Published online Jul 28, 2020. doi: 10.4329/wjr.v12.i7.137
Peer-review started: April 27, 2020
First decision: May 15, 2020
Revised: June 14, 2020
Accepted: June 17, 2020
Article in press: June 17, 2020
Published online: July 28, 2020
Processing time: 86 Days and 8.7 Hours
Coronavirus disease 2019 (COVID-19) is a novel very contagious infection which was designated a pandemic in all countries of the world in April 2020. Its presentation varies from mild to severe infection, but the majority of infected patients have mild manifestations. Many therapeutic choices have been suggested to treat the infection, but none are fully effective.
Herein we present a 26-year-old woman with a twin pregnancy at 36 wk and one day gestation with confirmed COVID-19 who responded dramatically to convalescent plasma therapy (CPT) and Favipiravir.
Although this case report shows the efficacy of CPT in addition to usual medications used for COVID-19, there are many questions that need to be answered regarding dosage, para-clinical efficacy, side effects and combination therapy.
Core tip: This is one of the first reports on the efficacy of convalescent plasma therapy (CPT) in a pregnant patient with coronavirus disease 2019 (COVID-19) pneumonia. Chest computed tomography scan findings and clinical parameters showed a dramatic response to the combination of Favipiravir and CPT, which may be an important choice for pregnant patients with COVID-19 pneumonia.
- Citation: Jafari R, Jonaidi-Jafari N, Dehghanpoor F, Saburi A. Convalescent plasma therapy in a pregnant COVID-19 patient with a dramatic clinical and imaging response: A case report. World J Radiol 2020; 12(7): 137-141
- URL: https://www.wjgnet.com/1949-8470/full/v12/i7/137.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i7.137
Coronavirus disease 2019 (COVID-19) caused by a novel Coronavirus was first identified in Wuhan (China) in December 2019. The spread of COVID-19 was so rapid that within a few months it became a pandemic[1,2]. The most common presentations of COVID-19 are cough, fever, dyspnea, and malaise, but some patients present severe manifestations such as respiratory failure, acute respiratory distress syndrome, septic shock, multi-organ failure, and disseminated intravascular coagulation (DIC)[3]. Comorbidities such as diabetes, hypertension, chronic cardiovascular diseases, pulmonary disease, and malignancies exacerbate the disease, particularly in older patients and pregnant women, especially in the third trimester[2,3]. Pregnant women are a sensitive group, therefore are susceptible to severe manifestations and complications of COVID-19. Thus, to avoid probable complications these patients should be treated appropriately and immediately.
A 26-year-old woman with a twin pregnancy at 36 wk and one day gestation (gravida 1, 0 abortions) was referred to our emergency unit.
The patient presented with malaise, fever, and a non-productive cough for 6 d prior to admission.
No remarkable past medical history.
No remarkable personal and family medical history.
On physical examination, she was febrile, tachycardic, hypoxic with O2 saturation of 92%, and diffuse rhonchi on auscultation.
The most significant laboratory finding was an increased erythrocyte sedimentation rate. A non-stress-test was reactive for both fetuses and both biophysical profiles were 6/8 due to decreased fetal movement.
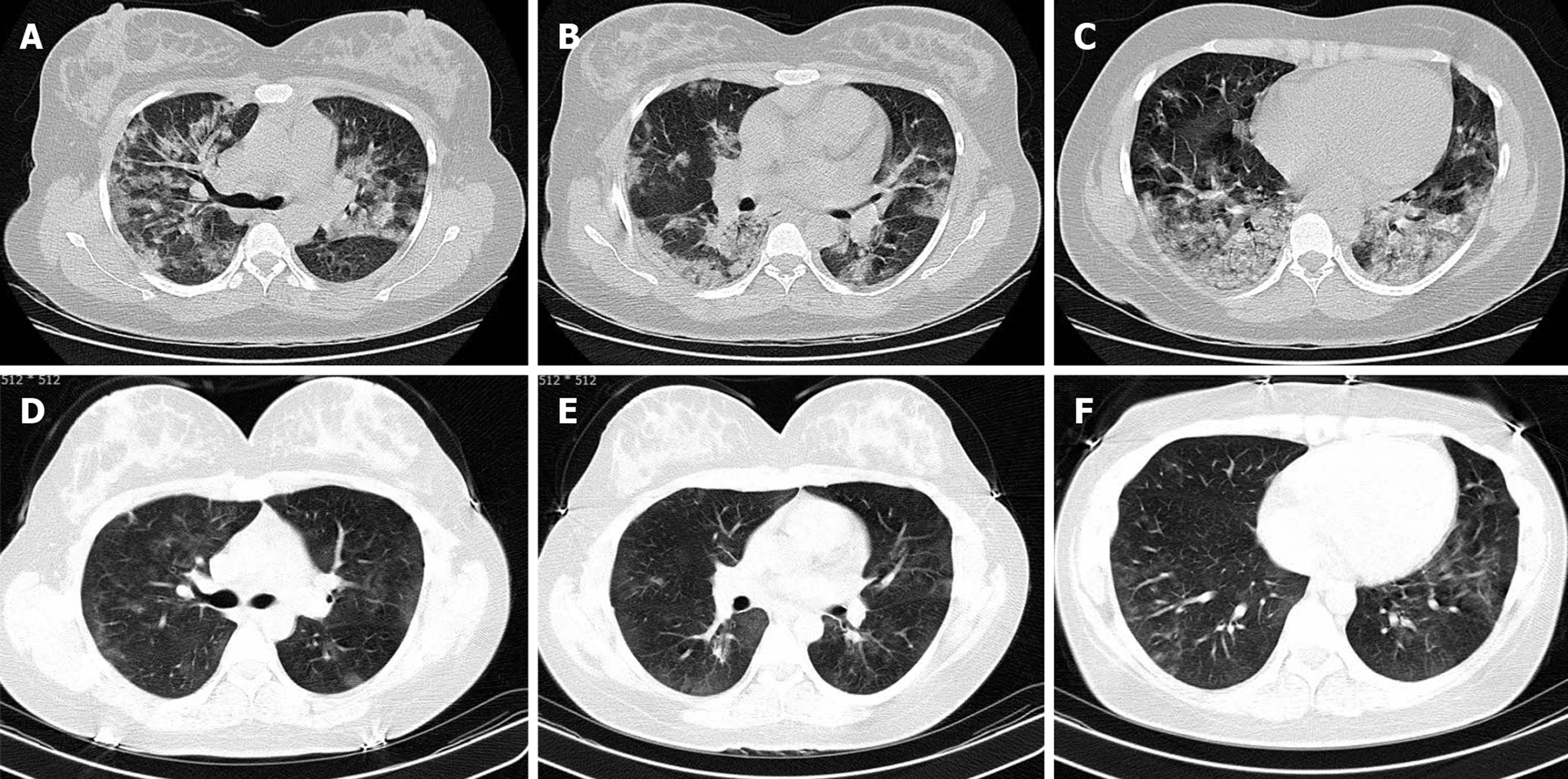
Chest computed tomography (CT) scan was performed for further evaluation and revealed multifocal sub-pleural patchy consolidative opacities on both lung fields highly suggestive of COVID-19 pneumonia (Figure 1A-C).
After initial preparation and oxygen therapy, the patient was immediately transferred to the operating room for a cesarean section. Both infants were in a good condition with a first minute APGAR score of 9/10. They were isolated from their mother and transferred to the Neonatal Intensive Care Unit for close observation. COVID-19 infection was confirmed in the mother using the reverse transcription-polymerase chain reaction (RT-PCR), while nasopharynx specimens were negative in both infants and they were completely asymptomatic at the two-week follow-up. Meropenem, azithromycin, and hydroxychloroquine as well as supplemental oxygen were started after delivery. Due to the lack of a favorable response to treatment, on the sixth day after the admission, the patient received one plasma transfusion obtained from cured COVID-19 patients. Favipiravir was added to her medications. Her clinical course during hospitalization improved, particularly during the second week.
After twelve days, another CT scan was performed, and the results showed only very faint residual ground-glass opacities. Therefore, the dramatic response to this therapeutic regimen was notable (Figure 1E-G).
COVID-19 with pulmonary involvement.
The patient was effectively treated with Favipiravir and convalescent plasma therapy (CPT).
The patient was discharged after 2 weeks with a dramatic response to therapy. Both newborns had no COVID-19 symptoms and negative PCR results.
Pregnant women, especially at the end of pregnancy, are more susceptible to severe manifestations of infections, including COVID-19, probably due to changes in the immune system and physical stature[4]. However, few studies have investigated the influence of COVID-19 on pregnancy and its possible complications. However, evidence from similar viral infections from the Coronaviridae family such as Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome has indicated more maternal and fetal complications, such as spontaneous miscarriage, intrauterine growth retardation, preterm parturition, maternal renal failure, sepsis, DIC, and even death[1-3,5]. Therefore, to reduce probable complications, pregnant women should be monitored for early diagnosis and given appropriate and immediate treatment[6].
The course of COVID-19 in pregnant women is unpredictable. Chen et al[7] reported nine pregnant women in the third trimester with an improved clinical course during hospitalization without complications. On the other hand, Liu et al[8] investigated thirteen pregnant women with COVID-19, and reported that during the study period six of these patients were transferred to the intensive care unit. Karami et al[3] reported a 27-year-old pregnant woman at 30 wk and 3 d gestation with COVID-19 and a deteriorating clinical course who subsequently died due to multi-organ failure. Fortunately, clinical and imaging findings in our patient indicated improved health status during hospitalization and she responded to treatment, especially during the second week and as mentioned, only very faint residual ground-glass opacities were visible in the second chest CT scan, in comparison with multifocal sub-pleural patchy consolidative opacities in her first chest CT.
Based on available published data, vertical transmission during pregnancy does not occur or occurs rarely[9]. The presented cases by Chen et al[7] and Liu et al[8] confirmed this hypothesis; however, Yu et al[10] reported one RT-PCR positive test for COVID-19 in seven neonates who were born by cesarean section, while all maternal tests were positive for COVID-19.
Many therapeutic opinions such as Chloroquine, Kaletra, Remdesivir, Azithromycin, Tocilizumab, and Favipiravir have been suggested for such cases, with no clear evidence of their effectiveness and their efficacy was confirmed by a few reports[11-13]. Recently, the effectiveness of CPT for severe COVID-19 patients was confirmed and Iran (Islamic Rep. of) is a pioneer country in using this therapeutic strategy[14]. Passive immunization using plasma obtained from recovered patients is the basis of this treatment, and the FDA has also approved this strategy, particularly for critically ill cases of COVID-19[15]. Duan et al[14] investigated the administration of a single dose of convalescent plasma in 10 adults with severe COVID-19. They reported a positive clinical outcome after 3 d and radiological outcome after 7 d. Huo et al[16] reported that CPT can save as many as 6451 lives (ranging from 3074 to 26500 depending on the containment effectiveness) in the early period of the infection. However, as recently reported, “it seems that the convalescent plasma treatment discontinues the COVID-19 shedding, and cannot reduce mortality in critically end-stage COVID-19 patients”[17]. Although this is not good news for critical cases, it helps early stage-cases to avoid intensive care units and slow progression of the disease[18].
There is a query in the present case, and it is the simultaneous prescription of Favipiravir and CPT. This may confuse the efficacy of each medication, while the efficacy of Favipiravir has been previously confirmed[19]. A randomized clinical trial is recommended to include three groups of patients, one group treated with Favipiravir and CPT, and the other two groups treated with Favipiravir and CPT alone.
Furthermore, in our case this novel therapy diminished the clinical and radiologic features of COVID-19. The effects of Oxytocine should also be considered, as it was previously stated that “oxytocin decreases the neuroendocrine and cytokine activation and might be a candidate for the therapy of inflammatory diseases and conditions associated with high cytokine and vascular endothelial growth factor levels”[20].
Our knowledge on this novel coronavirus infection in pregnancy and its probable complications is insufficient, and more accurate investigations, particularly regarding biologic treatments, are necessary.
We would like to thank Professor Mostafa Ghanei for valuable comments.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu YC, Rismanbaf A S-Editor: Dou Y L-Editor: Webster JR E-Editor: Liu JH
| 1. | Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, Liu Y. Infants Born to Mothers With a New Coronavirus (COVID-19). Front Pediatr. 2020;8:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 2. | Asadi L, Tabatabaei RS, Nejad HS, Mohammadi M. New Corona Virus (COVID-19) Management in Pregnancy and Childbirth. Arch Clin Infect Dis. 2020; In press: e102938. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Karami P, Naghavi M, Feyzi A, Aghamohammadi M, Novin MS, Mobaien A, Qorbanisani M, Karami A, Norooznezhad AH. WITHDRAWN: Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020: 101665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Luo Y, Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. 2020;20:513-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 625] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 6. | Khan MA, Khan A, Mustagir G, Rana J, Haque R, Rahman M. COVID-19 infection during pregnancy: A systematic review to summarize possible symptoms, treatments, and pregnancy outcomes. medRxiv preprint 2020. [DOI] [Full Text] |
| 7. | Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2400] [Cited by in RCA: 2272] [Article Influence: 454.4] [Reference Citation Analysis (0)] |
| 8. | Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 9. | Mimouni F, Lakshminrusimha S, Pearlman SA, Raju T, Gallagher PG, Mendlovic J. Perinatal aspects on the covid-19 pandemic: a practical resource for perinatal-neonatal specialists. J Perinatol. 2020;40:820-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, Liu Y, Xiao J, Liu H, Deng D, Chen S, Zeng W, Feng L, Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 11. | Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 678] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 12. | Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 14. | Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490-9496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1251] [Cited by in RCA: 1314] [Article Influence: 262.8] [Reference Citation Analysis (0)] |
| 15. | Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 16. | Huo X, Sun X, Bragazzi N, Wu J. Effectiveness and Feasibility of Convalescent Blood Transfusion to Reduce COVID-19 Fatality Ratio. SSRN Electronic Journal. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, Xu JH, Lin WB, Cui GL, Zhang MM, Li C, Wang ZS, Zhang ZH, Liu ZS. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in COVID-19 Patients. J Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 18. | Rubin R. Testing an Old Therapy Against a New Disease: Convalescent Plasma for COVID-19. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 774] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 20. | Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686-E691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |