Published online Feb 28, 2020. doi: 10.4329/wjr.v12.i2.10
Peer-review started: August 2, 2019
First decision: November 6, 2019
Revised: November 21, 2019
Accepted: December 5, 2019
Article in press: December 5, 2019
Published online: February 28, 2020
Processing time: 201 Days and 10.1 Hours
Giant cavernous malformation (GCM) is rarely found in intraventricular or paraventricular locations.
We present two cases of 6-mo and 21-mo boys with intraventricular and paraventricular GCMs including a literature review focused on location and imaging findings. Characteristic magnetic resonance imaging findings such as multicystic lesions and a hemosiderin ring or bubbles-of-blood appearance can assist in the differential diagnosis of a hemorrhagic intraventricular and/or paraventricular mass.
Multifocal intraventricular and/or paraventricular GCM in small children is rare. The characteristic magnetic resonance imaging findings can help to differentiate GCMs from other intraventricular tumors.
Core tip: We present two rare cases of multifocal intraventricular and/or paraventricular giant cavernous malformation (GCM) in small children including a literature review focused on location and imaging findings. The GCM is a rare entity. Intraventricular cavernous malformations are very rare and account for 2.5% of all brain cavernous malformations and are mostly found in the third ventricle. Characteristic magnetic resonance imaging findings such as multicystic lesions and a hemosiderin ring or bubbles-of-blood appearance can assist to differentiate GCMs from other intraventricular tumors/masses.
- Citation: Eng-Chuan S, Kritsaneepaiboon S, Kaewborisutsakul A, Kanjanapradit K. Giant intraventricular and paraventricular cavernous malformations with multifocal subependymal cavernous malformations in pediatric patients: Two case reports. World J Radiol 2020; 12(2): 10-17
- URL: https://www.wjgnet.com/1949-8470/full/v12/i2/10.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i2.10
The overall prevalence of intracranial cavernous malformation (CM), cavernous hemangioma, cavernous angioma or cavernoma is 0.4%-0.6% and the mean age of presentation is 30.6 years[1]. Its prevalence in children is very rare at 0.37%-0.53%[2,3]. The most common location is the cerebral hemisphere[1]. Intraventricular CMs are very rare and account for 2.5% of all brain CMs and are mostly found in the third ventricle[4,5]. The size of the CMs varies between 1 mm and 75 mm (mean size 14.2 mm)[4-6]. The giant cavernous malformation (GCM) is a rare entity and there is no standard size definition, with some prior studies defining a GCM as having a diameter greater than 4-6 cm[7,8]. The most common presenting symptoms are seizure, neurological deficits, and hemorrhage[8,9]. The current standard treatment for symptomatic patient is surgical removal, particularly for lesions in noneloquent areas[8-10]. The surgical outcome is good despite the large size[10,11]. Herein we reported two cases of paraventricular and intraventricular GCMs with intraventricular hemorrhage in small children.
Chief complaints: A 6-mo-old boy presented with increased head circumference and delayed development.
History of present illness: Delayed gross motor development started a month ago with increased head circumference for 1 wk.
History of past illness: The patient had no previous medical history and uneventful perinatal and postnatal periods. He had no underlying disease or family history of brain tumor.
Physical examination: The patient had bulging anterior fontanel sized about 4 cm and his head circumference is about 48.5 cm (> P97).
Laboratory examinations: The patient’s routine laboratory investigations were within normal limits.
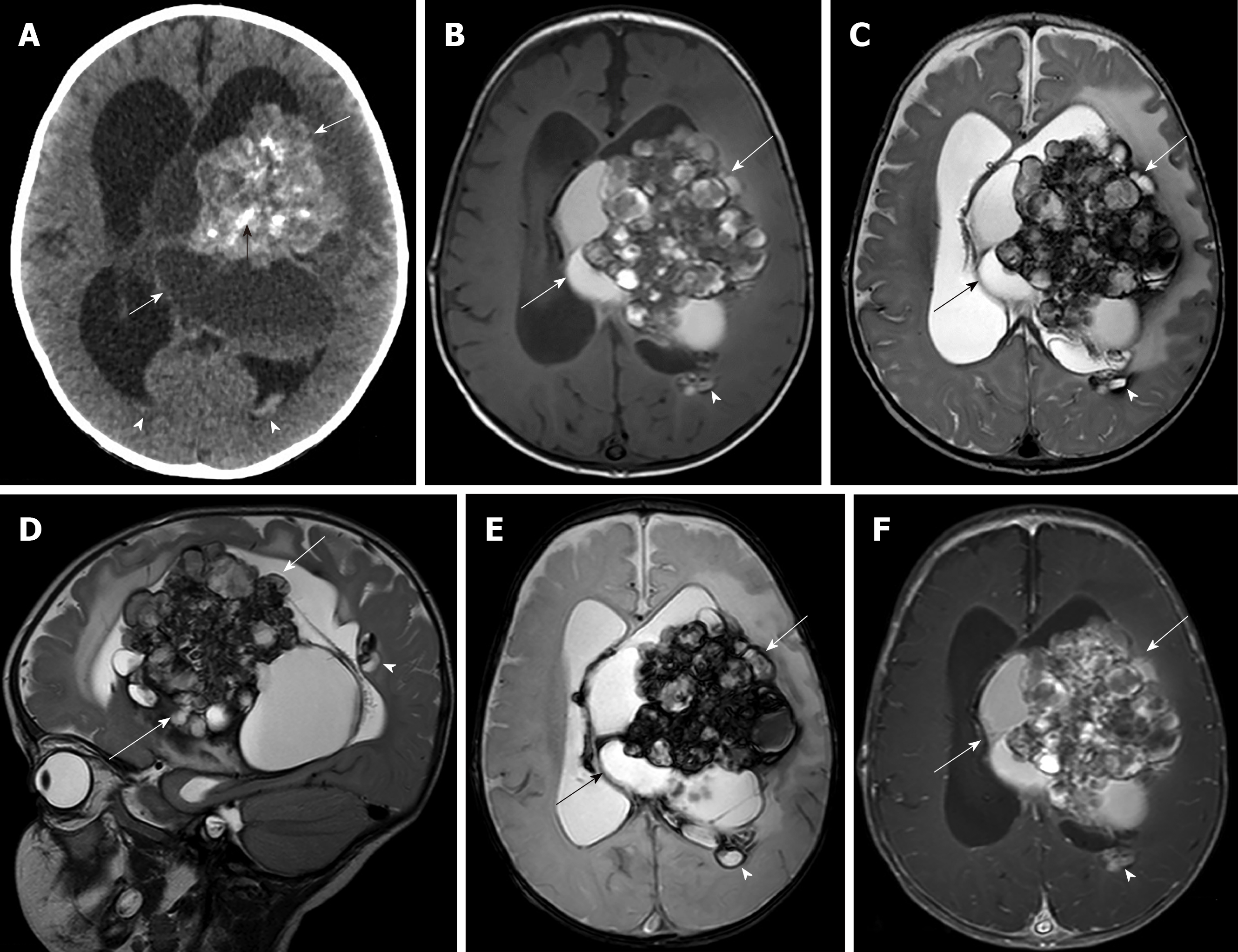
Imaging examinations: Brain computed tomography (CT) showed a mixed solid-cystic intraventricular tumor with multiple internal calcifications involving the body and trigone of the lateral ventricle and third ventricle with severe hydrocephalus (Figure 1A). Magnetic resonance imaging (MRI) of the brain revealed a large mixed minimal heterogeneous enhancing solid cystic intraventricular mass with internal calcification and perilesional brain edema mainly located at the left trigone and body of the left lateral ventricle resulting in moderate hydrocephalus, subfalcine and left descending transtentorial herniation. Two other small subependymal nodules at the posterior bodies of the left and right lateral ventricles were seen. Associated intraventricular hemorrhage and subarachnoid hemorrhage were found (Figure 1B-F). The provisional diagnosis from the MRI findings was choroid plexus papilloma.
Treatment: He underwent right frontal ventriculostomy with shunt placement and a left frontoparietal craniotomy with partial tumor removal. A hypervascular intraventricular tumor with lobulated shape was found in the left lateral ventricle with signs of an old hemorrhage.
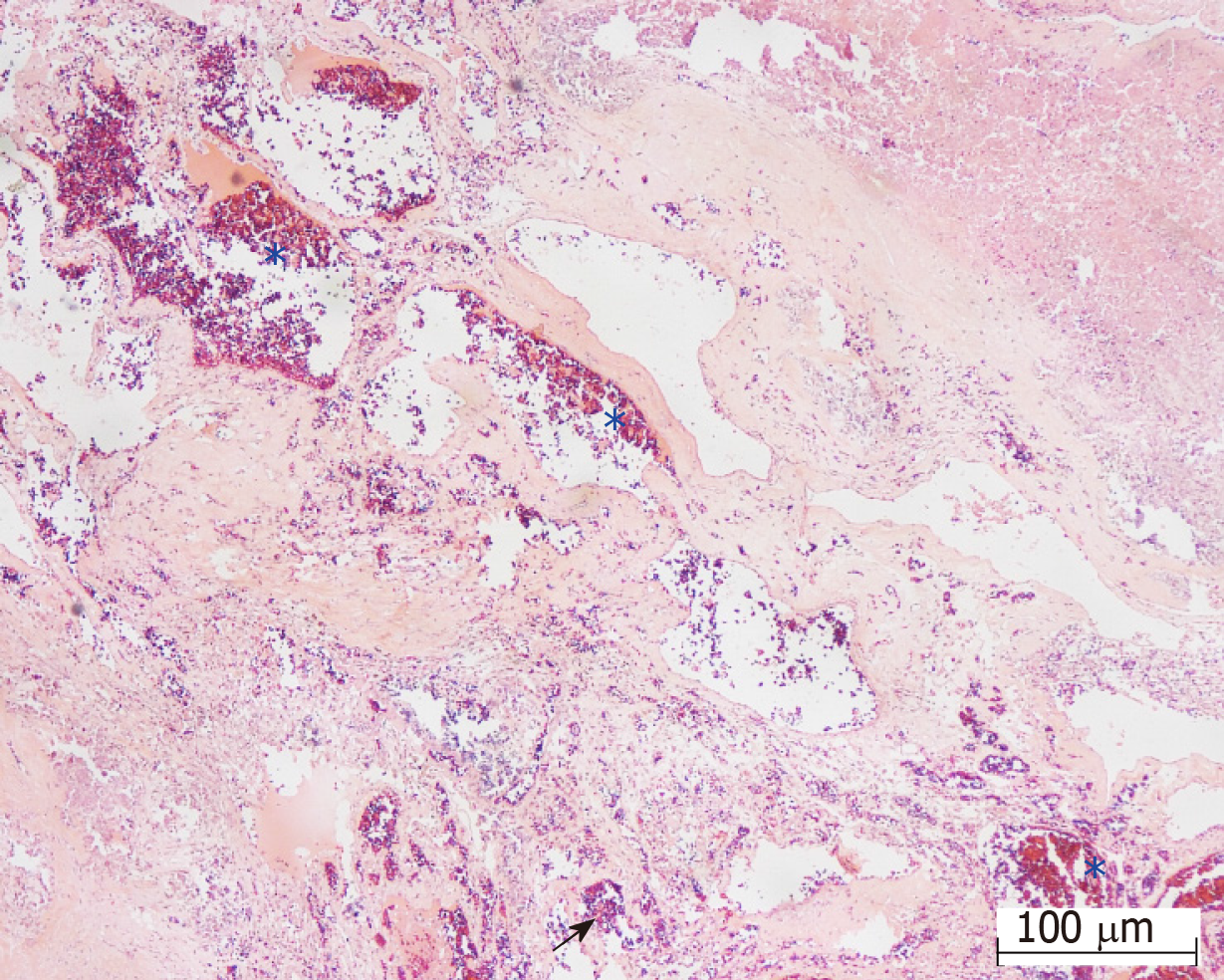
Final diagnosis: The pathological diagnosis was compatible with “cavernous hemangioma with hemorrhage” (Figure 2).
Outcome and follow-up: An MRI study 2 years later after the patient had developed chorea showed a larger size CM and. He was scheduled for a second surgical tumor removal.
Chief complaints: A 21-mo-old boy was referred to our institute due to left hemiparesis.
History of present illness: The left hemiparesis has been progressed for 5 mo.
History of past illness: The patient had no previous medical history and no relevant family history.
Physical examination: The patient had left hemiparesis grade 4. The other general conditions of the patients were normal.
Laboratory examinations: The patient’s routine laboratory investigations were within normal limits.
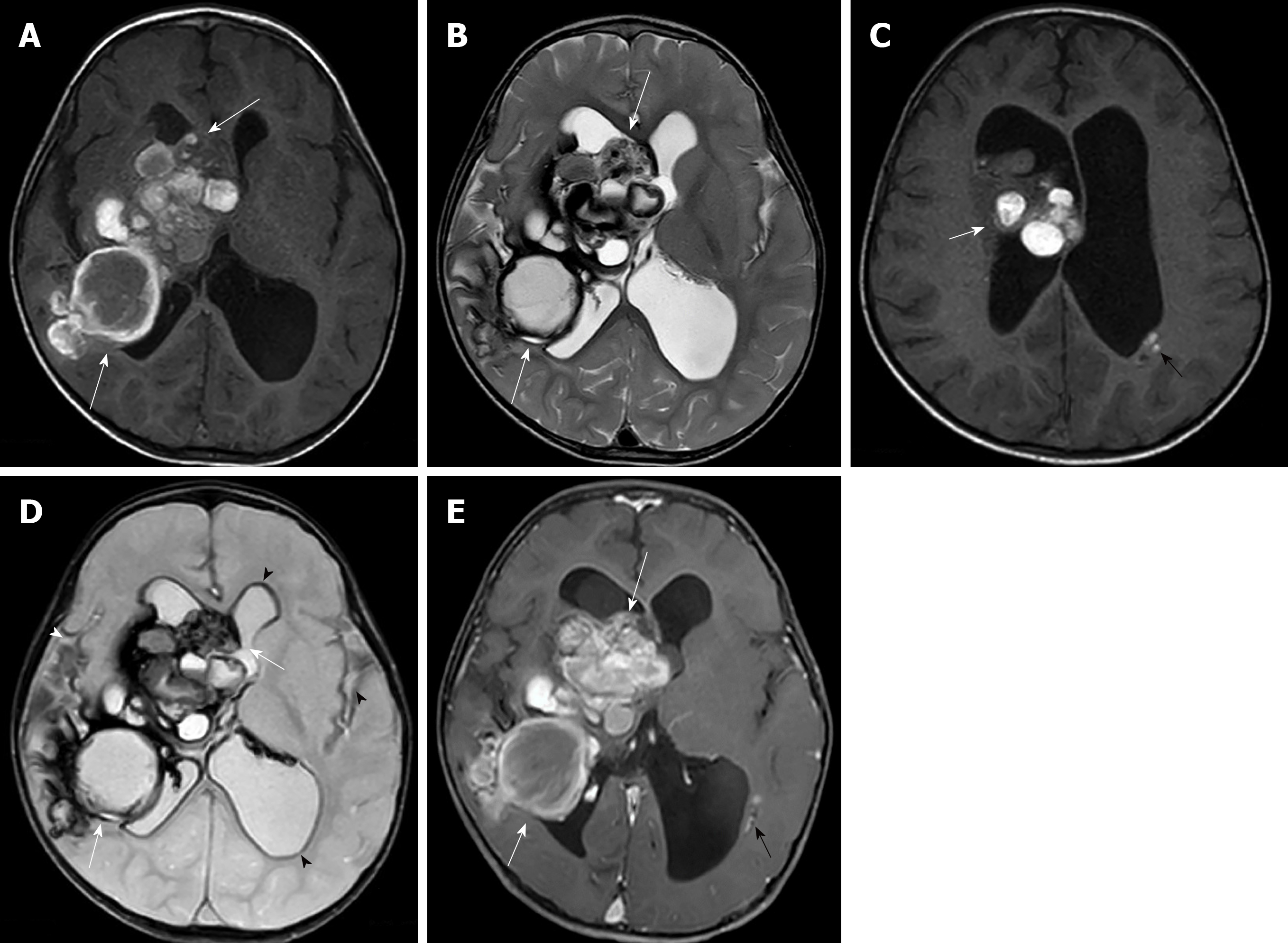
Imaging examinations: A brain MRI study showed multifocal multilobulated cystic masses with variously staged hemorrhages at the right periventricular region, right foramen of Monro, right ventricular trigone and occipital horn of the right lateral ventricle with perilesional brain edema. The largest mass involved the right basal ganglia and right thalamus. Old intraventricular hemorrhage and superficial hemosiderosis were evident. A mass effect caused subfalcine, mild right uncal herniation and moderate hydrocephalus. There was no associated venous angioma (Figure 3).
Treatment: The patient underwent a keyhole right parieto-temporal craniotomy with partially tumor removal. The operative findings were a mulberry-liked solid-cystic mass and old blood clot and mild cerebral edema with hemosiderin straining along the arachnoid plane.
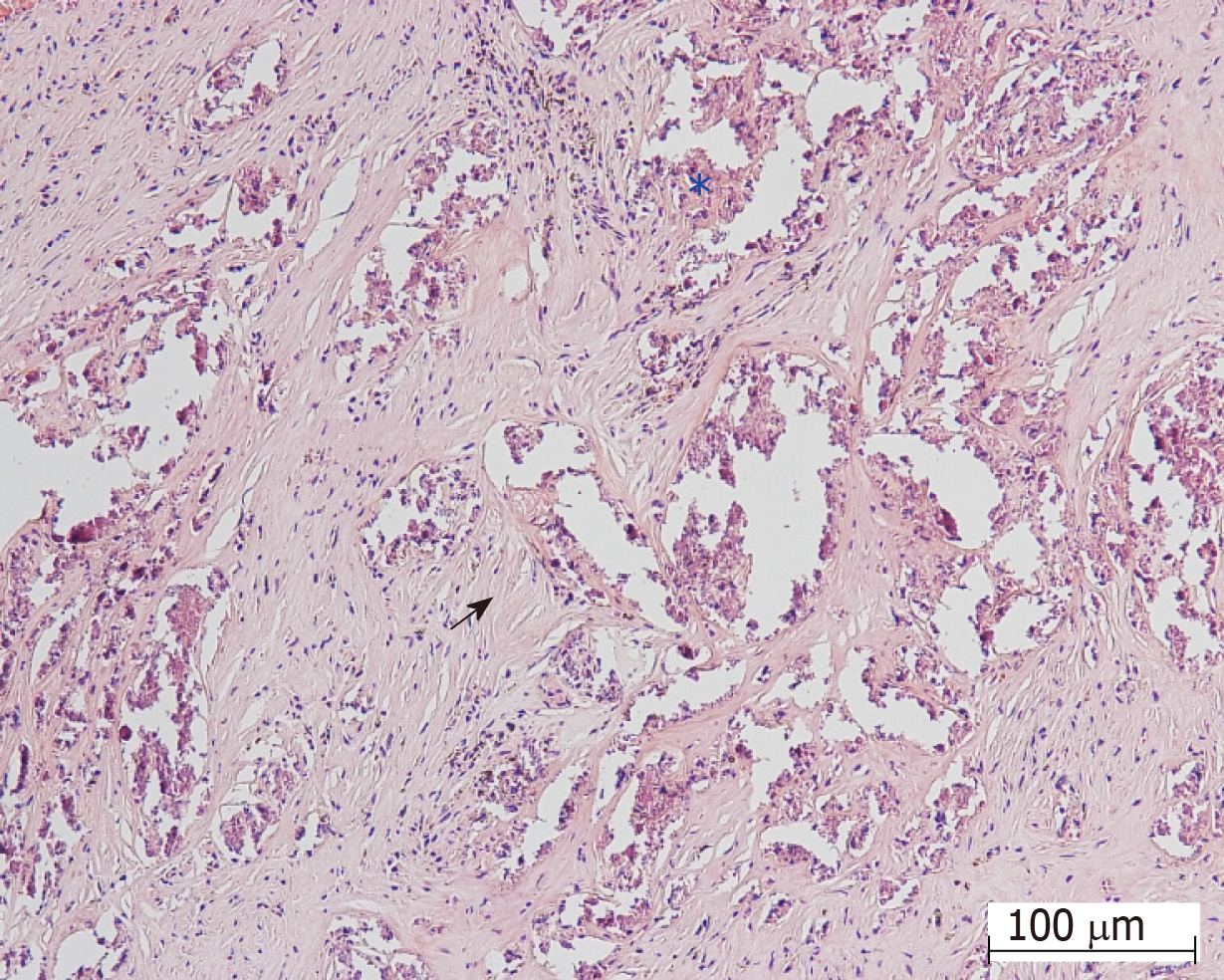
Final diagnosis: The pathological diagnosis was fibrous tissue, calcification, and hemorrhage (Figure 4) and compatible with cavernous hemangioma.
Outcome and follow-up: After the operation, his motor power was partially improved.
Relevant articles were searched for using the keywords GCM, pediatric, intraventricular, paraventricular and intraventricular hemorrhage. Seven articles were found, including 13 cases of intraventricular and paraventricular GCM[8,12-17]. The details are shown in Table 1. The GCM is rare and can occur along the neural axis as a CM with the most common location at supratentorial region[8,12]. Intraventricular and paraventricular GCMs are uncommon locations and 44% of them are located in the third ventricle, 27% in the lateral ventricle, 20% in the trigone, and 9% in the fourth ventricle[5].
| No. | Ref. | Age, sex | Size (cm) | Location | Lesion number | Symptoms | Computed tomography scan | Magnetic resonance imaging | Post- operative status |
| 1 | Kawagishi et al[11], 1993 | 11 mo, boy | 10 | Right paraventricle | Single | Left hemiparesis | Multilobular high density enhanced rim/septum | Heterogeneous signal intensity on T1w/T2w with hemosiderin rim | Improved |
| 2 | Van Lindert et al[10], 2007 | 3 mo, girl | 6-7 | Left paraventricle | Single | Generalized seizure | Left fronto-temporal multicystic | - | Improved |
| 3 | Gezen et al[12], 2008 | 10 mo, boy | 6 × 4 × 4.5 | Left paraven tricular parietal lobe | Single | Focal seizure | - | Lobulated, hemorrhages | Hemi-paresis |
| 4 | Nieto et al[13], 2003 | 11 yr, girl | 5 | Left trigone | Single | Seizure | - | Rounded and well-delineated | Improved |
| 5 | Kumar et al[14], 2006 | 8 yr, boy | 5 | Right trigone | Single | Mass effect | Hyperdense enhanced, calcifications | - | Improved |
| 6 | Kan et al[8], 2008 | 0.9 yr, boy | 9 × 6 | Left fronto-parietal, intraventricle | Single | Seizure, right hemiparesis | Hyperdense, heterogeneous calcifications | Multicystic with multiple hemosiderin rings | NA |
| 7 | Ozgen et al[15], 2011, 7 reported cases | 2 yr, girl | > 4 | Left parietal | Single | Seizure | - | Macroscopic cysts and huge calcification | NA |
| 8 | 4 yr, boy | > 4 | Medial temporal | Single | Seizure | - | Multicystic hemosiderin ring | NA | |
| 9 | 8 mo, girl | > 4 | Left parietal | Single | Seizure | - | Multicystic cysts with subacute hematoma | NA | |
| 10 | 18 mo, boy | > 4 | Peri-atrial of left parietal region | Multiple | Seizure | - | Multicystic with fluid level | NA | |
| 11 | 1 yr, girl | > 4 | Left parietal | Single | Vomiting, altered consciousness | - | Macroscopic cysts, huge calcification | NA | |
| 12 | 9 yr, girl | > 4 | Left parietal | Single | Seizure | - | Solid, well-defined | NA | |
| 13 | 8 yr, boy | > 4 | Intraventricle | Single | Headache | - | Solid, well-defined | NA | |
| 14 | Two present cases | 6 mo, boy | 9.4 | Left intraventricle | Multiple | Increased head circumference | Solid-cystic | Heterogeneous enhancing solid cyst | Hemi-paresis |
| 15 | 21 mo, boy | 8.2 | Right paraventricle | Multiple | Left hemiparesis | Multilobulate rim enhancement | Multicystic hemorrhage |
The pathogenesis of CMs is still unclear. Various studies have proposed that integrin-binding protein ICAP-1 and Krev interaction trapped protein 1 may play important roles in the pathogenesis of the cerebral CM (CCM) and GCM[18]. The increase in size of GCMs is thought to involve a process of cavernous proliferation from repetitive hemorrhages, pseudo-capsule formation, and expansion. The osmotic effect of the breakdown of blood products in cysts causes characteristic multicystic lesions with blood in various stages and surrounded by hemosiderin rings or bubbles of blood appearance on MRI[17]. One of our cases had a larger-than-normal size, and an intralesional hemorrhage was one possible reason for the growth of CM.
The papers also found that in children with CM, about 10% of cases were familial and about 17% had multiple lesions[1,19]. Most of the multiple-lesion cases were familial, up to 84%, and the rest were sporadic (30%)[17]. Only one case of intraventricular and paraventricular GCMs, which presented at 18 mo, had multiple lesions detected on T2* sequence (Table 1)[17]. One of our cases was first reported as multiple paraventricular GCMs at 6 mo of age. The studies also suggest that multiple CMs had a greater risk of de novo cavernoma-genesis than solitary CMs (7.1% and 0.6% per lesion per year)[19]. Both of our cases had multiple lesions, unfortunately genetic studies were not performed.
Initial CT imaging of our cases revealed solid-cystic masses with internal calcifications and small multifocal subependymal lesions that mislead us to suspect intra-/paraventricular tumors and subependymal seedings. MRI helped characterize the lesions by revealing bubbles of blood appearances and multi-stage hemorrhages mimicking solid components on CT. The MR imaging findings of our cases were characteristic imaging features of CMs, showing multicystic lesions with various stages of hemorrhages and hemosiderin rims. Minimally enhanced lesion in one of our cases could be from heterogeneity of CCMs relative to blood–brain barrier behavior[20].
Pediatric CMs are classified by imaging according to the Mottolese et al[2]’s study. The findings in our cases seem to fall-between Mottolese classification type IIIA (non-enhancing macroscopic cysts of mixed signal intensities surrounded by lower signal of hemosiderin rim on T2 weighted image) and IIIC (large solid mass with heterogeneous inner signal on T1 and T2 and peripheral low signal on T2* weighted image) that can mimic neoplasm. None of the paraventricular GCM in all of the previous reports had intraventricular hemorrhage as in both of our cases. CCMs in pediatric patients have about a 0.5% per lesion per year risk for hemorrhage[19]. The history of previous hemorrhage increases the annual hemorrhagic risk to 11.3%[19].
The differential diagnosis of a hemorrhagic intraventricular mass in small children includes choroid plexus tumor, teratoma and subependymal giant astrocytoma. All neoplasms reveal significant enhancement in post-contrast enhancement. Minimal enhancement, bubbles of blood and absence of associated findings of tuberous sclerosis such as cortical tubers may help to differentiate a GCM from intraventricular tumors.
The standard treatment of CCM is conservative or surgical removal[10]. Conservative treatment is preserved for asymptomatic patients. Most GCMs are symptomatic or occasionally indistinguishable from brain tumors, so the surgical removal is the treatment of choice. In our cases, the GCMs were partially removed because the tumors were located in eloquent areas and leading to limited choice of surgical technique[11].
In conclusion, we present two rare cases of multifocal intraventricular/ paraventricular GCM in small children. CT findings in GCMs can show a mixed solid-cystic mass with internal calcified solid components. The characteristic MRI findings of multicystic lesions and hemosiderin ring or bubbles of blood appearance can help to differentiate GCMs from other intraventricular tumors.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao BL, Movahed A S-Editor: Yan JP L-Editor: A E-Editor: Xing YX
| 1. | Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011;30:E24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Mottolese C, Hermier M, Stan H, Jouvet A, Saint-Pierre G, Froment JC, Bret P, Lapras C. Central nervous system cavernomas in the pediatric age group. Neurosurg Rev. 2001;24:55-71; discussion 72-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Acciarri N, Galassi E, Giulioni M, Pozzati E, Grasso V, Palandri G, Badaloni F, Zucchelli M, Calbucci F. Cavernous malformations of the central nervous system in the pediatric age group. Pediatr Neurosurg. 2009;45:81-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Tatagiba M, Schönmayr R, Samii M. Intraventricular cavernous angioma. A survey. Acta Neurochir (Wien). 1991;110:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Reyns N, Assaker R, Louis E, Lejeune JP. Intraventricular cavernomas: three cases and review of the literature. Neurosurgery. 1999;44:648-54; discussion 655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48:9-17; discussion 17-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 182] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Lawton MT, Vates GE, Quinones-Hinojosa A, McDonald WC, Marchuk DA, Young WL. Giant infiltrative cavernous malformation: clinical presentation, intervention, and genetic analysis: case report. Neurosurgery. 2004;55:979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kan P, Tubay M, Osborn A, Blaser S, Couldwell WT. Radiographic features of tumefactive giant cavernous angiomas. Acta Neurochir (Wien). 2008;150:49-55; discussion 55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Son DW, Lee SW, Choi CH. Giant cavernous malformation : a case report and review of the literature. J Korean Neurosurg Soc. 2008;43:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kivelev J, Niemelä M, Hernesniemi J. Treatment strategies in cavernomas of the brain and spine. J Clin Neurosci. 2012;19:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Ghali MG, Srinivasan VM, Mohan AC, Jones JY, Kan PT, Lam S. Pediatric cerebral cavernous malformations: Genetics, pathogenesis, and management. Surg Neurol Int. 2016;7:S1127-S1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | van Lindert EJ, Tan TC, Grotenhuis JA, Wesseling P. Giant cavernous hemangiomas: report of three cases. Neurosurg Rev. 2007;30:83-92; discussion 92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kawagishi J, Suzuki M, Kayama T, Yoshimoto T. Huge multilobular cavernous angioma in an infant: case report. Neurosurgery. 1993;32:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Gezen F, Karatas A, Is M, Yildirim U, Aytekin H. Giant cavernous haemangioma in an infant. Br J Neurosurg. 2008;22:787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Nieto J, Hinojosa J, Muñoz MJ, Esparza J, Ricoy R. Intraventricular cavernoma in pediatric age. Childs Nerv Syst. 2003;19:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kumar GS, Poonnoose SI, Chacko AG, Rajshekhar V. Trigonal cavernous angiomas: report of three cases and review of literature. Surg Neurol. 2006;65:367-371, discussion 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Ozgen B, Senocak E, Oguz KK, Soylemezoglu F, Akalan N. Radiological features of childhood giant cavernous malformations. Neuroradiology. 2011;53:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet. 2002;11:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Gross BA, Du R, Orbach DB, Scott RM, Smith ER. The natural history of cerebral cavernous malformations in children. J Neurosurg Pediatr. 2016;17:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Retta SF, Glading AJ. Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: Two sides of the same coin. Int J Biochem Cell Biol. 2016;81:254-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |