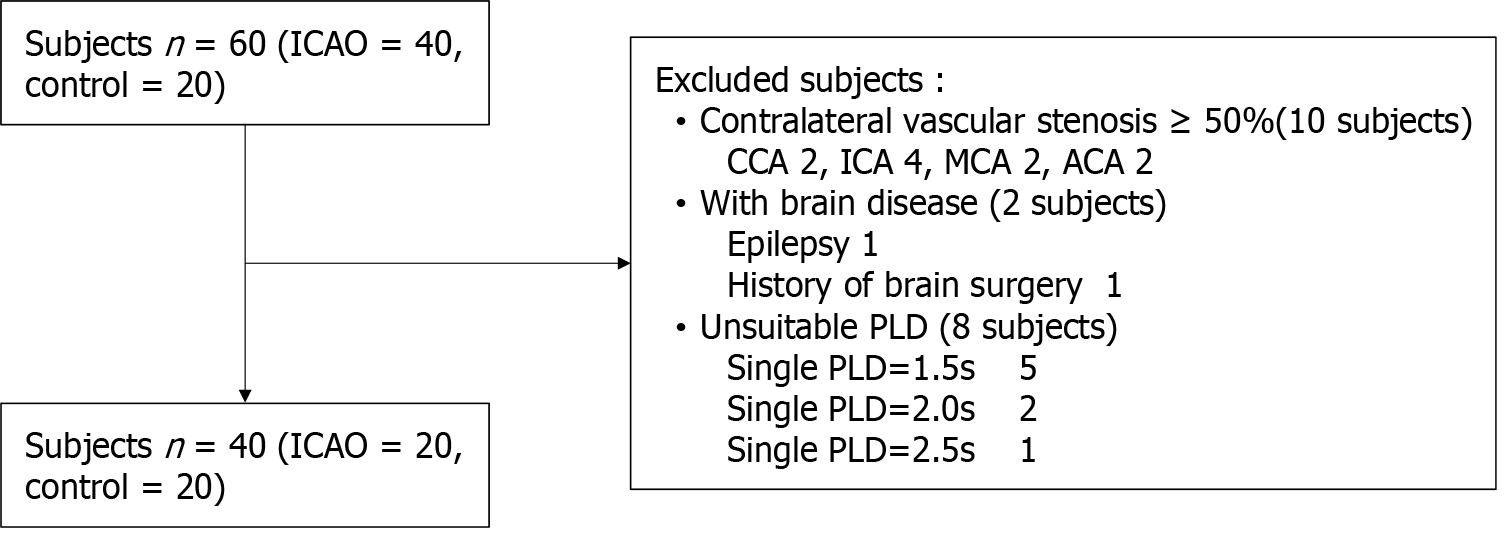
The hospital ethics committee approved the current retrospective study, and informed consent was obtained from all the participants. Between September 2021 and March 2024, patients who were diagnosed with unilateral ICAO confirmed by digital subtraction angiography and who underwent dual PLDs ASL imaging were included. The exclusion criteria were as follows: (1) Moderate or severe stenosis (≥ 50%) in contralateral vessels; (2) Poor image quality or ASL with single or other PLD; and (3) Brain injury, a history of brain surgery, psychiatric disorders, or other conditions affecting brain function. The patient enrolment process is illustrated in Figure 1.
Finally, twenty functionally independent ICAO patients (three women and seventeen men; mean age: 57.50 ± 10.841 years) and sex- and age-matched control subjects were examined.
All stroke patients had an ASPECTS score of 8-10 on diffusion weighted imaging (DWI), and the infarctions were located in the MCA territory. All stroke patients had subcortical watershed infarctions, 2 of them had cortical infarctions, and one of which with a basal area infarction. None of the patients presented infarct lesions exceeding 30 mm in diameter in the territory ipsilateral to the ICA occlusion.
The ASL and DWI sequences were scanned with a 3.0T scanner (HDxt Signa; GE Healthcare) outfitted with an 8-channel head coil for signal acquisition. A three-dimensional pseudo-continuous ASL (PCASL) sequence was used for the whole brain. The parameters of ASL were as follows: Repetition time (TR) 4599 ms (PLD 1525 ms), TR5294 ms (PLD 2525 ms), echo time (TE) 10.86 ms, labelling duration 1500 ms, field of view (FOV) 24 cm × 24 cm, layer thickness 4.0 mm, 36 slices, with background suppression. The parameters of DWI were as follows: TR, 5500 ms; TE, 75 ms; FOV, 22 cm × 22 cm; and layer thickness, 5.0 mm.
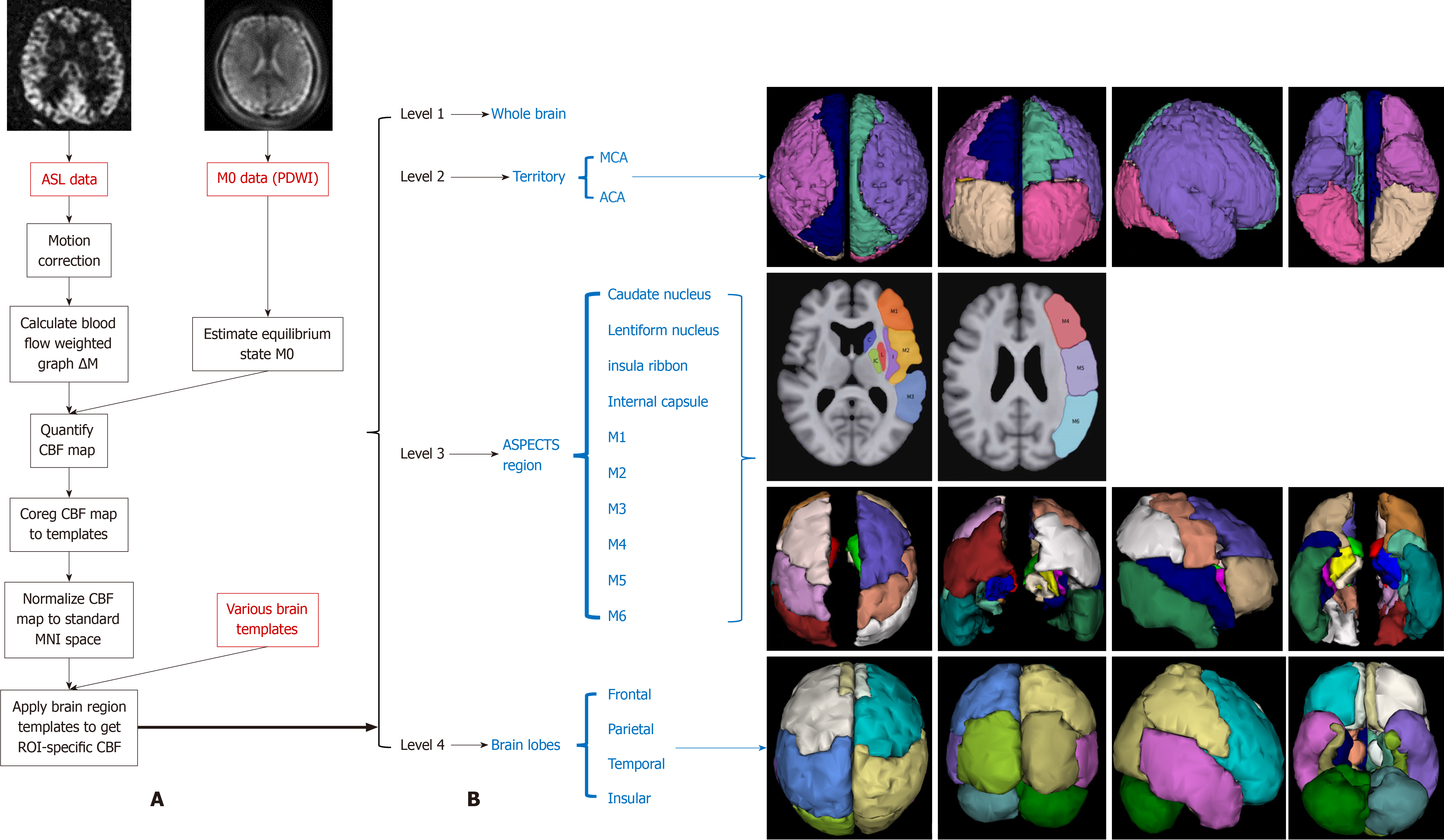
The original ASL sequence data (PLD 1.5 s and PLD 2.5 s were anonymized and uploaded to the Dr. Brain software ASL module (YIWEI Medical Technology, China). The Buxton hemodynamic model was processed with blood T1 and brain tissue T1 constants set at 1650 and 11665 ms, respectively. Figure 2 depicts the processing flow diagram and schematic for brain region segmentation.
