Published online Aug 28, 2018. doi: 10.4329/wjr.v10.i8.83
Peer-review started: May 4, 2018
First decision: June 6, 2018
Revised: June 26, 2018
Accepted: July 10, 2018
Article in press: July 10, 2018
Published online: August 28, 2018
Processing time: 117 Days and 20.2 Hours
To investigate the utility of renal diffusion tensor imaging (DTI) to detect early renal damage in patients with type 2 diabetes.
Twenty-six diabetic patients (12 with microalbuminuria (MAU), and 14 with normoalbuminuria) and fourteen healthy volunteers were prospectively included in this study. Renal DTI on 3.0 T MR was performed, and estimated glomerular filtration rate (eGFR) was recorded for each subject. Mean cortical and medullary fractional anisotropy (FA) values were calculated by placing multiple representative regions of interest. Mean FA values were statistically compared among groups. Correlations between FA values and eGFR were evaluated.
Both cortical and medullary FA were significantly reduced in diabetic patients compared to healthy controls (0.403 ± 0.064 vs 0.463 ± 0.047, P = 0.004, and 0.556 ± 0.084 vs 0.645 ± 0.076, P = 0.002, respectively). Cortical FA was significantly lower in diabetic patients with NAU than healthy controls (0.412 ± 0.068 vs 0.463 ± 0.047, P = 0.02). Medullary FA in diabetic patients with NAU and healthy controls were similar (0.582 ± 0.096 vs 0.645 ± 0.076, P = 0.06). Both cortical FA and medullary FA correlated with eGFR (r = 0.382, P = 0.015 and r = 0.552, P = 0.000, respectively).
FA of renal parenchyma on DTI might serve as a more sensitive biomarker of early diabetic nephropathy than MAU.
Core tip: Early diagnosis of diabetic nephropathy (DN) facilitates timely treatment, and therefore, improves patient outcomes. Microalbuminuria (MAU), a standard biomarker of DN, has limited sensitivity and reproducibility. Diffusion tensor imaging (DTI) quantifies the directional nature of water diffusion and is especially suited for highly oriented organs such as the kidney. DTI parameter changes were reported in several renal pathologic conditions. This study exhibited reductions of renal fractional anisotropy (FA) in diabetic patients, even with normoalbuminuria, which raises the possibility of detecting early DN with higher sensitivity than MAU. Furthermore, renal FA demonstrated statistical correlation with eGFR, making it a potential functional biomarker.
- Citation: Wang YT, Yan X, Pu H, Yin LL. In vivo evaluation of early renal damage in type 2 diabetic patients on 3.0 T MR diffusion tensor imaging. World J Radiol 2018; 10(8): 83-90
- URL: https://www.wjgnet.com/1949-8470/full/v10/i8/83.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i8.83
Diabetic nephropathy (DN) remains one of the most common causes of morbidity and mortality in patients with type 2 diabetes worldwide[1,2]. The mechanism of renal damage in DN is not fully understood. As type 2 diabetes progresses, the albuminuria of DN patients gradually progresses from intermittent to persistent. A sensitive and accurate biomarker of DN is needed for early detection and therefore early intervention to prevent irreversible renal damage in high-risk patients.
Although microalbuminuria (MAU) level measurement is currently the standard of care for the diagnosis of DN[3], it may remain difficult to detect until clinically significant renal damage occurs. Progression of DN precedes elevation of MAU levels and in a significant proportion of diabetics, the MAU levels may actually regress[4]. Other laboratory indicators such as estimated glomerular filtration rate (eGFR) are commonly used to evaluate the renal damage by DN, however, eGFR has a lower sensitivity[5].
Renal magnetic resonance (MR) diffusion-weighted imaging (DWI) has been used more frequently to identify early renal damage[6-9]. It allows noninvasive evaluation of molecular diffusion in vivo without gadolinium contrast, which is of significant concern to patients with risks of renal insufficiency. Diffusion tensor imaging (DTI) is a diffusion MRI protocol, which is more comprehensive than standard DWI, allowing the quantification of the directional nature of water diffusion. This ability makes it especially suited for analyzing organs with specific orientation, such as the kidneys, which have an organized internal structure with tubules, collecting ducts and vessels radially oriented towards the renal hilum[10].
DTI was first used by Ries et al[11] in the kidneys of healthy volunteers. As numerous studies reported that fractional anisotropy (FA) values measured from DTI differed significantly between the renal cortex and medulla, FA values may aid in the further detection of abnormalities in the microstructure of the kidney[4,12]. Further studies have defined the role of DTI to detect renal dysfunction in human allografts and early stages of chronic kidney disease[9,10,13,14]. Recent animal experiments showed that renal DTI reflected ischemia-reperfusion injury in which FA was significantly reduced[15]. Notably, histological changes induced by chronic hypoxia are considered major contributing factors to renal damage in diabetic patients[4], indicating that DTI might be a promising biomarker of DN. This was confirmed by a later study in a rat model of diabetic nephropathy showing that reduction of FA indicated pathological changes of diabetic nephropathy, such as interstitial fibrosis, glomerulosclerosis, and tubular damage[16]. However, the evaluation of renal damage by DTI in patients with DN is scarce.
The primary objective of this study was to explore the possibility of using renal DTI to detect early renal damage in patients with type 2 diabetes, and to verify whether DTI parameters of renal parenchyma correlate with clinical laboratory biomarkers of renal function (i.e., eGFR) in a cohort of diabetic patients compared to healthy control subjects.
This clinical study was approved by the ethics committee of our institution. Oral informed consents from the subjects were obtained. The consent included information regarding noninvasive, radiation-free scan, no interference with treatment, and anonymized clinical data for analysis.
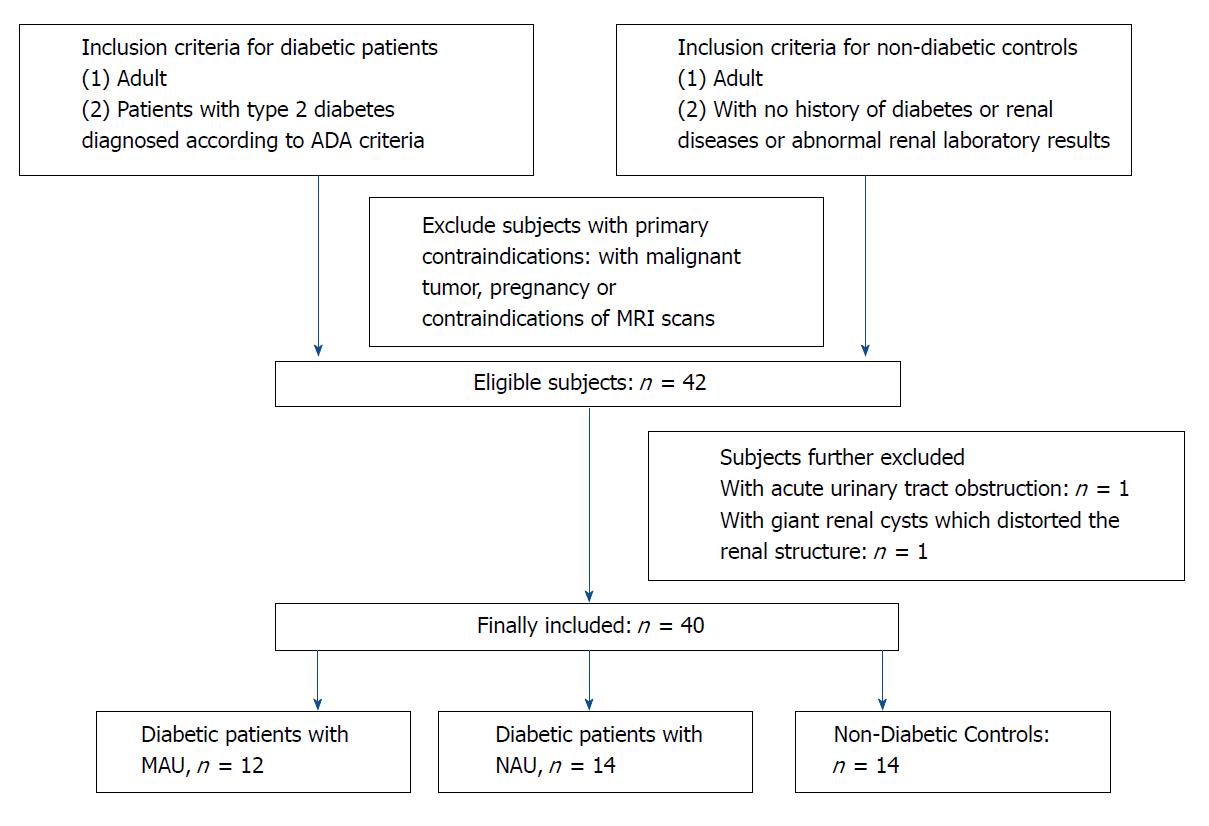
Patients with type 2 diabetes, diagnosed according to the American Diabetes Association 2014 criteria and healthy volunteers, who had no history of diabetes, kidney diseases, or abnormal renal laboratory results were included in the study from April 2017 to March 2018. Patients with malignant tumors, pregnancy, acute urinary tract obstruction, acute kidney failure, abnormal renal anatomy shown by MRI (including giant renal cysts which distorted the renal structure), or contraindications of MRI examination were excluded from the study as shown in Figure 1. The final study included 26 patients with type 2 diabetes (14 males and 12 females, aged 42 to 78 years, with a median age of 62 years) and 14 healthy subjects (8 males and 6 females, aged 40 to 77 years, with a median age of 60 years). The type 2 diabetic patients were further divided into two sub-groups according to their MAU test results: sub-group (1), MAU (urine albumin/creatinine ratio ranged from 30 to 300 μg/min, considered to be in the early stage of DN, n = 12); and sub-group (2), normoalbuminuria (NAU) (urine albumin/creatinine ratio < 30 mg/g, n = 14).
