Published online Dec 28, 2024. doi: 10.4329/wjr.v16.i12.771
Revised: October 24, 2024
Accepted: December 3, 2024
Published online: December 28, 2024
Processing time: 205 Days and 8 Hours
Invasive fungal sinusitis (IFS) can present as a mild disease to life-threatening infection. A recent surge in cases was seen due to the coronavirus disease 2019 (COVID-19) pandemic. Many patients require surgical debridement and hence imaging [contrast-enhanced computed tomography (CECT) of the paranasal sinuses (PNS)] to document the extent of the disease. However, there was no scoring system using CECT to describe the severity of IFS. This study proposes a computed tomography (CT) severity index (CTSI) to describe the severity of rhino-orbital-cerebral involvement in symptomatic COVID-19 patients and hypothesizes that higher CTSI correlates with disease severity and thus slow response/non-response to treatment.
To propose a scoring system using CECT to describe the severity of IFS and correlate it with clinical outcomes.
A prospective study on 66 COVID-19 positive patients with CECT PNS done for IFS was performed. Split-bolus single-phase CT technique was used. Based on the extent of involvement, a CTSI was designed. Disease in four major subsite areas was assessed. Each subsite involvement was given points according to this model and then summated. Based on the final summated CTSI, the disease was classified as mild, moderate, or severe. Two subsets were subsequently analyzed including survival and death; and responders and non-responders.
The study cohort was 66 COVID-19-positive patients with suspected IFS with a median age of 48.5 years. Mild disease was noted in 34 (51.52%), moderate in 28 (42.42%), and severe disease in 4 (6.06%) patients. There was a significant association of mortality and poor clinical response (P = 0.02) with disease bilaterality. Laterality and CTSI were significant predictors of response to treatment. The mean CTSI of responders was 6.3, of non-responders was 12.9 and the response to treatment was significantly associated with CTSI (t-test, P < 0.001). Receiver operating characteristic curve analysis (Liu method) to distinguish between responders and non-responders showed that the cut-off value for CTSI of 11 had a sensitivity of 78.26% and a specificity of 95.35% to predict response assessment.
CTSI can help in quantification of the disease burden, mapping out disease extent, triaging patients, and response assessment; especially patients with underlying comorbidities. A higher score would alert the clinician to initiate aggressive treatment, as severe disease correlates with slow response/non-response to the treatment.
Core Tip: This was a prospective study with 66 coronavirus disease 2019-positive patients in whom contrast-enhanced computed tomography (CECT) of the paranasal sinuses was done for invasive fungal sinusitis (IFS). It aimed to propose a scoring system [computed tomography severity index (CTSI)] using CECT to describe the severity of IFS and correlate it with clinical outcomes. Disease was categorized as mild, moderate, and severe based on CTSI. A higher score correlated with slow response/non-response to treatment in our study. Thus, CTSI can help in the quantification of the disease burden, triaging patients, and response assessment. A higher score would alert the clinician to initiate aggressive treatment, as severe disease correlates with slow response/non-response to treatment.
- Citation: Manchanda S, Bhalla AS, Nair AD, Sikka K, Verma H, Thakar A, Kakkar A, Khan MA. Proposed computed tomography severity index for the evaluation of invasive fungal sinusitis: Preliminary results. World J Radiol 2024; 16(12): 771-781
- URL: https://www.wjgnet.com/1949-8470/full/v16/i12/771.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i12.771
Invasive fungal sinusitis (IFS) due to mucormycosis/aspergillus can present with a clinical spectrum ranging from mild disease to life-threatening infection. Clinically, IFS can present with atypical signs and symptoms like complicated sinusitis, such as nasal blockage, crusting, proptosis, facial pain and edema, ptosis, chemosis, and even ophthalmoplegia[1]. Headache, fever, and various neurological signs and symptoms are seen if intracranial extension is present. Without early diagnosis and treatment, there may be a rapid progression of the disease, with reported mortality rates from intra-orbital and intracranial complications of 50-80 percent[2]. Many patients require surgical debridement and hence imaging to document the extent of the disease[3]. Contrast-enhanced computed tomography (CECT) of the paranasal sinuses (PNS) is the ideal modality in the emergency setting for adequate demonstration of the extra-sinus spread of disease to orbit, infratemporal fossa, skull base, and intracranial involvement along with bony erosions.
In the recent past, there was a pandemic due to coronavirus disease 2019 (COVID-19) and an associated rise in the cases of IFS. This was because COVID-19 infection and its treatment (mostly with steroids) predispose patients to many opportunistic infections at multiple sites including paranasal sinuses[4,5].
There has been no scoring system using CECT to describe the severity of IFS and its relation to the clinical severity in patients with COVID-19.
Therefore, we undertook this study to propose a computed tomography (CT) severity index (CTSI) to describe the severity of rhino-orbital-cerebral involvement in symptomatic COVID-19 patients using CECT paranasal sinuses and to correlate the radiological changes with the clinical outcomes.
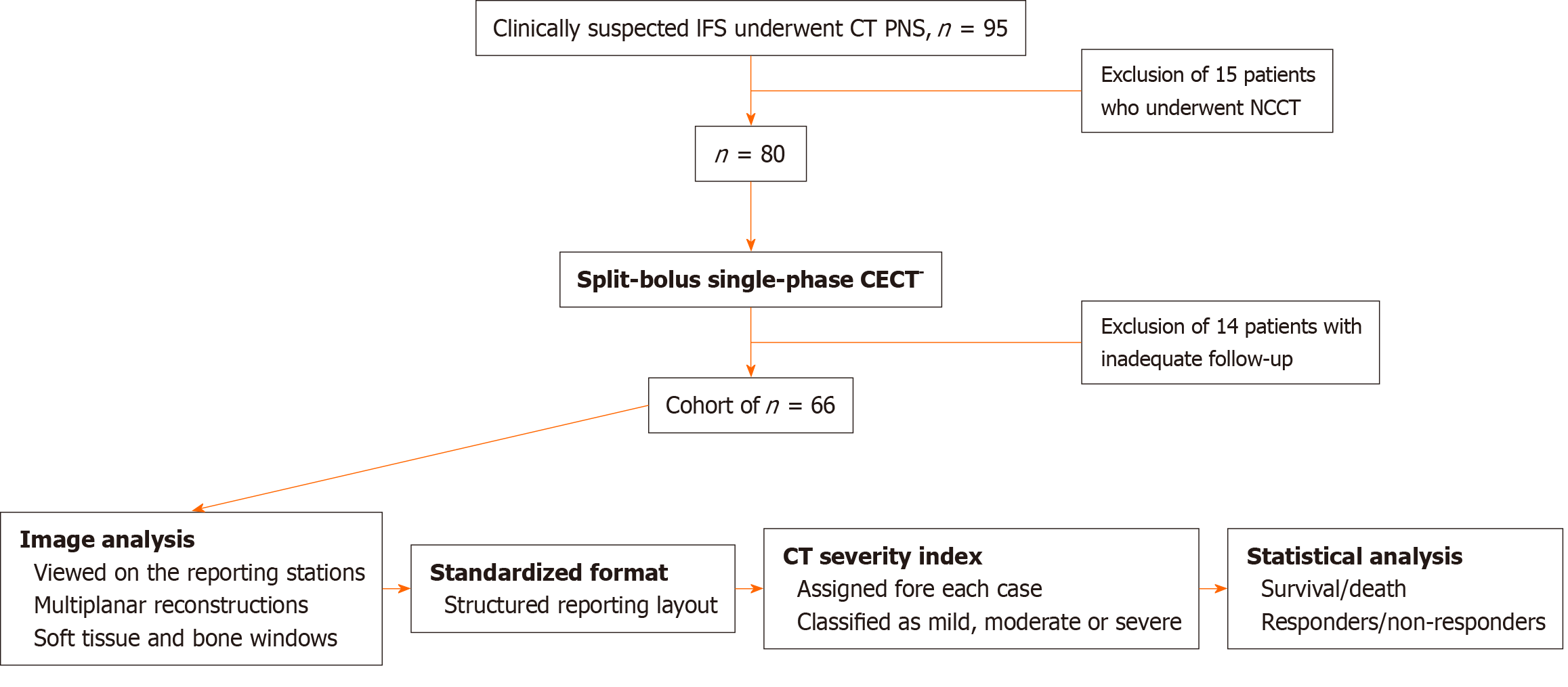
Ninety-five patients with diagnosed COVID-19 and suspected IFS underwent CT PNS during the study period of April 2021 to January 2022. Non-contrast scans were acquired for fifteen patients with deranged kidney function tests and were excluded from the study group. Fourteen other patients were also excluded due to inadequate information or follow-up. Thus, data from 66 COVID-19-positive patients with CECT PNS done for IFS was analysed (Figure 1).
Split-bolus single-phase CT technique[6] was used to optimally demonstrate the vascular (arterial and venous) as well as mucosal extent of the disease. This protocol was performed with 1 mL/kg of non-ionic contrast medium of concentration 300 mg iodine/mL (Iohexol) administered through an 18 to 20 G cannula in the antecubital vein using a pressure injector. Two-thirds of the contrast was given, 4 mL/sec followed by 30 seconds gap and then the rest of the contrast was given, 3.5 mL/sec followed by automatic bolus tracking and image acquisition. With the use of this technique, there was simultaneous opacification of the internal carotid artery and cavernous sinus along with optimal soft tissue demon
The scans were viewed on the reporting stations using multiplanar reconstructions in both soft tissue and bone windows. Further, a structured reporting layout was employed in each case to reflect the extent of the disease in a standardized format.
Based on the extent of involvement, a CTSI was designed (Table 1). Four major areas included were disease in nose/paranasal sinuses; adjacent soft tissue infiltration; orbit and intracranial involvement. The involvement of each subsite was given points according to this model and then summated. For each patient, the final CTSI was calculated, and the disease was further classified as mild (CTSI 1-8), moderate (9-16) or severe (17-25). Demographic analysis was done, including clinical presentation and presence of co-morbidities like diabetes and hypertension. Two subsets were subsequently analyzed including survival and death; and responders and non-responders. Residual clinical disease/ worsening (on monthly nasal cavity cleaning and clinical assessment) or persistent disease in the follow-up scans (performed at 1-3 months intervals) were taken as criteria of non-responder. Clinical staging was done as per the TALMI staging system[7].
| Items | Value |
| Disease limited to nose/PNS | |
| Mucosal disease in paranasal sinuses | 1 |
| Mucosal disease in nasal cavity and nasopharynx | 1 |
| Adjacent tissue invasion | |
| Hard palate erosion | 1 |
| Soft tissue infiltration anterior/posterior periantral fat | 1 |
| Soft tissue infiltration extending to PPF/SOF/IOF/Orbital apex/ITF | 2 |
| Significant bilateral disease | 2 |
| Orbit | |
| Soft tissue/fat/muscle/NLD involvement | 1 |
| Bone erosion | 2 |
| Intraocular/optic nerve involvement | 3 |
| Intracranial disease | |
| Skull base invasion (erosion) | 2 |
| Cavernous sinus involvement | 3 |
| Internal carotid artery narrowing/occlusion | 3 |
| Intracranial complications (meningitis/cerebritis/abscess/infarct) | 3 |
Data was recorded on a pre-designed proforma and managed on an Excel spreadsheet. Statistical analysis was done using the Stata 14.1. A P value of less than 0.05 was considered significant.
The study cohort was of 66 COVID-19-positive patients with suspected IFS. The age of the patients included in the study ranged from 26 to 71 years (median 48.5 years, Interquartile range 40-60.75 years). Males predominated the study cohort (male:female = 5.6:1) with most of the patients (69.70%) from the urban background. The clinical presentation included periorbital pain (75.76%), facial numbness (63.64%), periorbital swelling (56.06%), restricted extra-ocular movements (56.06%), reduced visual acuity (60.61%), proptosis (33.33%), palatal involvement (31.82%), ptosis (27.27%), and cranial nerve involvement (27.27%; Table 2). About three-fourths of the patients (n = 51) were known diabetic while a total of 56 patients had their glycosylated hemoglobin above 6.5 gm/dL (mean HbA1c of 9.11 ± 2.20 gm/dL). Thirty-five percent of patients were hypertensive. Six (9.09%) patients had bilateral disease.
| Parameter | Criteria | n (%) | |
| 1 | Gender | Male:Female | 56 (84.85):10 (15.15) |
| 2 | Periorbital swelling | Absent:Present | 29 (43.94):37 (56.06) |
| 3 | Periorbital pain | Absent:Present | 16 (24.24):50 (75.76) |
| 4 | Facial numbness | Absent:Present | 24 (36.36):42 (63.64) |
| 5 | Extra-ocular movements | Normal:Restricted:Frozen | 29 (43.94):20 (30.3):17 (25.76) |
| 6 | Vision status | Normal:Reduced:FCCF:PL Negative | 26 (39.39):21 (31.82):1 (1.52):18 (27.27) |
| 7 | Proptosis | Absent:Present | 44 (66.67):22 (33.33) |
| 8 | Palatal involvement | Absent:Present | 45 (68.18):21 (31.82) |
| 9 | Cranial nerve Involvement | Absent:Present | 48 (72.73):18 (27.27) |
| 10 | Ptosis | Absent:Present | 39 (59.09):27 (40.91) |
| 11 | H/o diabetes | Absent:Present | 15 (22.73):51 (77.27) |
| 12 | H/o hypertension | Absent:Present | 43 (65.15):23 (34.85) |
| 13 | HbA1c | < 6.5:≥ 6.5 | 10 (15.15) 56 (84.85) |
| 14 | Side involved | Unilateral:Bilateral | 60 (90.91):6 (9.09) |
| 15 | CT severity index | Mild (1-8):Moderate (9-16):Severe (≥ 17) | 34 (51.52):28 (42.42):4 (6.06) |
| 16 | Final organism | Mucormycosis:Aspergillosis:Both | 45 (68.18):9 (13.64):12 (18.18) |
| 17 | Clinical outcome | Responder:Non-responder | 43 (65.15):23 (34.85) |
| 18 | Survival | Survived:Expired | 56 (84.85):10 (15.15) |
| 19 | Diagnosis of COVID-19 before IFS | After:Before | 9 (13.63):57 (86.36) |
| 20 | Environment | Rural:Urban | 20 (30.3):46 (69.7) |
| 21 | TALMI staging | Stage 1:Stage 2:Stage 3:Stage 4 | 2 (3.03):31 (46.97):7 (10.61):26 (39.39) |
| 22 | Steroid given for COVID-19 (n = 57) | Not given:Given | 24 (42.1):33 (57.89) |
The CTSI was calculated and grouped as mild (score 1-8), moderate (score 9-16), or severe (score ≥ 17). Mild disease was noted in 34 (51.52%), moderate in 28 (42.42%), and severe disease in 4 (6.06%) patients (Table 2). As per the TALMI clinical scoring systems, more patients had Stage 2 disease (46.97%).
Out of the 66 patients, 57 had COVID-19 before diagnosis of IFS, ranging from a period of 1-36 days. Nine patients were diagnosed to be COVID-19 positive during the admission for IFS. No statistically significant difference between the CTSI and TALMI staging was seen in these two subgroups using the Pearson χ2 test.
Forty-five (68.18%) patients had fungi of the Mucorales order identified in the tissue specimen while Aspergillus sp. was identified in 9 (13.64%) patients as the sole causative agent. Twelve (18.18%) patients had coexistent infection. The difference in CTSI, survival rates and response to treatment between the subgroups of different etiological agents is summarised in Table 3. There was no statistically significant difference in the survival rates (P value = 1) or response to treatment (P value = 0.53) between the three subgroups.
| Parameter | Subclassification of parameter | Aspergillosis (n = 9) | Mucormycosis (n = 45) | Mixed (n = 12) |
| Diabetes mellitus | 3 (33.3) | 37 (82.2) | 11 (91.7) | |
| Hypertension | 2 (22.2) | 16 (35.6) | 5 (41.7) | |
| Laterality | Unilateral | 9 (100) | 40 (89.9) | 11 (91.7) |
| Bilateral | 0 (0) | 5 (10.1) | 1 (8.3) | |
| CTSI | Mild | 7 (77.8) | 21 (46.7) | 6 (50) |
| Moderate | 2 (22.2) | 21 (46.7) | 5 (41.7) | |
| Severe | 0 (0) | 3 (6.7) | 1 (8.3) | |
| Survival | Survival | 8 (88.9) | 38 (84.4) | 10 (83.3) |
| Mortality | 1 (11.1) | 7 (15.6) | 2 (16.7) | |
| Responder/non-responder | Responder | 7 (77.8) | 27 (60) | 9 (75) |
| Non-responder | 2 (22.2) | 18 (40) | 3 (25) |
There were ten deaths with a survival rate of 84.85%. Comparative analysis was done amongst survivors and patients who expired. There was no association found between survival and history of diabetes or hypertension, causative organism, CT severity score, or TALMI staging. However, there was a significant association of bilateral disease with mortality (Table 4). The level of glycosylated hemoglobin also had no significant effect on mortality (t-test, P = 0.79). Steroids were given to manage COVID-19 in 33 (57.89%) patients before onset of IFS. However, no significant correlation was observed between survival and steroid use.
| Parameter | Criteria | Survivor | Expired | Total | P value (Fischer's exact test) |
| Diabetes mellitus | Absent | 14 | 1 | 15 | |
| Present | 42 | 9 | 51 | 0.28 | |
| Hypertension | Absent | 36 | 7 | 43 | |
| Present | 20 | 3 | 23 | 0.52 | |
| Laterality | Unilateral | 54 | 6 | 60 | |
| Bilateral | 2 | 4 | 6 | 0.004 | |
| Organism | Mucormycosis | 38 | 7 | 45 | |
| Aspergillosis | 8 | 1 | 9 | ||
| Mixed | 10 | 2 | 12 | 1 | |
| Clinical response | Responder | 38 | 5 | 43 | |
| Non-responder | 18 | 5 | 23 | 0.23 | |
| TALMI staging | Stage 1 | 2 | 0 | 2 | |
| Stage 2 | 29 | 2 | 31 | ||
| Stage 3 | 5 | 2 | 7 | ||
| Stage 4 | 20 | 6 | 26 | 0.16 | |
| CTSI | Mild (1 to 8) | 29 | 5 | 34 | |
| Moderate (9 to 16) | 25 | 3 | 28 | ||
| Severe (17 or more) | 2 | 2 | 4 | 0.12 |
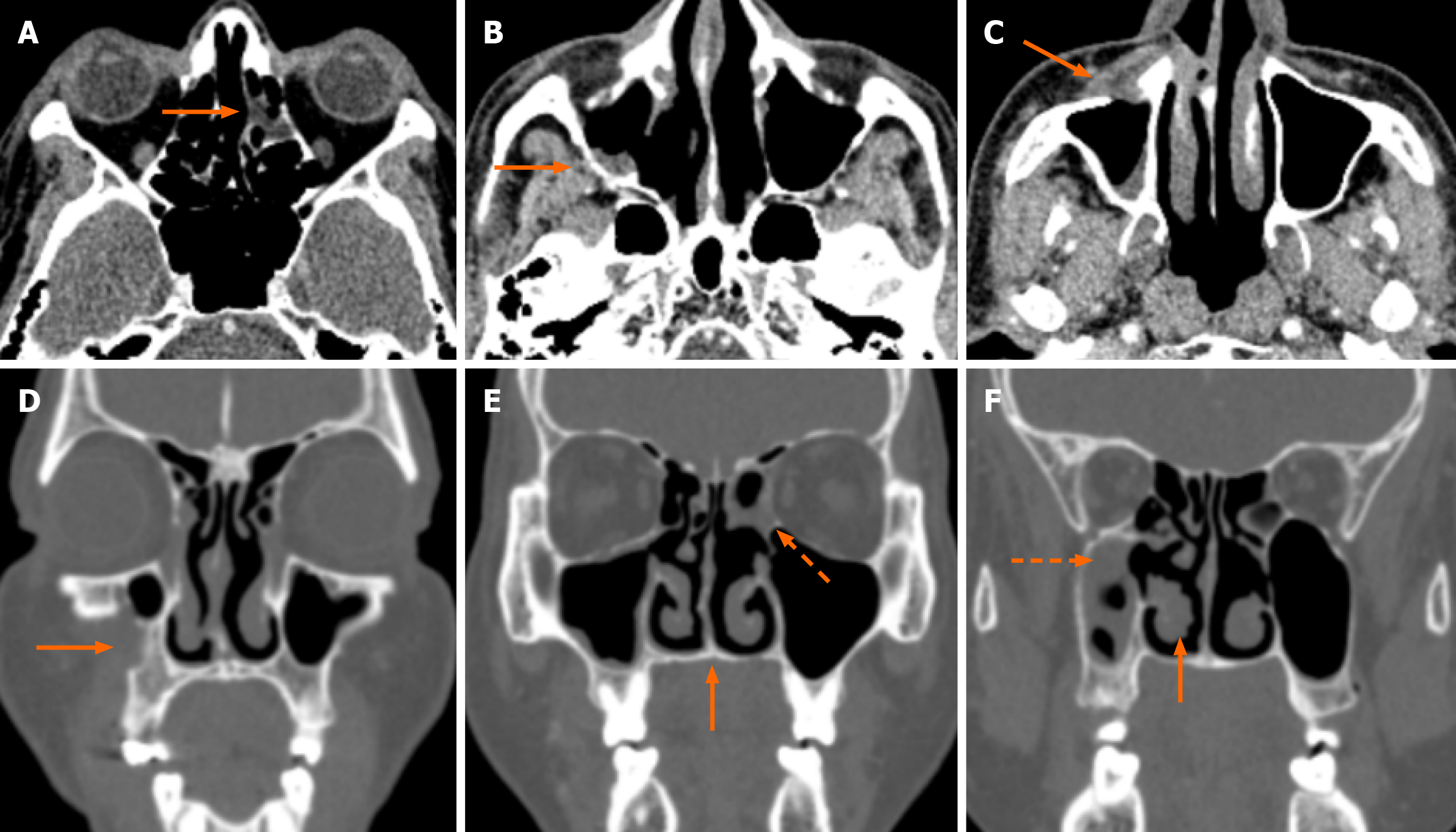
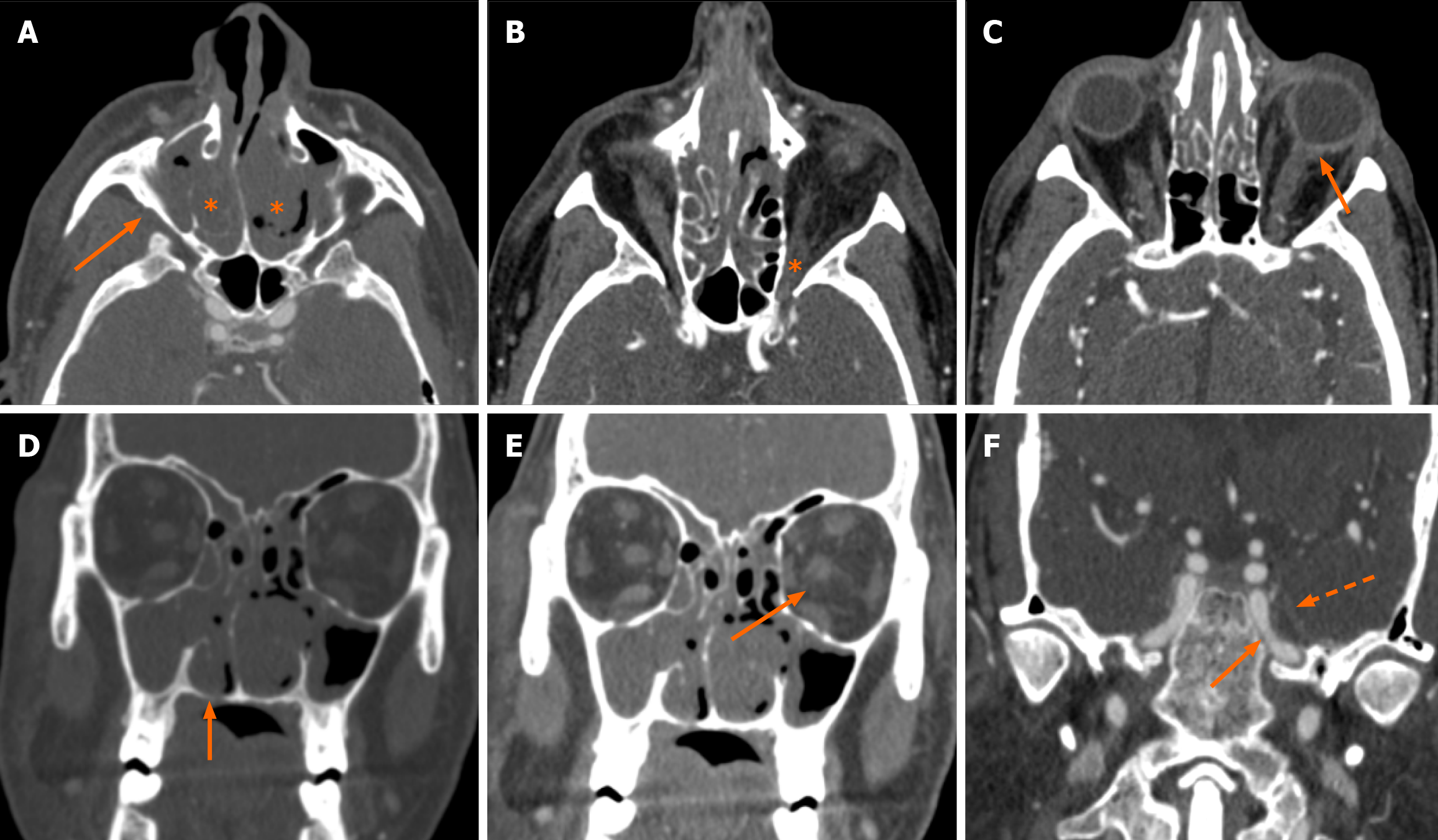
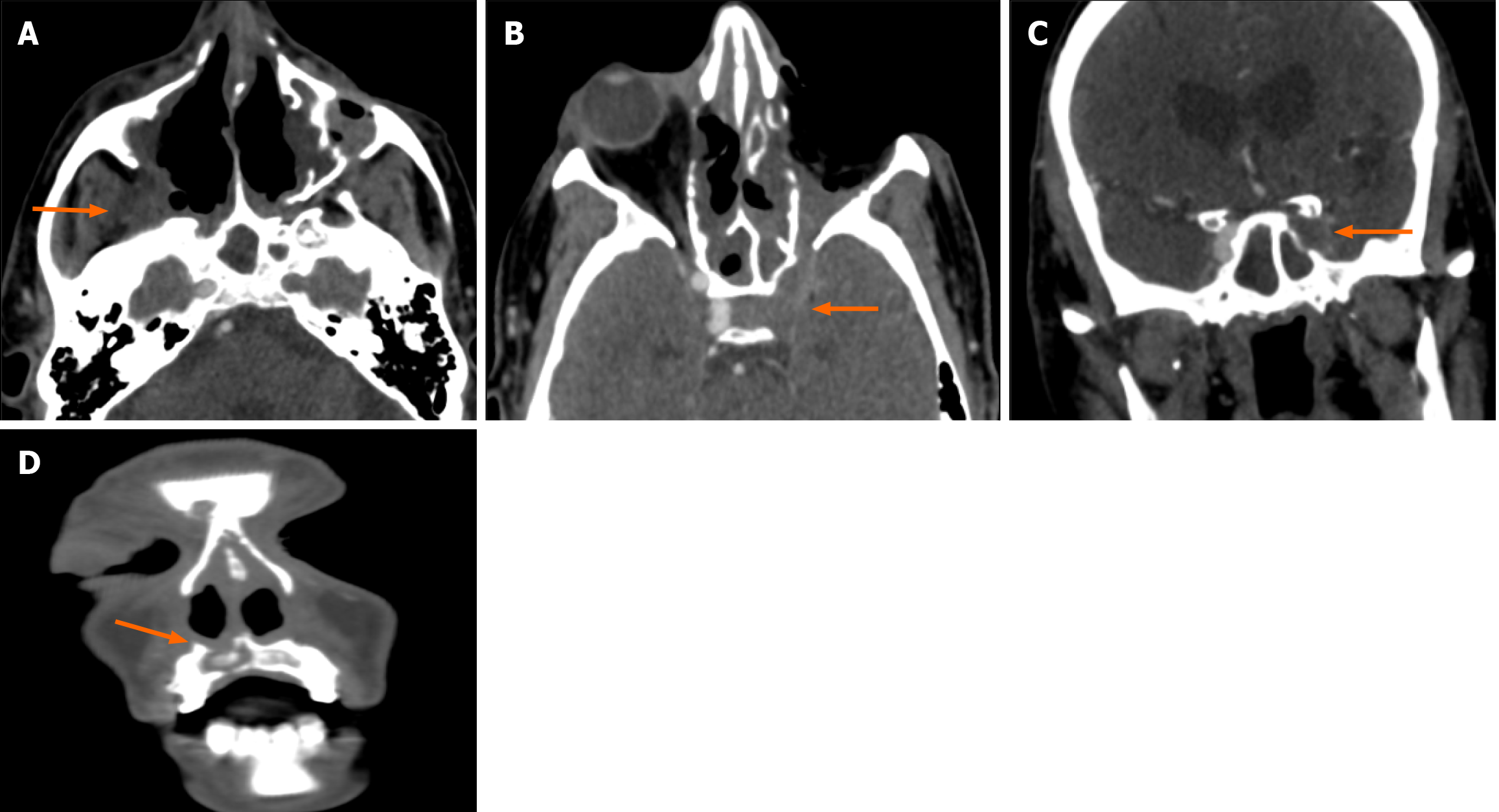
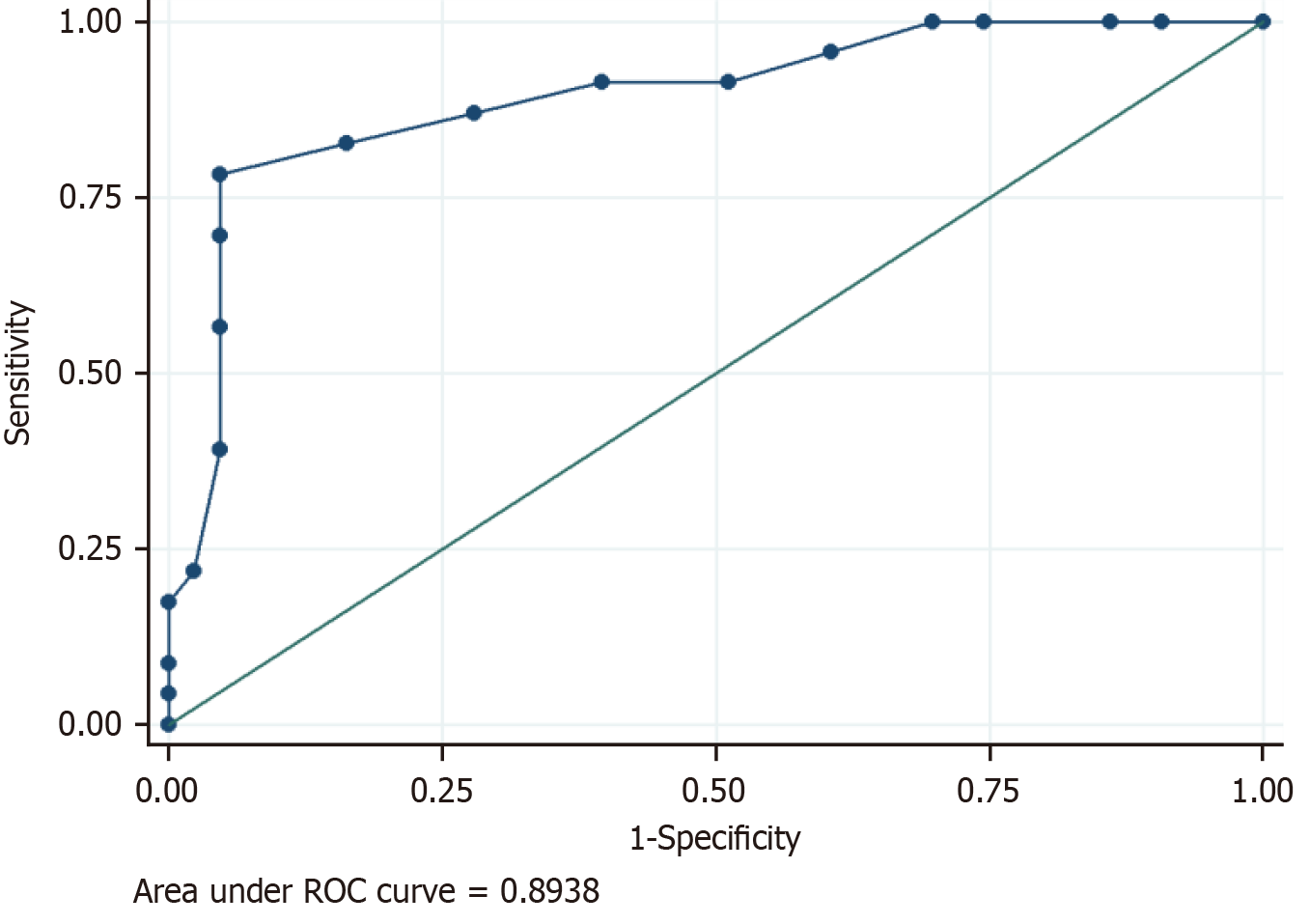
Clinico-radiological assessment was done for the follow-up of these patients. This was in the form of monthly nasal cavity cleaning and evaluation followed by radiology based on symptoms and signs. Residual clinical disease/ worsening and persistent disease in the follow-up scans (performed at 1-3 months intervals) were taken as criteria of non-responder. Forty-three patients showed clinico-radiological resolution whereas 23 patients were non-responders. Laterality and CTSI were significant predictors of response to treatment (Table 4). Out of the six patients who had bilateral disease, five were non-responders and bilaterality of disease had a significant association with poor clinical response (P = 0.02; Table 5). The mean CTSI of responders was 6.3, of non-responders was 12.9 and the response to treatment was significantly associated with CTSI (t-test, P < 0.001). On further categorizing the disease as mild, moderate, and severe on CTSI, it was seen that the majority (91%) of patients with mild disease were responders (Figure 2). Sixteen out of 28 patients (57%) with moderate disease and all the patients with severe disease (n = 4) were non-responders (Figure 3 and Figure 4). Receiver operating characteristic curve analysis (Figure 5; Liu method) to distinguish between responders and non-responders showed that the cut-off value for CTSI of 11 had a sensitivity of 78.26% and a specificity of 95.35% to predict response assessment.
| Parameter | Criteria | Responder | Non-responder | Total | P value (Fischer's exact test) |
| Diabetes mellitus | Absent | 12 | 3 | 15 | |
| Present | 31 | 20 | 51 | 0.14 | |
| Hypertension | Absent | 27 | 16 | 43 | |
| Present | 16 | 7 | 23 | 0.39 | |
| Laterality | Unilateral | 42 | 18 | 60 | |
| Bilateral | 1 | 5 | 6 | 0.02 | |
| Organism | Mucormycosis | 27 | 18 | 45 | |
| Aspergillosis | 7 | 2 | 9 | ||
| Mixed | 9 | 3 | 12 | 0.53 | |
| Survival | Survivor | 38 | 18 | 56 | |
| Expired | 5 | 5 | 10 | 0.23 | |
| TALMI Staging | Stage 1 | 2 | 0 | 2 | |
| Stage 2 | 22 | 9 | 31 | ||
| Stage 3 | 4 | 3 | 7 | ||
| Stage 4 | 15 | 11 | 26 | 0.58 | |
| CTSI | Mild (1 to 8) | 31 | 3 | 34 | |
| Moderate (9 to 16) | 12 | 16 | 28 | ||
| Severe (17 or more) | 0 | 4 | 4 | 0.00 |
No significant association however could be established between history of diabetes or hypertension, type of causative organism or TALMI staging; and the response to treatment. The level of glycosylated hemoglobin also could not predict response to treatment (t-test, P = 0.25). However, the HbA1c values were significantly associated with CTSI values (t-test, P < 0.01) but with a weak correlation (Pearson coefficient 0.34). Age was not significantly associated with CTSI (t-test, P > 0.05). In addition, there was no agreement (kappa < 0.1) between the CTSI and TALMI staging.
Acute IFS is characterized by the invasion of neurovascular structures resulting in necrosis and spread beyond the sinonasal cavity. The presence of ophthalmologic or neurological complications carries a worse prognosis and can be potentially life-threatening. The two common causative organisms of IFS are from the Aspergillus species and Zygomy
COVID-19 infection causes immune dysregulation and predisposes the patients to other infections. The factors associated with an increased incidence of IFS in COVID-19 patients include uncontrolled diabetes mellitus, excessive use of corticosteroids for immunosuppression, and prolonged stays in the intensive care unit[10].
In the emergency setup, CECT PNS is the ideal modality for delineation of the extra sinus spread of disease to orbit, infratemporal fossa, skull base, and intracranial involvement along with the extent of bony erosions. An accurate delineation of the extension of disease on CT allows timely management with antifungal agents and surgical debride
We have proposed a scoring system on CECT (CTSI) to describe the severity of IFS in COVID-19 patients. This scoring system evaluates the disease extent to the sinonasal cavity, adjacent tissues, orbit, and intracranial disease on CT and further categorizes the disease into mild, moderate, and severe (Table 1). The previously available staging system of rhino-orbital-cerebral mucormycosis as described by Talmi et al[7] is primarily based on clinical evaluation. The critical areas of involvement including pterygopalatine fossa, superior and inferior orbital fissures, orbital apex, and infratemporal fossa have been given a higher value. Similarly, optic nerve and intraocular involvement in the orbit and cavernous sinus invasion, internal carotid artery narrowing, and intracranial complications were given a higher value.
In our study cohort, most patients had mild to moderate disease (Table 2) and TALMI stage 2 disease (46.97%). The mortality rate was 15.15% which is similar to 14% of COVID-19-associated rhino-orbital-cerebral mucormycosis (ROCM)[11] and less than 34% of coronavirus associated mucormycosis[12]. It has been suggested that though COVID-19 predisposes an individual to fungal infections, it does not appear to alter the prognosis in such patients. Also, in cases of ROCM, timely sinonasal debridement with appropriate antifungal therapy has been associated with higher survival rates. There was a significant association of bilateral disease with mortality. Bilateral sinus involvement increases the imminent risk to both the orbits[11] and intracranial disease and hence has a worse prognosis.
Fifty-seven patients (86%) had COVID before the diagnosis of IFS, ranging from a period of 1-36 days in comparison to the collaborative OPAI-IJO study on mucormycosis in COVID-19 study group[11] where 56% of cases had onset of ROCM within 14 days of COVID-19 diagnosis. In this study, all positive rapid antigen tests (RATs) were confirmed by a molecular test i.e., reverse transcription polymerase chain reaction (RT-PCR). It is essential to understand that COVID-19 test results can be influenced by multiple factors, such as the timing of sample collection, the type of test used, and the prevalence of the virus in the population. False positive results have been reported in 0.5% to 5% of cases. False positivity is of low concern in high prevalence situations. As this study was conducted during the COVID-19 pandemic when the prevalence was high, it is likely to be low. Potential causes of false positivity include mislabeling of samples during processing, cross-contamination of samples during collection and processing. RT-PCR result positive for a single gene should be viewed with suspicion and repeat testing should be done[13]. When using RAT, cross-reactivity of test antibodies with rheumatoid factor in patients with autoimmune diseases may also give a false positive result[14].
About three-fourths of the patients (n = 51) were known diabetic while a total of 56 patients had their glycosylated hemoglobin above 6.5 gm/dL (mean HbA1c of 9.11 ± 2.20 gm/dL). This incidence is similar to previous literature and it is believed that COVID-19 worsens the glucose profile of the patients with diabetes further predisposing them to mucormycosis[15]. Despite the associated co-morbidities, no significant association was found between survival and history of diabetes or hypertension, causative organism, CT severity score, or TALMI staging. The level of glycosylated hemoglobin also had no significant effect on mortality (t-test, P = 0.79).
Non-responders were defined as patients with residual clinical disease/ worsening and persistent disease in the follow-up scans (performed at 1-3 month intervals). Laterality and CTSI were significant predictors of response to treatment and CTSI of 11 had a sensitivity of 78.26% and a specificity of 95.35% to predict response assessment.
Out of the six patients who had bilateral disease, five were non-responders and bilaterality of disease had a significant association with poor clinical response as has been seen in previous studies. The treatment received by our cohort of patients included intravenous amphotericin B, surgery (functional endoscopic sinus surgery/paranasal sinus debride
Collectively, these data indicated that the proposed radiological score is a useful guide in the diagnosis and follow-up of symptomatic patients with IFS. We believe that CTSI can help in the quantification of the disease burden and mapping out the extent of the disease for the surgeon. It can be useful in triaging patients at presentation and in response assessment during the hospital stay. A higher score on the initial CT would alert the clinician to initiate aggressive treatment, as severe disease correlates with slow response/non-response to treatment.
We encourage other institutions to test this scoring system and its correlation with the clinical status and response of patients of IFS (COVID-19 and non-COVID-19) to confirm its diagnostic efficacy.
The main limitation of our study was small sample size for comparison of radiological staging and clinical staging. Due to the limited sample size, comparison between mucormycosis and aspergillosis was also not possible in our study.
The CTSI is useful in quantification of the disease burden of IFS and response assessment in COVID-19 patients. It will also be useful in the triaging of patients at presentation, especially those with comorbidities like diabetes and hyper
| 1. | Honavar SG. Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian J Ophthalmol. 2021;69:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 2. | Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2022;41:616-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Al-Tawfiq JA, Alhumaid S, Alshukairi AN, Temsah MH, Barry M, Al Mutair A, Rabaan AA, Al-Omari A, Tirupathi R, AlQahtani M, AlBahrani S, Dhama K. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. 2021;49:833-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Raut A, Huy NT. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? Lancet Respir Med. 2021;9:e77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 6. | Bhalla AS, Manchanda S, Kabilan K, Thakar A, Sikka K, Verma H. Split-bolus, single-phase contrast enhanced CT: a one-stop shop for invasive fungal sinusitis. Emerg Radiol. 2023;30:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, Berkowicz M, Keller N, Kronenberg J. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Kurokawa M, Kurokawa R, Baba A, Kim J, Tournade C, Mchugh J, Trobe JD, Srinivasan A, Bapuraj JR, Moritani T. Deadly Fungi: Invasive Fungal Rhinosinusitis in the Head and Neck. Radiographics. 2022;42:2075-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Manchanda S, Semalti K, Bhalla AS, Thakar A, Sikka K, Verma H. Revisiting rhino-orbito-cerebral acute invasive fungal sinusitis in the era of COVID-19: pictorial review. Emerg Radiol. 2021;28:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Pal R, Singh B, Bhadada SK, Banerjee M, Bhogal RS, Hage N, Kumar A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses. 2021;64:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 11. | Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, Sharma M, Sachdev M, Grover AK, Surve A, Budharapu A, Ramadhin AK, Tripathi AK, Gupta A, Bhargava A, Sahu A, Khairnar A, Kochar A, Madhavani A, Shrivastava AK, Desai AK, Paul A, Ayyar A, Bhatnagar A, Singhal A, Nikose AS, Bhargava A, Tenagi AL, Kamble A, Nariani A, Patel B, Kashyap B, Dhawan B, Vohra B, Mandke C, Thrishulamurthy C, Sambare C, Sarkar D, Mankad DS, Maheshwari D, Lalwani D, Kanani D, Patel D, Manjandavida FP, Godhani F, Agarwal GA, Ravulaparthi G, Shilpa GV, Deshpande G, Thakkar H, Shah H, Ojha HR, Jani H, Gontia J, Mishrikotkar JP, Likhari K, Prajapati K, Porwal K, Koka K, Dharawat KS, Ramamurthy LB, Bhattacharyya M, Saini M, Christy MC, Das M, Hada M, Panchal M, Pandharpurkar M, Ali MO, Porwal M, Gangashetappa N, Mehrotra N, Bijlani N, Gajendragadkar N, Nagarkar NM, Modi P, Rewri P, Sao P, Patil PS, Giri P, Kapadia P, Yadav P, Bhagat P, Parekh R, Dyaberi R, Chauhan RS, Kaur R, Duvesh RK, Murthy R, Dandu RV, Kathiara R, Beri R, Pandit R, Rani RH, Gupta R, Pherwani R, Sapkal R, Mehta R, Tadepalli S, Fatima S, Karmarkar S, Patil SS, Shah S, Shah S, Shah S, Dubey S, Gandhi S, Kanakpur S, Mohan S, Bhomaj S, Kerkar S, Jariwala S, Sahu S, Tara S, Maru SK, Jhavar S, Sharma S, Gupta S, Kumari S, Das S, Menon S, Burkule S, Nisar SP, Kaliaperumal S, Rao S, Pakrasi S, Rathod S, Biradar SG, Kumar S, Dutt S, Bansal S, Ravani SA, Lohiya S, Ali Rizvi SW, Gokhale T, Lahane TP, Vukkadala T, Grover T, Bhesaniya T, Chawla U, Singh U, Une VL, Nandedkar V, Subramaniam V, Eswaran V, Chaudhry VN, Rangarajan V, Dehane V, Sahasrabudhe VM, Sowjanya Y, Tupkary Y, Phadke Y; members of the Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC) Study Group. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 12. | Kasatwar A, Shukla R, Rathod N, Nandanwar J, Mishra D, Dhobley A. Insights from Surgically treated Post Covid Acute Invasive Fungal Rhino-Orbital sinusitis in Chandrapur Study (SPAROS): A Population Based study of Coronavirus Associated Mucormycosis (CAM) characteristics in India. IJID Reg. 2022;5:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 13. | Healy B, Khan A, Metezai H, Blyth I, Asad H. The impact of false positive COVID-19 results in an area of low prevalence. Clin Med (Lond). 2021;21:e54-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Herbert C, McManus DD, Soni A. Persistent False Positive Covid-19 Rapid Antigen Tests. N Engl J Med. 2024;390:764-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 15. | John TM, Jacob CN, Kontoyiannis DP. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 16. | Al-Ani RM. Rhino-orbital-cerebral mucormycosis as a complication of coronavirus disease 2019. World J Virol. 2022;11:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Reference Citation Analysis (0)] |