This study evaluated differences in regional CBF across several areas: Global, MCA territory; ACA territory; ASPECTS regions (including the caudate nucleus, lentiform nucleus, insula ribbon, internal capsule, and M1-M6); and brain lobes (including the frontal, parietal, temporal, and insular lobes). This analysis compared CBF differences between the ipsi
Owing to the absence of differences in CBF between the left and right hemispheres in control subjects, the CBF values for these hemispheres were averaged for analysis.
The Statistical Package for the Social Sciences for Windows, Version 21.0 (SPSS, Chicago), was used for statistical ana
The fundamental features of the ICAO patients are shown in Table 1. Regional CBF data are shown in Table 2.
| Patient Number | Age (year) | Sex | Symptoms/Onset time | Imaging | Occlusion side | Occlusion site | Contralateral side | Collateral flow pathway |
| 1 | 47 | Female | Headache/1 month | R | Initial | ACoA | ||
| 2 | 51 | Male | Neuro-deficiency/5 days | Stroke | R | Ophthalmic | ACoA | |
| 3 | 50 | Male | Neuro-deficiency/1 week | Stroke | R | Ophthalmic | ACoA + PCoA + L | |
| 4 | 57 | Male | Neuro-deficiency/1 week | Stroke | L | Initial | ACoA + PCoA + OA | |
| 5 | 58 | Male | TIA/4 days | R | Initial | CCA 20% | ACoA + L + OA | |
| 6 | 55 | Male | Neuro-deficiency/2 weeks | Stroke | R | Clinoid | OA | |
| 7 | 82 | Male | TIA/1 month | L | Initial | ICA 20% | ACoA + PCoA | |
| 8 | 67 | Male | Neuro-deficiency/4 days | Stroke | L | Initial | ICA 20% | ACoA |
| 9 | 66 | Male | Neuro-deficiency/2 weeks | Stroke | R | Initial | ICA 20% | PCoA |
| 10 | 61 | Female | Headache/2 months | L | Ophthalmic | ACoA + PCoA + L | ||
| 11 | 56 | Male | Dizziness/1 month | Stroke | R | Initial | MCA 20% | ACoA + PCoA + OA |
| 12 | 57 | Male | Dizziness/3 years | L | Communicating | ICA 20% | PCoA | |
| 13 | 42 | Male | Dizziness/3 days | L | Ophthalmic | ACoA + PCoA | ||
| 14 | 59 | Male | TIA/1 week | R | Initial | ICA 20% | ACoA + PCoA + OA + L | |
| 15 | 35 | Male | Neuro-deficiency/2 months | Stroke | R | Terminal | New vessels + L | |
| 16 | 70 | Male | Neuro-deficiency/2 months | Stroke | R | Ophthalmic | ICA 20%-30% | ACoA + PCoA |
| 17 | 46 | Female | Neuro-deficiency/5 days | Stroke | R | Ophthalmic | ACoA | |
| 18 | 62 | Male | TIA/3 days | L | Initial | ICA 40% | OA | |
| 19 | 71 | Male | Neuro-deficiency/10 days | Stroke | L | Initial | ICA 40% | |
| 20 | 58 | Male | Neuro-deficiency/3 days | Stroke | L | Ophthalmic | ICA 20% | ACoA |
| Variables brain region | Ips-CBF at PLD 1.5 | Ips-CBF at PLD 2.5 | Con-CBF at PLD 1.5 | Con-CBF at PLD 2.5 | HC CBF at PLD 1.5 | HC CBF at PLD 2.5 |
| Anterior | 27.968 ± 7.941 | 32.687 ± 7.409 | 29.728 ± 4.828 | 32.24 (11.3) | 33.353 ± 7.966 | 38.226 ± 6.900 |
| Middle | 18.818 ± 6.485 | 29.09 (6.93) | 27.504 ± 4.243 | 30.71 (4.05) | 31.687 ± 7.907 | 35.710 ± 7.602 |
| Caudate nucleus | 17.57 ± 4.573 | 21.67 ± 5.603 | 22.175 ± 3.355 | 23.714 ± 5.898 | 26.442 ± 4.510 | 31.393 ± 5.603 |
| Lentiform nucleus | 19.812 ± 7.299 | 25.054 ± 5.531 | 28.816 ± 4.572 | 27.412 ± 4.750 | 33.222 ± 6.785 | 29.205 ± 6.178 |
| Insula ribbon | 24.313 ± 8.122 | 31.186 ± 5.886 | 33.001 ± 3.984 | 32.176 ± 3.502 | 38.537 ± 8.69 | 36.881 ± 8.136 |
| Internal capsule | 24.464 ± 3.627 | 24.449 ± 4.307 | 20.248 ± 5.343 | 23.861 ± 5.089 | 29.292 ± 4.519 | 24.958 ± 4.429 |
| M1 | 19.764 ± 6.796 | 28.01 (5.47) | 27.172 ± 4.373 | 29.05 (4.35) | 31.417 ± 7.361 | 33.463 ± 7.962 |
| M2 | 19.735 ± 9.102 | 28.088 ± 7.164 | 31.633 ± 4.568 | 32.035 (5.61) | 37.329 ± 9.097 | 36.49 ± 8.590 |
| M3 | 20.208 ± 7.954 | 29.376 ± 7.442 | 29.249 ± 6.268 | 33.245 ± 5.028 | 32.575 (7.83) | 38.132 ± 9.422 |
| M4 | 19.293 ± 6.837 | 29.59 (9.53) | 25.399 ± 4.554 | 29.64 (5.81) | 28.413 ± 7.149 | 35.048 ± 6.726 |
| M5 | 16.271 ± 7.443 | 25.099 ± 7.604 | 25.688 ± 5.449 | 29.03 (4.48) | 26.45 (4.22) | 33.933 ± 6.944 |
| M6 | 15.902 ± 6.986 | 24.869 ± 7.918 | 25.057 ± 5.284 | 30.209 ± 3.750 | 29.28 (5.58) | 36.683 ± 7.377 |
| Frontal lobe | 19.933 ± 6.746 | 29.98 (7.99) | 27.238 ± 4.513 | 30.21 (4.62) | 30.712 ± 7.051 | 34.558 ± 7.166 |
| Parietal lobe | 19.471 ± 6.988 | 26.906 ± 7.273 | 26.378 ± 5.135 | 30.42 (2.81) | 28.698 (4.82) | 36.092 ± 6.995 |
| Temporal lobe | 19.931 ± 7.940 | 28.557 ± 7.084 | 29.646 ± 5.311 | 32.257 ± 4.582 | 33.393 ± 8.285 | 36.518 ± 8.837 |
| Insular lobe | 25.732 ± 6.496 | 32.083 ± 7.257 | 30.485 ± 4.243 | 32.29 (6.4) | 34.371 ± 7.031 | 35.689 ± 6.415 |
The global CBF of ICAO patients was significantly lower than that of the control group at PLD 1.5 s (24.152 ± 4.517 vs 30.194 ± 7.164 mL/min/100 g, P = 0.011) and PLD 2.5 s (28.904 ± 4.564 vs 34.028 ± 6.730 mL/min/100 g, P = 0.024).
Compared with that of the control subjects, the CBF in the occluded hemisphere was significantly lower in 15 brain regions at PLD 1.5 s (P < 0.05), but no differences were found in the ACA territory (P > 0.05) (as shown in Table 3). The CBF in the occluded hemisphere was lower in the MCA territory, insula ribbon, M2, M3, M4, M5, M6, parietal lobe (P < 0.05), and temporal lobe at PLD 2.5 s (P < 0.05), but no differences were found in the ACA territory, caudate nucleus, len
| Variables brain region | P value | P value | P value | P value | P value | P value | P value | P value |
| Anterior | 0.088 | 0.054 | 0.147 | 0.095 | 0.001e | 0.000f | 0.454 | 1.000 |
| Middle | 0.000a | 0.009b | 0.083 | 0.078 | 0.000e | 0.002f | 0.000g | 0.175 |
| Caudate nucleus | 0.000a | 0.176 | 0.008c | 0.715 | 0.001e | 0.191 | 0.003g | 0.323 |
| Lentiform nucleus | 0.000a | 0.073 | 0.050 | 0.393 | 0.000e | 0.117 | 0.001g | 0.206 |
| Insula ribbon | 0.000a | 0.041b | 0.068 | 0.073 | 0.001e | 0.267 | 0.003g | 0.567 |
| Internal capsule | 0.004a | 0.762 | 0.000c | 0.557 | 0.988 | 0.003f | 0.014g | 0.727 |
| M1 | 0.000a | 0.063 | 0.068 | 0.137 | 0.000e | 0.006f | 0.001g | 0.546 |
| M2 | 0.000a | 0.009b | 0.246 | 0.098 | 0.000e | 0.577 | 0.001g | 0.061 |
| M3 | 0.001a | 0.011b | 0.286 | 0.088 | 0.000e | 0.006f | 0.001g | 0.095 |
| M4 | 0.002a | 0.014b | 0.185 | 0.070 | 0.000e | 0.000f | 0.006g | 0.291 |
| M5 | 0.002a | 0.004b | 0.577 | 0.099 | 0.000e | 0.000f | 0.000g | 0.090 |
| M6 | 0.000a | 0.000b | 0.086 | 0.005d | 0.000e | 0.001f | 0.000g | 0.035h |
| Frontal lobe | 0.000a | 0.070 | 0.124 | 0.286 | 0.000e | 0.000f | 0.001g | 0.291 |
| Parietal lobe | 0.002a | 0.002b | 0.178 | 0.051 | 0.000e | 0.000f | 0.003g | 0.152 |
| Temporal lobe | 0.000a | 0.014b | 0.157 | 0.109 | 0.000e | 0.036f | 0.000g | 0.090 |
| Insular lobe | 0.002a | 0.184 | 0.080 | 0.286 | 0.000e | 0.042f | 0.002g | 0.985 |
Compared with that of the control subjects, the CBF in the contralateral hemisphere was significantly lower in the caudate nucleus and internal capsule at PLD 1.5 s (P < 0.05), but no differences were found in the other 14 brain regions (P > 0.05) (as shown in Table 3). The CBF in the contralateral hemisphere was lower in M6 at PLD 2.5 s (P < 0.05), but no differences were found in the other 15 brain regions (P > 0.05) (as shown in Table 3).
The global CBF at PLD 1.5 s was significantly lower than that at PLD 2.5 s (24.152 ± 4.517 vs 28.904 ± 4.564 mL/min/100 g, P = 0.000).
When comparing PLD 1.5 s with PLD 2.5 s, the CBF on the occluded side was significantly lower in 15 brain regions (P < 0.05), but no differences were found in the internal capsule (P > 0.05) (as shown in Table 3). The CBF of the nonoccluded side was significantly lower in 12 brain regions (P < 0.05), but no differences were found in the caudate nucleus, lentiform nucleus, insula ribbon, or M2 region (P > 0.05) (as shown in Table 3).
At PLD 1.5 s, the CBF in 15 brain regions ipsilateral to the ICAO was significantly lower than that in the contralateral hemisphere (P < 0.05), but no differences were found in the ACA territory (P > 0.05) (as shown in Table 3).
At PLD 2.5 s, the CBF in the M6 region ipsilateral to the ICAO was significantly lower than that in the contralateral hemisphere (P < 0.05), but no differences were found in the other 15 brain regions at PLD 2.5 s (P > 0.05) (as shown in Table 3).
ASL and dynamic susceptibility contrast (DSC) MRI perfusion imaging are commonly used to measure CBF; DSC re
The labeling duration and PLDs are the most important information that can be used to interpret (quantify) CBF images[10]. ASL permits noninvasive estimation of CBF but relies upon the arterial transit time (ATT)[11]. While ATT is well defined for healthy vasculature, it can clearly differ in disease states, potentially making conventional single PLD ASL techniques unsuitable[12]. Owing to the formation of collateral blood flow, the transit time is prolonged in cerebral artery steno-occlusive diseases, which restricts the accurate measurement of CBF by ASL[12-14]. However, the use of a solitary traditional PLD results in an underestimation of CBF[9,12], which can be partially mitigated by using long PLD[2]. In addition to the PLD of 1.5 seconds, we employed a relatively longer PLD of 2.5 seconds, as seen in previous studies. Akiyama et al[11] reported the efficacy of using dual PLDs with 1.5- and 2.5-second techniques to evaluate the slow collateral blood flow that sustains the cerebrovascular reserve (CVR) in stenotic or occlusive ICA conditions[11,15].
Compared with those in the control group and contralateral hemisphere, the current findings indicate that nonacute unilateral atherosclerotic ICAO results in decreased CBF in the global, MCA territory and across most ASPECTS areas of the ipsilateral hemisphere at both PLD 1.5 s and PLD 2.5 s. ICAO leads to the interruption of blood flow on the occluding side. Owing to its anatomical characteristics, the MCA territory is most affected, and the ASPECTS area is located in the MCA territory, so it is also affected. This finding is consistent with previous research; for example, Jeroen Hendrikse's study demonstrated reduced CBF in the grey matter of the ipsilateral MCA territory[16]. Bokkers et al[17] reported significantly decreased CBF in the frontal and frontal parietal regions on the occluded side of the ICA[17]. Furthermore, studies using contrast-enhanced perfusion imaging have consistently shown reduced CBF in both grey and white matter of the occluded hemisphere of the ICA[18-20].
The CBF in the ACA territory was not affected in this study, which is inconsistent with previous research. Hartkamp et al[3] reported that in patients with ICAO, the CBF was significantly lower in the ACA and MCA territories on the occluded side of the ICA than in control subjects[3]. This may be related to the fact that 70% of the ICAO patients in
The ASPECTS was originally developed to assess the volume of acute MCA infarction on non-contrast CT scans, but its applicability extends to multimodal MRI techniques as well. In the present study, all cerebral infarction patients had ASPECTS values ranging from 8 to 10. A previous meta-analysis revealed that higher ASPECTS values (8-10) are as
ASPECTS can be divided into subcortical (including the caudate nucleus, lentiform nucleus, insula ribbon, and internal capsule) and cortical regions (including M1-M6)[23]. The findings of the current study indicate that the hypoperfusion areas in ASPECTS regions are located mainly in the cortex, a detail not previously emphasized. Another study indicated that a higher baseline cortical ASPECTS was predictive of favorable clinical outcomes in patients with ASPECTS < 6 and large vessel occlusion treated with EVT[24]. Therefore, the ASPECTS has significant clinical importance, and perfusion evaluation is necessary.
The CBF at PLD 1.5 s was significantly lower than that at PLD 2.5 s globally, in MCA territories, and in most ASPECTS areas, and the hypoperfusion areas from PLD 1.5 s to PLD 2.5 s were reduced in the occluded hemisphere. These findings indicate the existence of slow flow and redistribution to compensate for ischemia in unilateral ICAO. The current results indicate that the selection of the parameter PLD for ASL can affect the evaluation of CBF correctly because of the presence of slow flow in ICAO patients, and a shorter PLD of 1.5 seconds may result in an underestimation of regional CBF in ICAO patients. An increase in collateral flow in ICAO patients results in longer blood flow pathways and a prolonged blood arrival time[25,26]. A shorter delay does not allow the labelled blood water to be fully delivered to the tissue, and a longer PLD must be included. Our results indicate that dual PLD settings can improve the quantification of CBF in ICAO patients. Presently, many clinical studies have been performed utilizing several PLDs with ATT correction to improve accurate quantification of CBF[12,14,25,26], and the latest work has shown a multi-PLD technique that gauges CBF and ATT, building regional ATT parametric maps to better display pathological tissue[27]. Single-PLD ASL is often sufficient for rapid evaluation of steno-occlusive disease hemodynamics, whereas multi-PLD potentially increases CBF accuracy and provides regional ATT information, and the ATT artefacts can be corrected[10].
Previous studies have shown various findings regarding CBF patterns in the contralateral hemisphere of ICAO patients. For example, one study reported no significant difference in grey matter CBF within the MCA territory between the hemisphere contralateral to the ICAO and a matched control group[16]. Conversely, Bokkers et al[17] reported significantly lower CBF in the anterior frontal region of the nonoccluded hemisphere in ICAO patients than in controls[17]. Additionally, Hartkamp et al[3] demonstrated that the CVR was significantly lower in the ACA and MCA territories of the nonoccluded hemisphere in ICAO patients than in control subjects[13]. Given these discrepancies in previous findings, the results of our current study hold particular significance. We found that the CBF was significantly lower in some ASPECTS areas, such as the lower CBF in the caudate nucleus and internal capsule at PLD 1.5 s and in M6 at PLD 2.5 s, than in the control subjects. Furthermore, we observed that the CBF at PLD 1.5 s was significantly lower than that at PLD 2.5 s. The difference in CBF between PLD 1.5 s and PLD 2.5 s in the contralateral hemisphere represents the existence of slow flow and redistribution to compensate for ischemia. Occlusion of an artery results in a decrease in pressure in that territory, and this pressure gradient will cause blood to flow from healthy arteries to the territory of the occluded artery. Because the collateral blood flow path is long, when the blood flow arrives late, it cannot be detected with a short PLD, and the CBF measured with a long PLD can truly reflect cerebral perfusion. These findings suggest that unilateral ICAO not only affects the occluded side but also triggers regional redistribution of CBF in the contralateral hemisphere to compensate for cerebral ischemia. This finding was not recognized in the past because of the lack of detailed seg
In our current study, patients with internal ICAO presented with a spectrum of clinical manifestations ranging from asymptomatic to TIA to small infarcts within the MCA territory. Despite similar vascular occlusions, outcomes vary significantly on the basis of the ability to recruit collateral pathways that restore blood flow to the ischemic region during the minutes and hours after an acute event[9,11]. Collateral blood flow plays a crucial role in steno-occlusive ICA disease by preventing irreversible ischemic damage[3]. All patients in this study received collateral compensation, either through the primary pathway of the circle of Willis or secondary pathways involving the leptomeningeal and ophthalmic arteries or through neovascularization. Different clinical manifestations indicate that the quality of collateral status varies widely in patients with ICAO. A previous study suggested that the collection of secondary collateral is related to more severe damage, and its existence might be viewed as a marker of insufficiency of the primary collateral routes[28,29]. Owing to the limited sample size in this study, we were unable to explore collateral circulation extensively or assess differences in CBF according to symptoms, which will be a critical direction for future research.
Due to the limited sample size in our study, caution is warranted when interpreting and generalizing the current results to other populations. Future studies will continue to accumulate larger samples to increase the robustness and applicability of the findings. The CBF measured in our study primarily reflects a combination of regional brain grey and white matter. It is known that CBF values differ between grey and white matter, with generally lower CBF values ob
In conclusion, unilateral ICAO results in hypoperfusion in the global and MCA territories, affecting most ASPECTS areas on the occluded side. The ACA territory was not significantly affected. Perfusion deficits were also observed in some ASPECTS areas on the nonoccluded side. Using a single PLD of 1.5 seconds underestimates regional CBF; dual PLD settings prove more suitable for accurate CBF quantification in ICAO cases.
| 1. | Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD Jr. Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke. 2004;35:e349-e352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Paciaroni M, Caso V, Venti M, Milia P, Kappelle LJ, Silvestrelli G, Palmerini F, Acciarresi M, Sebastianelli M, Agnelli G. Outcome in patients with stroke associated with internal carotid artery occlusion. Cerebrovasc Dis. 2005;20:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Hartkamp NS, Petersen ET, Chappell MA, Okell TW, Uyttenboogaart M, Zeebregts CJ, Bokkers RP. Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. J Cereb Blood Flow Metab. 2018;38:2021-2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Malhotra K, Goyal N, Tsivgoulis G. Internal Carotid Artery Occlusion: Pathophysiology, Diagnosis, and Management. Curr Atheroscler Rep. 2017;19:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1624] [Cited by in RCA: 1805] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 6. | Sorensen C. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. J Emerg Med. 2015;49:258-259. [DOI] [Full Text] |
| 7. | Lloyd M. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. J Emerg Med. 2015;48:521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3829] [Article Influence: 382.9] [Reference Citation Analysis (0)] |
| 9. | Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez‐garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lindner T, Bolar DS, Achten E, Barkhof F, Bastos-Leite AJ, Detre JA, Golay X, Günther M, Wang DJJ, Haller S, Ingala S, Jäger HR, Jahng GH, Juttukonda MR, Keil VC, Kimura H, Ho ML, Lequin M, Lou X, Petr J, Pinter N, Pizzini FB, Smits M, Sokolska M, Zaharchuk G, Mutsaerts HJMM; on behalf of the ISMRM Perfusion Study Group. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med. 2023;89:2024-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 11. | Akiyama T, Morioka T, Shimogawa T, Haga S, Sayama T, Kanazawa Y, Murao K, Arakawa S. Arterial Spin-Labeling Magnetic Resonance Perfusion Imaging with Dual Postlabeling Delay in Internal Carotid Artery Steno-occlusion: Validation with Digital Subtraction Angiography. J Stroke Cerebrovasc Dis. 2016;25:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | MacIntosh BJ, Lindsay AC, Kylintireas I, Kuker W, Günther M, Robson MD, Kennedy J, Choudhury RP, Jezzard P. Multiple inflow pulsed arterial spin-labeling reveals delays in the arterial arrival time in minor stroke and transient ischemic attack. AJNR Am J Neuroradiol. 2010;31:1892-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Yun TJ, Sohn CH, Han MH, Kang HS, Kim JE, Yoon BW, Paeng JC, Choi SH, Kim JH, Song IC, Chang KH. Effect of delayed transit time on arterial spin labeling: correlation with dynamic susceptibility contrast perfusion magnetic resonance in moyamoya disease. Invest Radiol. 2013;48:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Wang R, Yu S, Alger JR, Zuo Z, Chen J, Wang R, An J, Wang B, Zhao J, Xue R, Wang DJ. Multi-delay arterial spin labeling perfusion MRI in moyamoya disease--comparison with CT perfusion imaging. Eur Radiol. 2014;24:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Haga S, Morioka T, Shimogawa T, Akiyama T, Murao K, Kanazawa Y, Sayama T, Arakawa S. Arterial Spin Labeling Perfusion Magnetic Resonance Image with Dual Postlabeling Delay: A Correlative Study with Acetazolamide Loading (123)I-Iodoamphetamine Single-Photon Emission Computed Tomography. J Stroke Cerebrovasc Dis. 2016;25:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Hendrikse J, van Osch MJ, Rutgers DR, Bakker CJ, Kappelle LJ, Golay X, van der Grond J. Internal carotid artery occlusion assessed at pulsed arterial spin-labeling perfusion MR imaging at multiple delay times. Radiology. 2004;233:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Bokkers RP, van Laar PJ, van de Ven KC, Kapelle LJ, Klijn CJ, Hendrikse J. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol. 2008;29:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Kluytmans M, van der Grond J, Viergever MA. Gray matter and white matter perfusion imaging in patients with severe carotid artery lesions. Radiology. 1998;209:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Apruzzese A, Silvestrini M, Floris R, Vernieri F, Bozzao A, Hagberg G, Caltagirone C, Masala S, Simonetti G. Cerebral hemodynamics in asymptomatic patients with internal carotid artery occlusion: a dynamic susceptibility contrast MR and transcranial Doppler study. AJNR Am J Neuroradiol. 2001;22:1062-1067. [PubMed] |
| 20. | van Osch MJ, Rutgers DR, Vonken EP, van Huffelen AC, Klijn CJ, Bakker CJ, van der Grond J. Quantitative cerebral perfusion MRI and CO2 reactivity measurements in patients with symptomatic internal carotid artery occlusion. Neuroimage. 2002;17:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Phan K, Saleh S, Dmytriw AA, Maingard J, Barras C, Hirsch JA, Kok HK, Brooks M, Chandra RV, Asadi H. Influence of ASPECTS and endovascular thrombectomy in acute ischemic stroke: a meta-analysis. J Neurointerv Surg. 2019;11:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, Zhang J, Zhou Z, Yao X, Wang G, Song W, Cai X, Nan G, Li D, Wang AY, Ling W, Cai C, Wen C, Wang E, Zhang L, Jiang C, Liu Y, Liao G, Chen X, Li T, Liu S, Li J, Gao F, Ma N, Mo D, Song L, Sun X, Li X, Deng Y, Luo G, Lv M, He H, Liu A, Zhang J, Mu S, Liu L, Jing J, Nie X, Ding Z, Du W, Zhao X, Yang P, Liu L, Wang Y, Liebeskind DS, Pereira VM, Ren Z, Wang Y, Miao Z; ANGEL-ASPECT Investigators. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N Engl J Med. 2023;388:1272-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 470] [Article Influence: 235.0] [Reference Citation Analysis (0)] |
| 23. | Shin DH, Shin DJ, Kim JR. Do All ASPECT Score Regions have the Same Predictive Power for Functional Outcomes? J Stroke Cerebrovasc Dis. 2020;29:104516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Xing PF, Zhang YW, Zhang L, Li ZF, Shen HJ, Zhang YX, Li H, Hua WL, Liu P, Liu P, Yang PF, Hong B, Deng BQ, Liu JM. Higher Baseline Cortical Score Predicts Good Outcome in Patients With Low Alberta Stroke Program Early Computed Tomography Score Treated with Endovascular Treatment. Neurosurgery. 2021;88:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 806] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 26. | Wang DJ, Alger JR, Qiao JX, Gunther M, Pope WB, Saver JL, Salamon N, Liebeskind DS; UCLA Stroke Investigators. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke - Comparison with dynamic susceptibility contrast enhanced perfusion imaging. Neuroimage Clin. 2013;3:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Bokkers RP, Bremmer JP, van Berckel BN, Lammertsma AA, Hendrikse J, Pluim JP, Kappelle LJ, Boellaard R, Klijn CJ. Arterial spin labeling perfusion MRI at multiple delay times: a correlative study with H(2)(15)O positron emission tomography in patients with symptomatic carotid artery occlusion. J Cereb Blood Flow Metab. 2010;30:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis. 2002;14:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 781] [Article Influence: 35.5] [Reference Citation Analysis (0)] |