Urine and blood samples were collected except during the menstruation in female subjects. All subjects were instructed to avoid strenuous activity and high-fat diets the night before sample collection. MAU was detected using the immune transmission turbidimetric method. The Modification of Diet in Renal Disease (MDRD) formula was employed to calculate the estimated GFR (eGFR, mL/min per 1.73 m2) = 170 × (serum creatinine)-0.999 × (age)-0.176 × (urea nitrogen)-0.170 × (albumin)0.318 × 0.762 (if female)[17,18]. The serum creatinine and urea nitrogen concentrations were determined on a C16000 (Abbott, United States) using a creatinine assay kit (Maccura, China) and a reagent kit (Biosino, China).
All MRI scans were performed using a 3.0 T system (Magnetom Verio; Siemens, Erlangen, Germany) with phased array body and spine surface coils. Conventional sequences included axial T1-, T2-, and coronal T2-weighted sequences. DTI was performed using coronal single-shot echo-planar imaging sequence with breath-held method, and diffusion images were obtained through fat saturation sequences. The parameters were as follows, TR = 2200 ms, TE = 70 ms; slices thickness = 3 mm, intersection gap = 1 mm; diffusion directions = 6, b = 0, 200 s/mm2 (which can exhibit better cortical-medullary contrast according to our preliminary study results); phase encoding direction = left to-right, field-of-view = 38 cm × 38 cm, matrix size = 154 × 154, and the time for acquisition was 24 s.
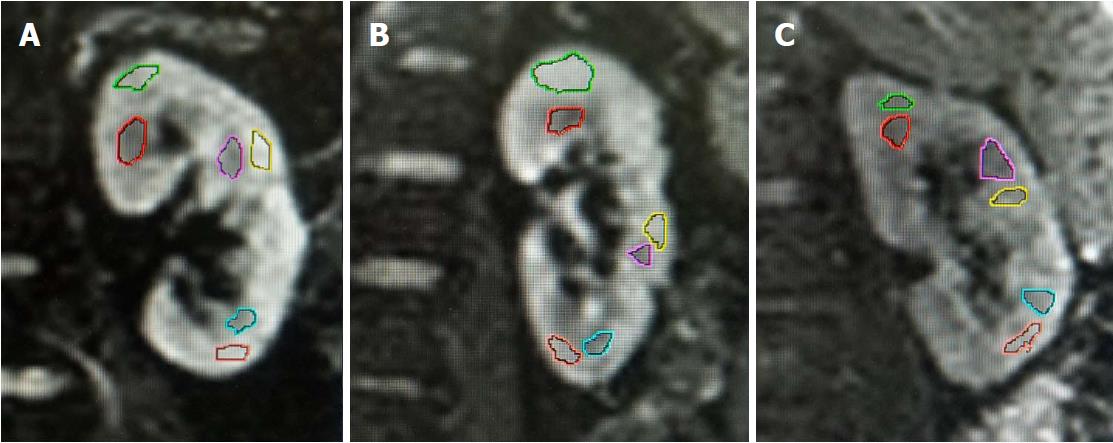
The source images were used to generate coronal DWI trace images, apparent diffusion coefficient (ADC) maps, and FA maps of kidneys automatically on a workstation (Siemens Healthcare Sector, Erlangen, Germany). The diffusion tensor, which represents the true mean diffusivity, was determined by the orientation of water molecules and magnitude of diffusion. The degree of diffusion anisotropy was calculated and represented by FA on a convenient index that ranged from 0 (isotropic-no preferred direction) to 1 (full anisotropy-only one direction)[19]. Regions of interest (ROI) were then drawn and analyzed by the same operator for all subjects under the supervision of an experienced radiologist. Both doctors were blinded to the eGFR results during post-processing. ROIs were drawn on the diffusion-weighted images (b = 0 s/mm2) to exhibit adequate anatomic details. A freehand method was employed to better adapt to the contours of renal cortex and medulla. ROIs were automatically overlaid on ADC and FA maps for quantification. In order to measure the cortical and medullary FA separately and sample the representative parenchyma accurately, the ROIs were located within the upper, middle, and lower portions of bilateral renal cortex and medulla on the coronal images, bypassing the renal pelvis, visible blood vessels, and small cysts. Two contiguous slices nearest to the renal hila of both kidneys were used for the drawing of ROIs. The mean cortical and medulla FA values for each subject were calculated from averaging the measurements from both kidneys. This method has been widely used in previous studies evaluating renal DTI[20-23].
Statistical analysis was performed using SPSS 19.0 statistical software. Data were indicated as the mean ± SD. Comparison of FA values between diabetic and healthy control groups, and between MAU and NAU groups were performed by two-tailed student t test. Cortical and medullary FA values with eGFR were correlated using Pearson’s correlation test. A level of P value < 0.05 was considered to be statistically significant.
Twenty-six patients with type 2 diabetes, consisting of twelve with MAU and fourteen with NAU, and fourteen healthy controls were included in this study. The demographic and clinical characteristics are summarized in Table 1.
| Characteristics | Type 2 diabetic patients with MAU | Type 2 diabetic patients with NAU | Healthy volunteers |
| No. | 12 | 14 | 14 |
| Medium age (yr) | 61 | 68 | 60 |
| Sex (male/female) | 10/4 | 6/8 | 8/6 |
| Serum creatinine (μmol/L) | 92.0 ± 66.1 | 65.9 ± 19.9 | 54.3 ± 10.3 |
| eGFR (mL/min·1.73 m2) | 88.6 ± 9.1 | 94.4 ± 7.9 | 104.8 ± 14.8 |
Image quality was satisfactory and free of significant artifacts for depiction of renal contour and anatomic details in all included subjects. There was clear differentiation between the renal cortex and medulla in axial T1-, T2-weighted, and b0 images. No remarkable differences in renal anatomic details were noted among the healthy volunteer group, diabetic patients with NAU group, and diabetic patients with MAU group by visual observation (Figure 2).
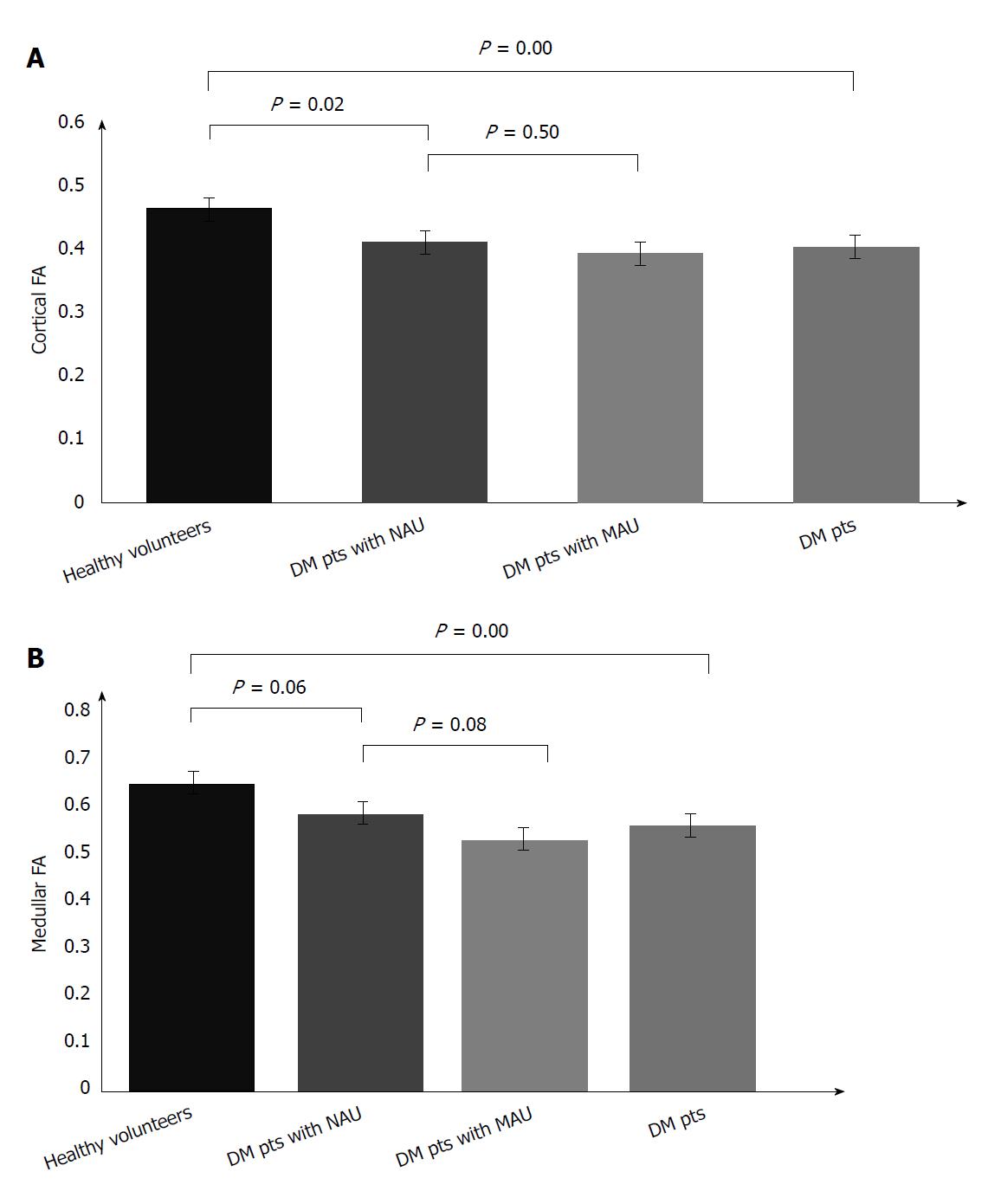
The mean FA of the renal cortex and medulla in diabetic patients with MAU, NAU, and healthy volunteers is displayed in Table 2. There was a statistically significant difference in cortical and medullary FA between the diabetic patients compared to healthy volunteers (P = 0.00, P = 0.00 respectively). The cortical FA was significantly lower in diabetic patients with NAU than in healthy volunteers (P = 0.02). The difference of the medullary FA between patients with NAU and healthy volunteers was not statistically significant, with a marginal P value of 0.06. There was no significant difference of either cortical or medullary FA between MAU and NAU groups (P = 0.50 and 0.08, respectively) (Figure 3).
| FA values | Cortical FA | P value | Medullary FA | P value |
| Type 2 diabetic patients with MAU | 0.393 ± 0.062 | 0.526 ± 0.047 | ||
| Type 2 diabetic patients with NAU | 0.412 ± 0.068 | 0.582 ± 0.096 | ||
| Type 2 diabetic patients with either MAU or NAU | 0.403 ± 0.064 | 0 | 0.556 ± 0.084 | 0 |
| Healthy volunteers | 0.463 ± 0.047 | 0.645 ± 0.076 |
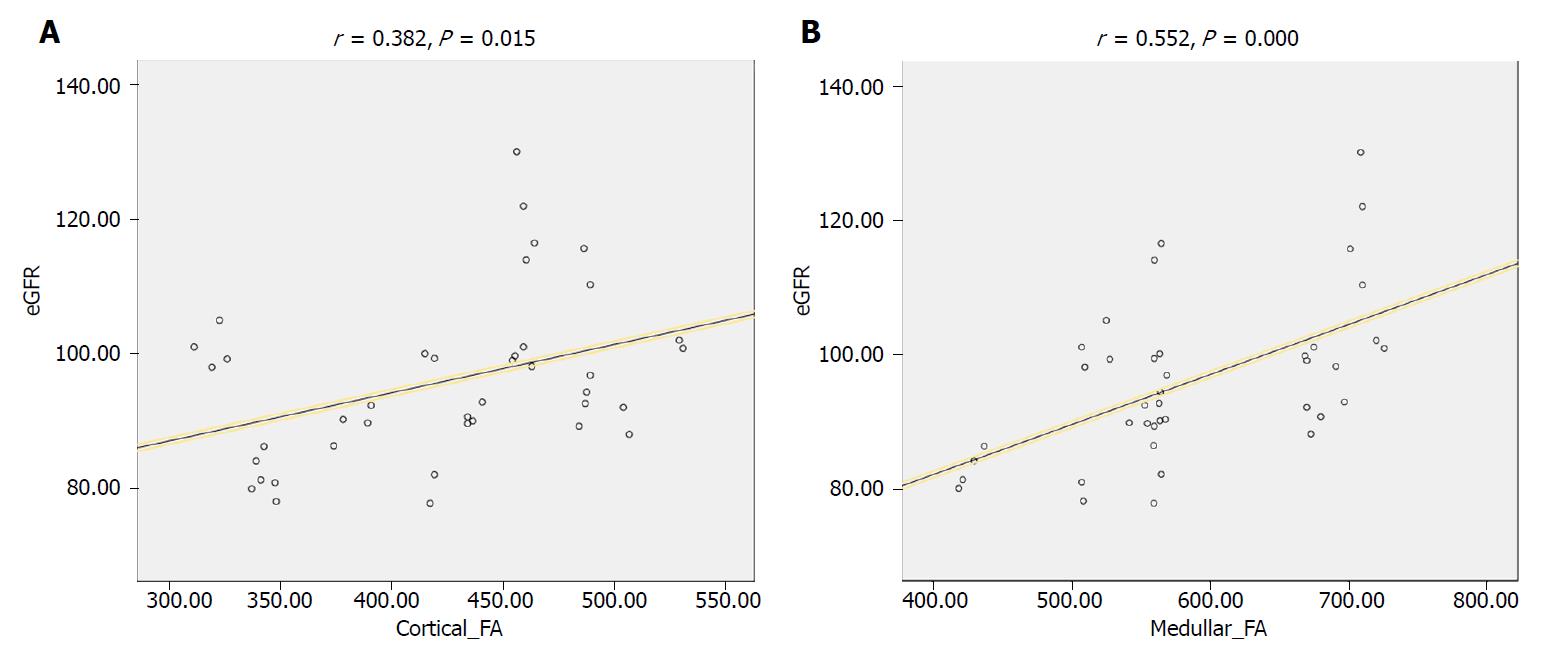
Significant correlation was observed between medullary FA and eGFR in all included patients (r = 0.519, P = 0.001). A similar significant correlation was observed between cortical FA and eGFR (r = 0.322, P = 0.043), as shown in Figure 4.
Our study used quantitative DTI techniques to demonstrate significant differences between the renal cortical and medullary FA among healthy volunteers and diabetic patients (with either NAU or MAU), and between healthy volunteers and diabetic patients with NAU. These differences were detected despite a lack of demonstrable difference in renal anatomic structures on conventional sequences. In addition, significant correlations were detected between renal cortical FA and eGFR and between medullary FA and eGFR.
Previous studies have shown that early recognition and diagnosis of renal damages caused by diabetes mellitus could facilitate a timely and proper treatment protocol for this irreversible process, and therefore improve patient outcomes[4,24]. Currently, although MAU is considered to be a standard biomarker of DN, it has limited sensitivity and clinical reproducibility. The study by Lu et al[5] indicated that diabetic patients without decreases in eGFR had significantly lowered renal FA than healthy controls, and the study by Wang et al[22] showed that diabetic patients with MAU had the lowest FA compared to diabetic patients with NAU and healthy controls. This study showed a significant reduction of cortical FA and a decreasing tendency of medullary FA in patients with type 2 diabetes with NAU compared to healthy controls, raising the possibility that DTI parameters might serve as a more sensitive biomarker for DN than MAU.
As an organ with a highly directional structure with tubules, collecting ducts, and vessels radially oriented towards the pelvis, kidneys have been shown to be one of the ideal organs for evaluation by DTI in previous studies[10]. Furthermore, such diffusion anisotropy of kidneys is necessary to maintain normal function of glomerular filtration, reabsorption, and concentration, as the cortical-medullary solute gradient is driven by the directional perfusion of renal blood flow[12]. Such renal ultrastructure could be compromised in pathological processes, such as chronic kidney diseases, tumors, or obstructive diseases[15,20,21]. Currently, few studies have explored the relationship between DTI parameters and clinical biomarkers of DN. This study indicates that both cortical and medullary FA values correlate with eGFR in a cohort of subjects with normal or diabetic kidneys, and therefore could potentially detect functional changes in diabetic patients even before the gross structural changes appear on conventional sequences.
The study by Lu et al[5] suggested that medullary FA correlated significantly with eGFR, while cortical FA did not. However, a later study by Razek et al[25] showed that the cortical FA in diabetic patients correlated with urinary and serum biomarkers such as urinary albumin and creatinine. This study exhibited that both cortical and medullary FA correlated with eGFR, suggesting that both the renal cortex and medulla could be involved in the pathogenesis of DN and renal functional damage. Several previous articles reported the correlation of FA with eGFR in chronic kidney diseases, with a concordant trend shown in this study[20,21]. However, the correlation coefficients (r) of this study were lower. This might be attributed to the fact that we included patients with early nephropathy during which the eGFR has not yet decreased significantly.
The pathophysiologic process underlying the observed reduction of renal FA in diabetic patients is worth discussion. The b value plays a critical role in MR diffusion models, which encodes different tissue properties into the diffusion signals. As the b value increases, the sensitivity to detect molecular diffusion increases and the MR signal to noise ratio decreases[26]. According to the literature[19,27], the renal ADC was approximately three times of brain ADC (brain DTI usually employs b values of 0, 1000 s/mm2). Therefore, many studies used a b value of 0, 200-400 s/mm2 in renal DTI in order to get similar signal intensity, which can be interpreted as low to intermediate b values in MR diffusion models[19,27]. This b value region is sensitive to both tissue vascularity and cellularity.
Several potential mechanisms of FA decrease in pathological conditions of DN can be postulated, and more than one might be involved. These include potential vascular abnormalities, reduced tubular flow rate, and tubular ultrastructural damage[5]. Furthermore, some studies suggest that medullary FA correlates with interstitial fibrosis, while cortical FA correlates with glomerulosclerosis in other pathological processes. Both the interstitial fibrosis and glomerulosclerosis can be present in the early stage of DN[9,10,14,19,21,28]. Despite the explicit overall FA changes in renal damage, further studies are required to distinguish these pathophysiologic changes of tissue properties in DN.
This study had some limitations. First, the number of patients included was relatively small, which limits the statistical analysis. Further studies with larger sample sizes are needed to further validate the results. Second, renal pathological results were not collected for correlation. Further experimental or clinical studies with pathological findings will be useful to reveal the specific underlying mechanism of FA reduction in DN. Third, the FA values were calculated under the assumption that the selected ROIs can effectively represent the overall status of renal parenchyma. Although multiple ROIs in different locations were drawn to increase the sample size, biases and errors might still be present. Fourth, some subjects might have other unknown potential pathological conditions, such as hypertension and coagulation disorders. Other factors such as hydration status might also influence the DTI results[29].
In summary, both cortical and medullary FA could potentially exhibit early renal damage in type 2 diabetic patients, and therefore help to differentiate diabetic kidneys from healthy controls even before the clinical detection of MAU.
Diabetic nephropathy (DN) remains one of the most common causes of morbidity and mortality in patients with type 2 diabetes worldwide. Early diagnosis of DN facilitates timely treatment, and therefore, improving patient outcomes. Although microalbuminuria (MAU) level is currently the primary standard for the diagnosis of DN, it may lag behind DN progression and has limited sensitivity.
Diffusion tensor imaging (DTI) quantifies the directional nature of water diffusion and is especially suited for specifically oriented organs such as the kidney. DTI parameter changes were reported in several renal pathologic conditions presenting itself as a potential biomarker of renal damage.
The main objective of this study was to explore the possibility of using renal DTI to detect early renal damage in patients with type 2 diabetes.
This prospective study included 26 diabetic patients (12 with MAU, and 14 with normoalbuminuria) and 14 healthy volunteers. Renal DTI on 3.0 T MR was performed and estimated glomerular filtration rate (eGFR) was recorded for each subject. Mean cortical and medullary fractional anisotropy (FA) values were separately calculated by placing multiple representative regions of interest. Mean FA values were statistically compared among groups, and correlations between FA values and eGFR were evaluated. The high-field MR offered high signal-to-noise ratio and the multiple sampling of renal parenchyma ensured the representativeness of the underlying pathological changes.
The results showed that both cortical and medullary FA were significantly reduced in diabetic patients compared to healthy controls. Cortical FA was significant lower in diabetic patients with NAU than healthy controls, indicating that the renal damage reflected by the FA changes occurred even earlier than the clinical detection of MAU. Both cortical FA and medullary FA correlated with eGFR, indicating that both renal cortex and medulla could be involved in the pathogenesis of DN.
In conclusion, both cortical and medullary FA could potentially exhibit early renal damage in type 2 diabetic patients, and therefore help to differentiate diabetic kidneys from healthy controls even before the clinical detection of MAU.
Based on these findings, renal DTI could be developed as an early biomarker in addition to the current examinations in the clinical practice. Further experimental or clinical studies with pathological results can be designed to reveal the specific underlying mechanism of FA reduction in DN.
The authors thank Professor Aamer Chughtai for help editing the language. The authors also thank Dr. Ling Ma for help preparing the figures.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hasegawa H, Sijens PE, Villela-Nogueira C, Yong D S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3153] [Cited by in RCA: 2863] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 2. | Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63:S39-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Mogensen CE, Keane WF, Bennett PH, Jerums G, Parving HH, Passa P, Steffes MW, Striker GE, Viberti GC. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 306] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Chen X, Xiao W, Li X, He J, Huang X, Tan Y. In vivo evaluation of renal function using diffusion weighted imaging and diffusion tensor imaging in type 2 diabetics with normoalbuminuria versus microalbuminuria. Front Med. 2014;8:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Lu L, Sedor JR, Gulani V, Schelling JR, O’Brien A, Flask CA, MacRae Dell K. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol. 2011;34:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Gilet AG, Kang SK, Kim D, Chandarana H. Advanced renal mass imaging: diffusion and perfusion MRI. Curr Urol Rep. 2012;13:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Desar IM, ter Voert EG, Hambrock T, van Asten JJ, van Spronsen DJ, Mulders PF, Heerschap A, van der Graaf WT, van Laarhoven HW, van Herpen CM. Functional MRI techniques demonstrate early vascular changes in renal cell cancer patients treated with sunitinib: a pilot study. Cancer Imaging. 2012;11:259-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Yu X, Lin M, Ouyang H, Zhou C, Zhang H. Application of ADC measurement in characterization of renal cell carcinomas with different pathological types and grades by 3.0T diffusion-weighted MRI. Eur J Radiol. 2012;81:3061-3066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Feng Q, Ma Z, Wu J, Fang W. DTI for the assessment of disease stage in patients with glomerulonephritis--correlation with renal histology. Eur Radiol. 2015;25:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R. Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol. 2013;23:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Ries M, Jones RA, Basseau F, Moonen CT, Grenier N. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging. 2001;14:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Zheng Z, Shi H, Zhang J, Zhang Y. Renal water molecular diffusion characteristics in healthy native kidneys: assessment with diffusion tensor MR imaging. PLoS One. 2014;9:e113469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Lanzman RS, Ljimani A, Pentang G, Zgoura P, Zenginli H, Kröpil P, Heusch P, Schek J, Miese FR, Blondin D. Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology. 2013;266:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 14. | Hueper K, Gutberlet M, Rodt T, Gwinner W, Lehner F, Wacker F, Galanski M, Hartung D. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol. 2011;21:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Cheung JS, Fan SJ, Chow AM, Zhang J, Man K, Wu EX. Diffusion tensor imaging of renal ischemia reperfusion injury in an experimental model. NMR Biomed. 2010;23:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Hueper K, Hartung D, Gutberlet M, Gueler F, Sann H, Husen B, Wacker F, Reiche D. Magnetic resonance diffusion tensor imaging for evaluation of histopathological changes in a rat model of diabetic nephropathy. Invest Radiol. 2012;47:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11817] [Article Influence: 454.5] [Reference Citation Analysis (0)] |
| 18. | Klee GG, Schryver PG, Saenger AK, Larson TS. Effects of analytic variations in creatinine measurements on the classification of renal disease using estimated glomerular filtration rate (eGFR). Clin Chem Lab Med. 2007;45:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Notohamiprodjo M, Dietrich O, Horger W, Horng A, Helck AD, Herrmann KA, Reiser MF, Glaser C. Diffusion tensor imaging (DTI) of the kidney at 3 tesla-feasibility, protocol evaluation and comparison to 1.5 Tesla. Invest Radiol. 2010;45:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Wang WJ, Pui MH, Guo Y, Wang LQ, Wang HJ, Liu M. 3T magnetic resonance diffusion tensor imaging in chronic kidney disease. Abdom Imaging. 2014;39:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Liu Z, Xu Y, Zhang J, Zhen J, Wang R, Cai S, Yuan X, Liu Q. Chronic kidney disease: pathological and functional assessment with diffusion tensor imaging at 3T MR. Eur Radiol. 2015;25:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Wang YC, Feng Y, Lu CQ, Ju S. Renal fat fraction and diffusion tensor imaging in patients with early-stage diabetic nephropathy. Eur Radiol. 2018;28:3326-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Gürses B, Kiliçkesmez O, Taşdelen N, Firat Z, Gürmen N. Diffusion tensor imaging of the kidney at 3 Tesla MRI: normative values and repeatability of measurements in healthy volunteers. Diagn Interv Radiol. 2011;17:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Atkins RC, Polkinghorne KR, Briganti EM, Shaw JE, Zimmet PZ, Chadban SJ. Prevalence of albuminuria in Australia: the AusDiab Kidney Study. Kidney Int Suppl. 2004;S22-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Razek AAKA, Al-Adlany MAAA, Alhadidy AM, Atwa MA, Abdou NEA. Diffusion tensor imaging of the renal cortex in diabetic patients: correlation with urinary and serum biomarkers. Abdom Radiol (NY). 2017;42:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1551] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 27. | Kido A, Kataoka M, Yamamoto A, Nakamoto Y, Umeoka S, Koyama T, Maetani Y, Isoda H, Tamai K, Morisawa N. Diffusion tensor MRI of the kidney at 3.0 and 1.5 Tesla. Acta Radiol. 2010;51:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 29. | Sigmund EE, Vivier PH, Sui D, Lamparello NA, Tantillo K, Mikheev A, Rusinek H, Babb JS, Storey P, Lee VS. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology. 2012;263:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |