Peer-review started: January 2, 2018
First decision: January 15, 2018
Revised: January 24, 2018
Accepted: February 25, 2018
Article in press: February 25, 2018
Published online: February 28, 2018
Processing time: 56 Days and 17.1 Hours
Hepatocellular carcinoma (HCC) usually develops in the setting of chronic liver disease. In the adequate clinical context, both multiphasic contrast-enhanced CT and magnetic resonance imaging are non-invasive modalities that allow accurate diagnosis and staging of HCC, although the latter demonstrates greater sensitivity and specificity. Imaging criteria for HCC diagnosis rely on hemodynamic features such as hyperenhancement in the arterial phase and washout in the portal or equilibrium phase. However, imaging performance drops considerably for small (< 20 mm) nodules because their tendency to exhibit atypical enhancement patterns. In order to improve accuracy in the diagnosis and staging of HCC, particularly in cases of atypical nodules, ancillary features, i.e., imaging characteristics that modify the likelihood of HCC, have been described and incorporated into clinical reports, especially in Liver Imaging Reporting and Data System. In this paper, ancillary imaging features will be reviewed and illustrated.
Core tip: Hepatocellular carcinoma (HCC) usually develops in the setting of chronic liver disease. Imaging criteria for HCC diagnosis rely on hemodynamic features, however, imaging diagnostic performance drops considerably for small (< 20 mm) nodules because of their tendency to exhibit atypical enhancement patterns. In order to improve accuracy in these non-typical lesions, the use of ancillary features has been incorporated into clinical reports, especially in Liver Imaging Reporting and Data System. In this paper, ancillary imaging features will be reviewed and illustrated.
- Citation: Campos-Correia D, Cruz J, Matos AP, Figueiredo F, Ramalho M. Magnetic resonance imaging ancillary features used in Liver Imaging Reporting and Data System: An illustrative review. World J Radiol 2018; 10(2): 9-23
- URL: https://www.wjgnet.com/1949-8470/full/v10/i2/9.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i2.9
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fifth most common neoplasm in the world. It ranks third among reasons for cancer-related mortality[1,2].
HCC develops in a cirrhotic background in the setting of chronic hepatic inflammation. Approximately 80% of HCC cases are associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections[1,3].
Early stage recognition of HCC make these patients eligible for potentially curative therapies, including surgical resection, liver transplantation or thermoablative treatments[4,5], with 5-year survival rates ranging between 50%-70%[6]. Conversely, advanced HCCs confer a very poor prognosis.
Gadolinium-enhanced MRI has been shown to represent the mainstay of noninvasive evaluation of the cirrhotic liver, with higher sensitivity than CT for the detection of HCC (77%-100% and 68%-91% respectively)[7-8], independent of lesion size.
MR imaging also provides high soft-tissue contrast, improving both lesion detection and characterization. Multiparametric data can be obtained through T1-weighed (T1-WI) and T2-weighted (T2-WI) sequences, functional sequences [diffusion-weighted imaging (DWI)], and contrast uptake. The increasingly frequent integration of hepatobiliary contrast agents in MR imaging protocols is emerging as the foremost method in the diagnosis and staging of HCC.
Imaging criteria for the diagnosis of HCC are based on the hemodynamic features of the nodule (arterial hyperenhancement and venous/delayed washout)[9]. Thus, precise timing of the arterial phase is crucial for optimal HCC detection. Subtle arterial enhancement may be detected using post-processed subtraction arterial images, which are especially useful for nodules with intrinsic high T1 signal intensity[8]. Imaging plays an important role in the differentiation of HCC from nonmalignant cirrhosis-associated nodules, (e.g., regenerative nodules, low-grade dysplastic nodules, high-grade dysplastic nodules) and non-hepatocellular malignancies that may occur in the cirrhotic liver, mainly intrahepatic cholangiocarcinoma (ICC), which account for up to 5% of cancers in cirrhotic patients[7].
Recent recommendations by the American Association for the Study of Liver Diseases (AASLD) state that, among patients at risk, the non-invasive diagnosis of HCC can be confidently made if a nodule larger than 10 mm shows the typical hemodynamic features of HCC on either CT or MRI[10]. The current diagnostic approach has been structured in clinical practice guidelines and includes biopsy of suspicious hepatic lesions that do not meet typical HCC imaging criteria[7]. Nevertheless, biopsy comprises specific drawbacks including high interobserver variability among pathologists when interpreting small hepatic nodules in cirrhotic livers, and possible risks and complications such as needle track seeding and hemorrhage[5].
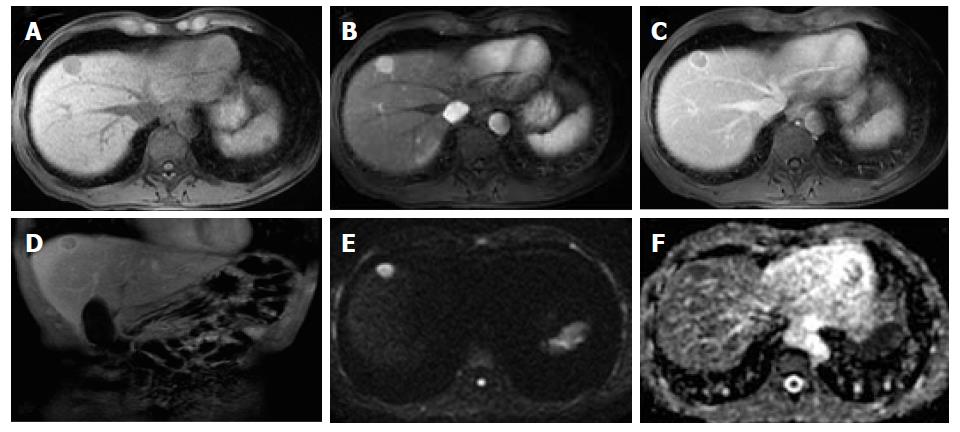
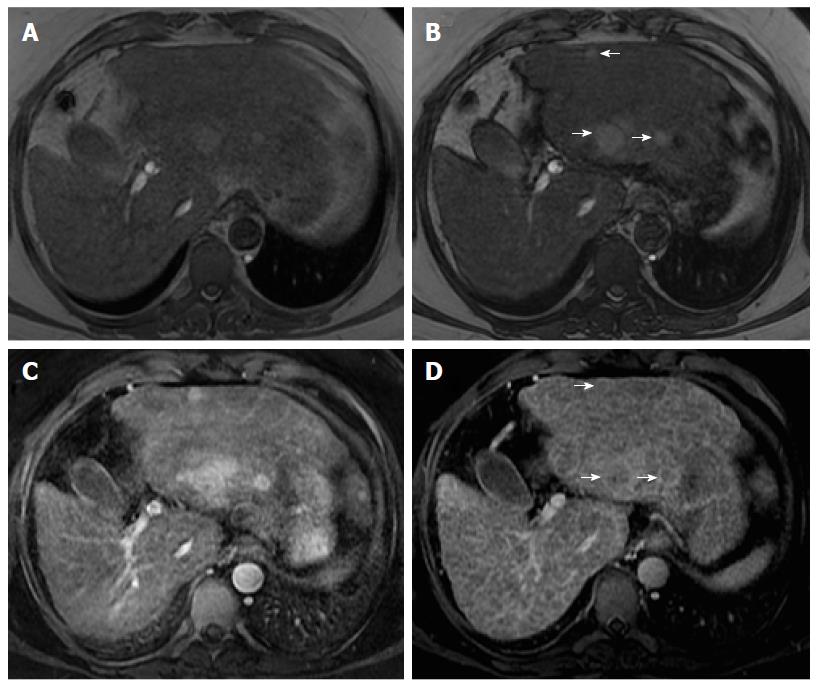
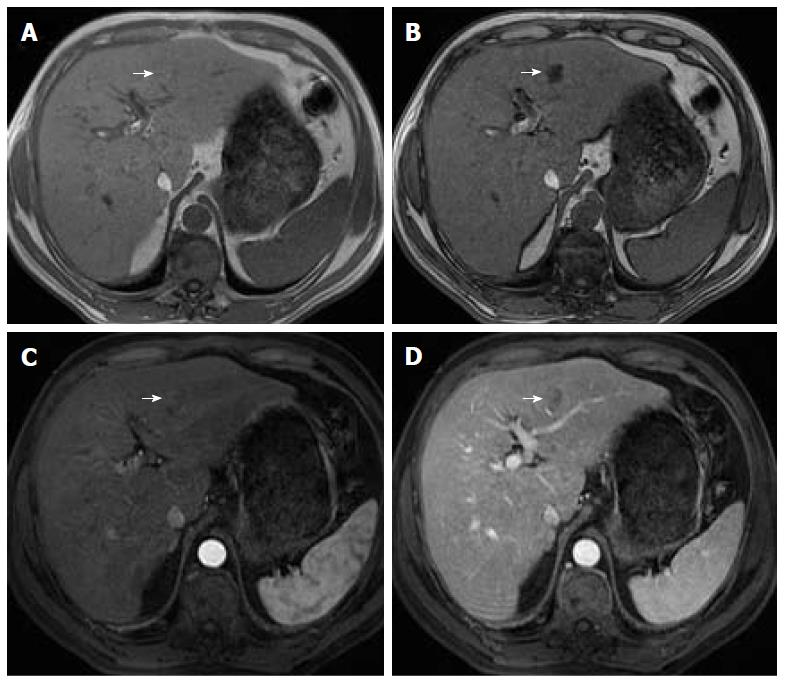
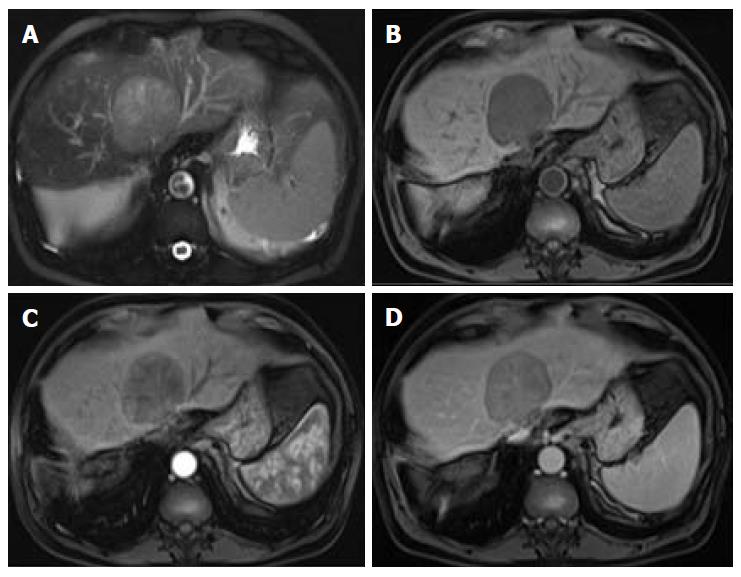
Organizations such as the AASLD, the European Association for the Study of the Liver (EASL) or the Organ Procurement and Transplantation Network (OPTN), have defined rigid and highly specific criteria for the diagnosis of HCC, which are credited to a shift in tumor blood supply from primarily portal venous to primarily arterial branches, recruited during neoangiogenesis. As a result, hyperenhancement relative to the background liver parenchyma is observed in the arterial phase[11], and as blood typically passes through HCCs into the venous outflow more rapidly than normal hepatic parenchyma, a characteristic “washout” appearance (tumor hypoenhancement in comparison to the liver parenchyma in the portal venous or delayed phase) may also be detected[7,11,12] (Figure 1). The combination of arterial hyperenhancement followed by washout in the portal venous/delayed phase has a sensitivity ranging between 64%-89%, specificity of 96% and positive predictive value of 93% for the diagnosis of HCC among patients at risk[12].
The capsule appearance is another important feature considered by some guidelines and attributed to compressed parenchyma, fibrosis, dilated sinusoids/blood vessels around the mass, or a combination of the above characteristics, which are observed in advanced HCCs (Figure 1). A peripheral rim of smooth and progressive enhancement with hyperenhancement in the portal venous or delayed phase can be seen. In at-risk patients, the capsule appearance holds a high positive predictive value for HCC, with specificity reportedly ranging between 83%-96%. However, sensitivity is only moderate, ranging between 43%-55%[13,14]. The capsule appearance should not be mistaken for peripheral enhancement of ICC and fibrous tissue surrounding cirrhotic nodules. Even in the absence of a typical washout appearance, the combination of arterial phase hyperenhancement and capsule appearance strongly suggests the diagnosis of HCC[7,15] (Figure 2). Zhang et al[16] showed that CT against MR produced false-negative findings of pseudo-capsule by 42.9%, with subsequent underestimation of LI-RADS scores by 16.9% for LR-3, 37.3% for LR-4, and 8.5% for LR-5. Corwin et al[17] compared CT and MR in grading LI-RADS and reported that 42% of the findings were upgraded on MRI when compared with CT. Nearly one-third of the upgrades were to categories 4, 5, or 5 V. This was mainly due to the detection of arterial hyperenhancement or a delayed enhancing capsule not previously recognized on CT[17].
The American College of Radiology (ACR) developed LI-RADS with the purpose of improving standardization in the performance and interpretation of CT and MRI for the diagnosis of HCC among high-risk patients[15]. In addition to major imaging features (wash-in, washout and peripheral capsule) LI-RADS, contrary to other classification systems, uses ancillary imaging features to adjust the LI-RADS category. Ancillary imaging features result due to a combination of radiologists’ experience and the necessity to complement a diagnosis in cases where typical dynamic characteristics are lacking. Although they modify the likelihood of HCC, in isolation, they do not allow for the reliable categorization of observations. Ancillary features have been described and included in the LI-RADS v2014 classification system[15]. Some of these features are specific to MRI, and those that are common to both CT and MRI are generally more easily identifiable on MRI. Ancillary features can be divided into those that favor the diagnosis of benignity and those that favor malignancy, the latter being further subdivided into non-specific and specific to HCC[7,15]. The different LI-RADS categories: LR-1 (definitely benign), LR-2 (probably benign), LR-3 (indeterminate probability of HCC), LR-4 (probably HCC), and LR-5 (definitely HCC)[15] are based on the combination of major and/or ancillary imaging features favoring benignity or malignancy. As a rule, ancillary features may be used at the radiologist’s discretion for improved detection, increased confidence, or category adjustment. For category adjustment, one or more ancillary features favoring malignancy may upgrade category up to LR-4, but cannot be used to upgrade a lesion to a LR-5 category[5,15].
LI-RADS, like any other imaging-based diagnostic systems, do possess limitations and problems that need to be addressed. Nevertheless, LI-RADS is being continually refined and updated in response to advances in research and technology. At its current level of development, LI-RADS are mainly used to separate the diagnosis of HCC (LR-5) and non-HCC nodules (LR-1 and 2). Another very important application is reserved for LR-4 and LR-3 categories, specifically to adapt patient management, including adopting a more aggressive approach or undergoing elective biopsy among patients with ancillary findings that favor HCC or malignancy.
In this article, we will review and illustrate each of the different ancillary MRI findings of malignancy by subdividing them into: (1) Non-specific features suggesting malignancy, i.e., those that may be frequently found among other malignancies (Table 1); and (2) HCC-specific features suggesting malignancy, which are mostly found in HCCs (Table 2).
| Feature | Critical details |
| Mild to moderate T2 hyperintensity | Well-differentiated HCCs and small moderately differentiated HCCs can be iso or hypointense in T2-WI |
| Metastasis and ICC can show mild to moderate T2 hyperintensity | |
| Restricted diffusion | Cirrhotic parenchyma may show restricted diffusion (due to abundance of fibrotic tissue) |
| Various HCCs, especially those < 20 mm, tend not to show diffusion restriction | |
| Lesional fat sparing | Applicable in the setting of fatty liver infiltration |
| Must be differentiated from hepatic fat sparing areas | |
| Lesional iron sparing | Applicable in the setting of iron-overloaded liver |
| May also be observed in other malignancies such as ICC and non-malignant processes such as confluent fibrosis | |
| Diameter increase less than threshold growth | Defined by a diameter increase of a lesion by a minimum of 5 mm, ≥ 50% over ≤ 6 mo or ≥ 100% over > 6 mo |
| Corona (peri-lesional) enhancement | May also be found in other hypervascular lesions, such as metastasis |
| Usually not present in early stage HCCs |
| Feature | Critical details |
| Intra-lesional fat | Characteristic of early HCC; Exception: Steatohepatitic variant of HCC, which may exhibit marked intralesional fat even if they represent progressed cancer |
| Nodule-in-nodule architecture | “Subnodule” corresponding to the HCC typically shows enhancement characteristics similar to other HCCs; “Parent” nodule is typically hyperintense on T1-WI, hypointense on T2-WI and hypo- or isoenhancing on the arterial phase (atypical of HCC) |
| Mosaic architecture | Extremely unusual in other tumors; Uncommon in small HCCs; May be helpful for the diagnosis of large hypovascular HCCs |
| Blood products | Associated with HCC expansion; Previous biopsy should be discarded |
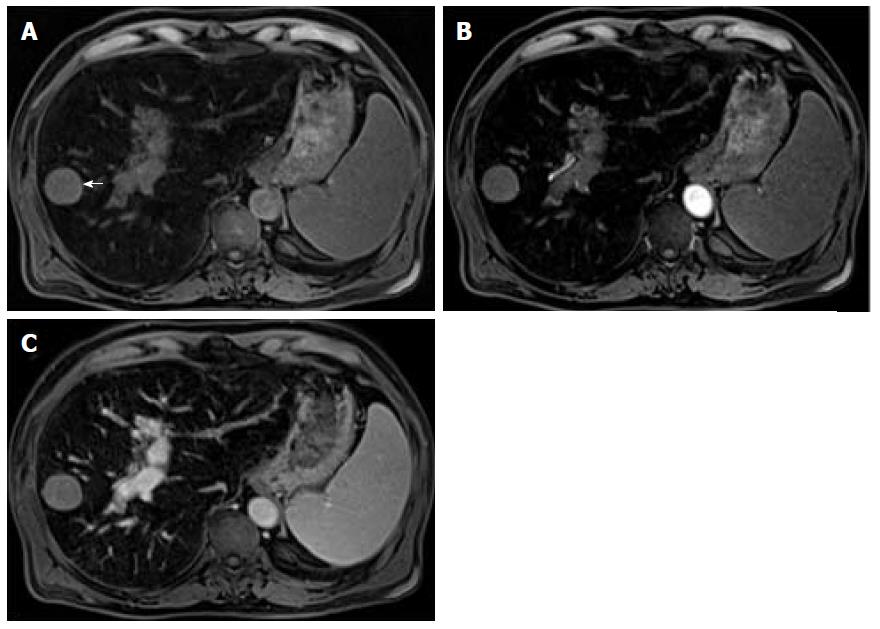
Mild or moderate T2 hyperintensity comparing to the liver is highly suggestive of malignancy (Figure 2)[15]. Cirrhotic and dysplastic nodules are characteristically iso or hypointense on T2-WI, and only very rarely appear mild/moderately hyperintense. This feature can be useful for the differentiation between a dysplastic nodule and a small HCC, especially when the latter presents as a hypervascular nodule without washout (Figure 3). HCCs tend to show minimal to mildly increased signal intensity on T2-WI, with specificity and a positive predictive value ranging between 73%-100% and 72%-100% respectively[14,18,19]. The addition of T2-WI hyperintensity to the AASLD criteria improved the detection rate of HCC, especially in nodules < 20 mm, by increasing sensitivity from 67.6% to 79%[20,21]. Sofue et al[22] evaluated the imaging features in MRI associated with the upgrade to a LI-RADS 5 category and verified that the risk factors in the 56 LR-4 observations, which were upgraded to LR-5, included mild to moderate T2 hyperintensity (P < 0.001) and growth (P < 0.001). Hyperintensity on T2-WI and DWI could also be useful in differentiating hypovascular HCCs from dysplastic nodules, which are perceived as hypointense nodules in the hepatobiliary phase[23].
Mild T2 hyperintensity may also have prognostic value, being found in 77% of HCCs larger than 30 mm[7]. However, many well-differentiated HCCs and some small, moderately differentiated HCCs can be iso or hypointense in T2-WI, and both metastasis and intrahepatic cholangiocarcinomas (ICC) can show mild T2 hyperintensity[7].
DWI has been increasingly used as a cancer-imaging tool in clinical practice. DWI measures the random motion of water molecules inside a voxel of tissue. DWI hyperintensity (relative to liver parenchyma) is expected within an HCC due to its highly cellular tissue (see Figure 1)[3,24]. In contrast, benign nodules usually present a similar microstructure to their surrounding tissues, and therefore free water movement is preserved. As such, DWI may be useful in detecting HCC amongst benign nodules and arterially enhancing pseudolesions, predicting histological grade, and assessing response to treatment, since treatment-induced necrotic and inflammatory tissue result in a loss of cellular density and an increase in membrane permeability with subsequent diminished restriction to diffusion[24].
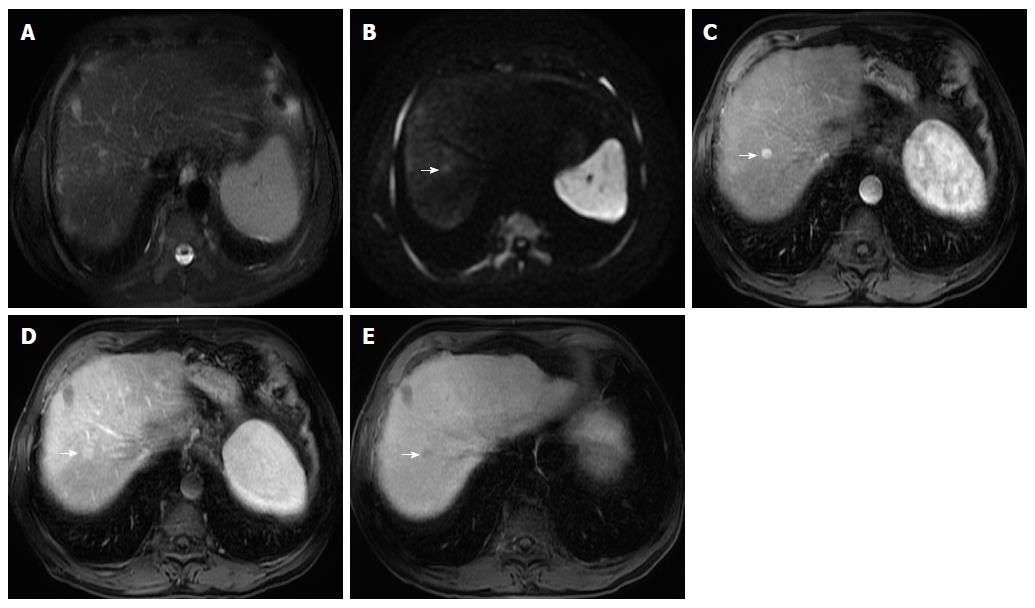
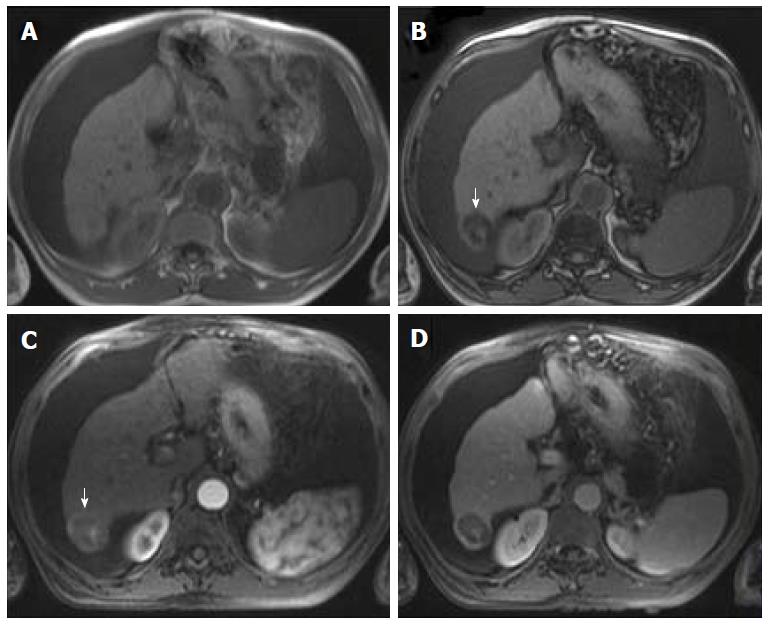
Some studies compared the HCC diagnostic sensitivity and specificity of DWI and contrast-enhanced sequences, reporting lower sensitivity of DWI for lesions < 20 mm and similar results for lesions > 20 mm[25,26]. However, all reports seemed to agree that DWI, as a diagnostic criterion for HCC, improved the sensitivity of conventional MRI, and the greatest benefit lied in the combined use of both (pooled sensitivity and specificity: 93% and 84% combined, respectively)[27] (Figure 4). However, cirrhotic parenchyma may itself show restricted diffusion, presumably owing to the abundance of fibrotic tissue, which results in reduced HCC conspicuity in DWI[18].
The limitation of this feature is its sensitivity, since many HCCs, especially those smaller than 20 mm, do not tend to show restricted diffusion.
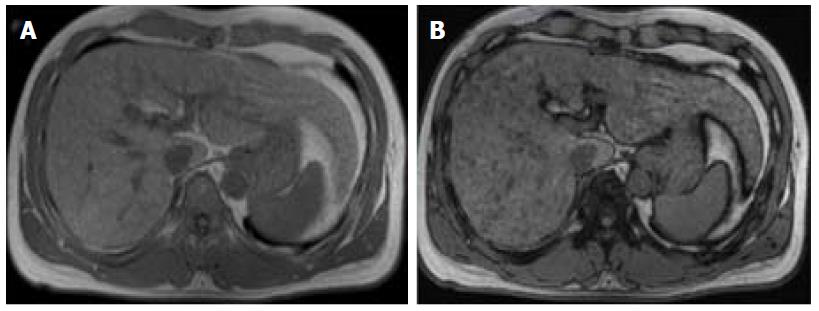
In patients at risk of HCC with fatty liver infiltration, if a suspicious lesion has a lower fractional fat content than that of the background liver, then it is an ancillary feature favoring HCC (Figure 5). However, this only applies in the presence of fatty liver infiltration, and must be differentiated from hepatic fat sparing areas by way of confirmation of the different enhancement pattern from that of background liver, which is better performed using subtraction technique[3,12,15].
As with lesional fat sparing, the absence of iron deposition in a suspicious lesion within an iron-overloaded liver parenchyma is highly suggestive of premalignancy or malignancy, since high-grade dysplastic nodules and HCC cells lose the ability to concentrate iron (Figure 6). This feature applies only to solid masses appearing in iron-overloaded livers that unequivocally have a lower fractional iron content than background liver. Liver iron deposition can be shown as signal loss on the second echo of a dual-echo sequence or as abnormal hypointensity on T2 or T2*-weighted images[8,10,15]. MRI is more sensitive and specific in detecting lesional iron sparing than CT[7]. A limitation of this feature is that it is only relevant to solid nodules found in iron-overloaded livers and not specific to HCC, as it may also be observed in other malignancies, such as ICC, and non-malignant processes such as confluent fibrosis[7].
Hepatobiliary contrast agents (HSA) have been increasingly recognized as an important tool for HCC diagnosis. Hepatocyte-specific MR contrast agents initially distribute in the extracellular fluid compartment, allowing for multiphasic dynamic post-contrast imaging, which is comparable to extracellular agents. They are subsequently taken up by functioning hepatocytes and excreted in bile, allowing for a late hepatobiliary phase (HBP) acquisition[3,28,29]. Due to the action of cellular membrane transporters named Organic Anionic Transporting Polypeptides (OATP) and Multidrug Resistance-associated Proteins (MRPs), only normal functioning hepatocytes take up HSA and excrete them into the biliary tree. In hepatobiliary phase images, both the liver and the bile ducts appear markedly enhanced. Blood vessels, as well as all non-hepatocellular lesions and lesions with impaired hepatocytes appear hypointense[28].
Two HSAs are currently available: Gadoxetic acid disodium (Eovist®/Primovist®; Bayer Healthcare) and gadobenate dimeglumine (MultiHance®; Bracco Diagnostics). Gadoxetic acid is highly liver-specific with approximately 50% of the injected dose taken up by functioning hepatocytes and excreted in bile, allowing for delayed uptake imaging within 20 min from the time of injection, compared to the uptake of 3%-5% for gadobenate dimeglumine, which allows for delayed uptake imaging within 2-3 h[3,28]. Gadoxetic acid is currently widely used due to the rapid acquisition of HBP images (10-20 min after contrast injection) and more intense HBP enhancement[29]. The aforementioned differences in biliary elimination of gadoxetic acid compared to other contrast agents affect lesion appearance, and consequently image interpretation. Despite the advantages of lesion detection and characterization, it may also generate new pitfalls in imaging interpretation, which are related to the less favorable behavior as an extracellular agent, and the “pseudowashout” phenomenon observed in benign lesions. Generally, washout is synonymous with malignant lesions, particularly certain hypervascular metastases and HCC, but when gadoxetic acid is used, the “pseudowashout” phenomenon may, in lesser-experienced hands, lead to misinterpretation of benign lesions as malignant. This agent does not provide a conventional delayed vascular phase, instead it provides a transitional phase that overlaps the portal venous and the hepatobiliary phase (transition from extracellular-dominant to intracellular-dominant enhancement), leading to intense hepatic parenchymal enhancement in late dynamic postcontrast phases. Therefore, hypointensity relative to the liver in the transitional phase may reflect hyperenhancement of the liver rather than lesion washout[8,28].
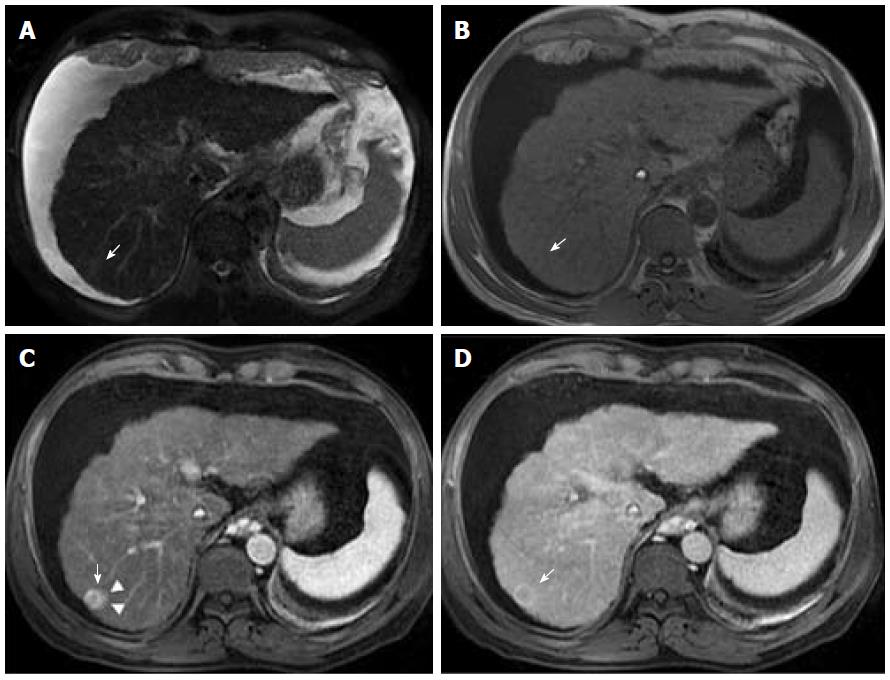
As OATP expression decreases during hepatocarcinogenesis, iso signal intensity (SI) to high SI on HBP images is generally suggestive of benign lesions (RNs or low-grade DNs), whilst low SI on HBP images is a strong predictor of premalignancy or malignancy (high-grade DNs or HCCs)[7,19,29]. When it comes to HBP images, most HCCs, including many early-stage HCCs, usually express low SI in strongly enhanced normal hepatic parenchyma due to the absence of functioning hepatocytes or decreased expression of OATP. One important benefit of the hepatobiliary phase is that it helps to distinguish arterially enhancing pseudolesions (such as arterio-portal shunts) from HCC, and identify early HCCs that are frequently isoenhancing in the vascular phases, and cannot therefore be reliably detected with the help of extracellular agents[7]. Nevertheless, approximately 10%-12% of HCCs show high SI on HBP images. These usually represent well-differentiated HCCs that may retain enough hepatocellular and OATP expression to allow the uptake of hepatobiliary-specific agents[7,19,29].
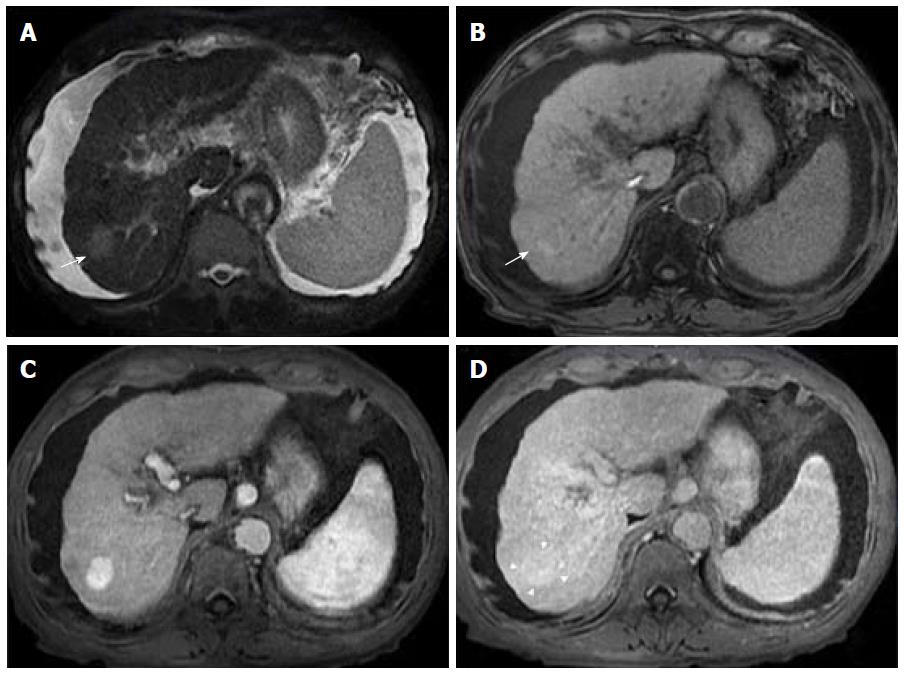
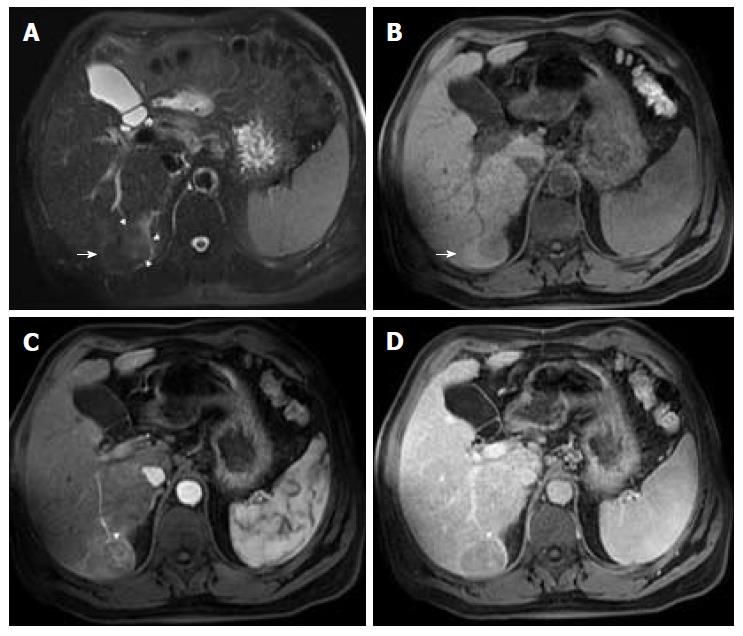
Advanced cirrhosis may also limit the detection of early HCC when using gadoxetic acid-enhanced MRI due to compromised uptake of hepatobiliary-specific agents by damaged hepatocytes, resulting in a decreased distinction between the background liver parenchyma and the lesion (Figure 7)[16].
The combination of routine dynamic and hepatobiliary imaging has been reported to be both sensitive and specific to HCC, with two recent meta-analyses describing a pooled sensitivity of 91% and specificity of 93%[27,30].
Peri-tumoral hypointensity seen on gadoxetic acid-enhanced hepatobiliary phase images was also seen in a retrospective study to be a specific, although insensitive, marker for microvascular invasion; the authors attributed this phenomenon to altered expression of OATP or MRP2 receptors in the hepatic parenchyma caused by hemodynamic changes associated with tumor obstruction of minute portal veins[31].
Therefore, gadoxetic acid-enhanced MRI has a higher sensitivity with regard to the diagnosis of HCC, (especially for lesions ≤ 20 mm), improves the characterization of arterially enhancing lesions that do not exhibit definite washout on subsequent imaging, helps differentiate arterially enhancing pseudolesions from HCC (Figures 4 and 7), and aids in the detection of lesions that are isointense to the background hepatic parenchyma on all other sequences, but has a high risk of transforming into hypervascular HCCs[3,10].
HCCs frequently pose diagnostic dilemmas due to differences in technique between the two subsequent examinations and measurement inequalities, hence rendering an increase in diameter, which is often difficult to ascertain.
Using the LI-RADS guidelines, the threshold growth is defined by a diameter increase of a lesion by a minimum of 5 mm, of ≥ 50% over ≤ 6 mo, or ≥ 100% over > 6 mo[15]. A new lesion measuring ≥ 10 mm also represents threshold growth, regardless of the time interval. These are major features of HCC. An increase in diameter less than the threshold growth is an ancillary feature that favors HCC. However, LI-RADS do not stipulate a minimum increase in diameter for its use as an ancillary feature. A note should be made that when measuring lesion diameter, the largest dimension should be used, i.e., outer edge to outer edge- and it should be measured in the sequence, phase, and imaging plane in which the margins are most sharply defined, and in which there is no (or less) anatomic distortion (preferably not in the arterial phase, due to the possible presence of peri-lesional enhancement).
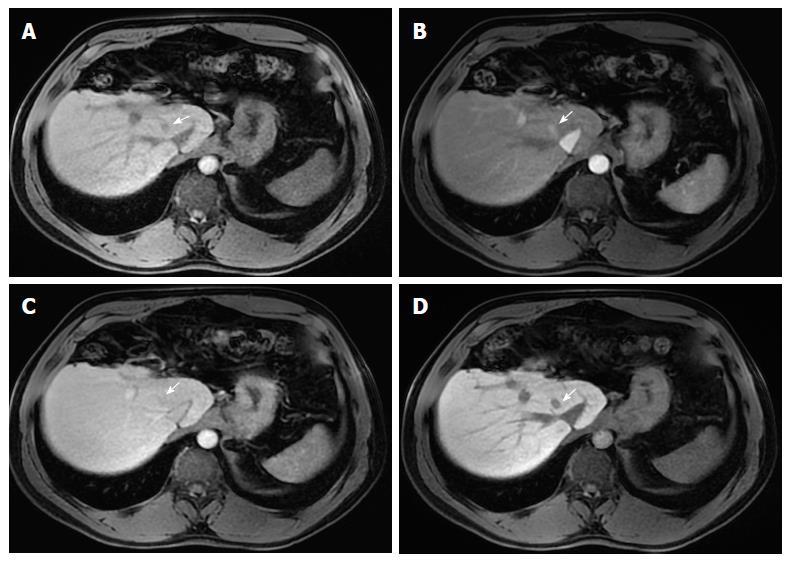
Corona enhancement is defined as a zone (or rim) of peri-lesional enhancing parenchyma seen in hypervascular and progressed HCC in the late arterial or early portal venous phase, which fades to isoenhancement in the subsequent phases (Figure 8)[15]. It represents the rapid drainage of contrast material from the arterially hyperenhancing lesion to the peri-tumoral parenchyma, carried by the tumor draining vessels, just a few seconds after the tumor itself begins to enhance[7,15].
This feature is not specific to HCC, since it may also be found in other hypervascular lesions, such as metastasis. However, its presence may be useful in distinguishing between small HCCs and vascular pseudolesions (e.g., arterio-portal shunts).
Corona enhancement may also have some prognostic value as it is not usually present in early stage HCCs, which have hepatic vein drainage, but in less differentiated lesions. In addition, some studies have suggested that large, irregular or distorted corona enhancement predicts microvascular invasion[32,33]. Metastatic satellite nodules and local recurrences after resection or ablation also frequently develop corona-like enhancement areas[34].
In lesions with diffuse arterial phase hyperenhancement, corona enhancement and capsule appearance may overlap in imaging. If rim enhancement occurs in the arterial phase or portal venous phase and subsequently fades in later phases, it is suggestive of corona enhancement. If rim enhancement increases in portal venous phase and delayed phase, it suggests capsule appearance[15].
Intra-lesional fat indicates the presence of fat in higher concentration within a lesion than background liver tissue. Fatty metaplasia is frequently histologically observed in early HCCs, and its detection in imaging favors this diagnosis (Figure 9). It has, however, limited sensitivity, ranging from 12%-37% in reported series[14,35]. Furthermore, its specificity is only moderate, ranging from 68%-91%[14,35], so it cannot be used to establish the diagnosis of HCC, since high-grade and occasionally low-grade dysplastic nodules also show intralesional fat[10,12]. Nevertheless, in a high-risk patient, the identification of intralesional fat in a suspicious nodule raises concerns about malignancy or premalignancy and should prompt, at least, a closer follow-up[10,12,15].
The evaluation of intralesional microscopic and macroscopic fat can be achieved using T1 weighted “in and out of phase” chemical shift imaging and fat-suppressed images respectively (Figure 10). Whether the lesion has a higher fractional fat content than the liver or its enhancement is different from that of background liver parenchyma must be confirmed by the presence of hepatic steatosis[10,12,15].
As it is characteristic of early but not progressed HCC, intralesional fat may also have prognostic value. It generally denotes a longer time for progression and a lesser risk of developing metastases than non–fat-containing HCCs[12].
The steatohepatitic variant of HCC, a newly described variant, however, is an exception. Strongly associated with underlying steatohepatitis and metabolic syndrome, steatohepatitic HCCs may exhibit marked intralesional fat even if they represent progressed cancers with an advanced tumoral grade[12,36].
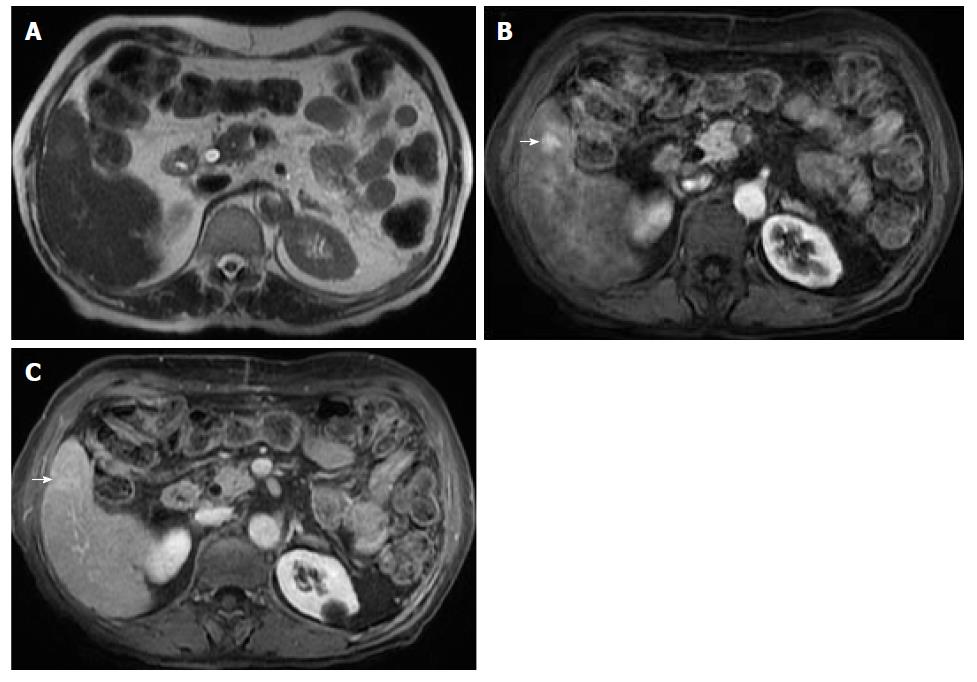
Remarkably, in a cirrhotic liver, the presence of intralesional fat in a single nodule is characteristic of early HCC, especially if larger than 15 mm (Figure 9). On the other hand, it is an unspecific finding, since low and high-grade dysplastic nodules may also contain fat. In addition, the presence of numerous fat-containing nodules measuring < 10 mm is suggestive of a benign nature[37] (Figure 11).
This finding represents the development of a progressed HCC within a dysplastic nodule (Figure 12). The “subnodule” corresponding to the HCC typically exhibits enhancement characteristics similar to other HCCs. The surrounding “parent” nodule, corresponding to more well-differentiated tissue, is typically hyperintense in T1-WI, hypointense in T2-WI and arterial phase hypo- or isoenhancing, which is atypical of HCC.
Fat or iron deposition can also occur in the parent nodule, and less frequently in the subnodule, reflecting the inability of neoplastic hepatocytes to concentrate iron and fat[12,15]. To our knowledge, its sensitivity to HCC diagnosis has not yet been defined, but it constitutes an uncommon finding in CT or MRI, and its value is therefore unclear.
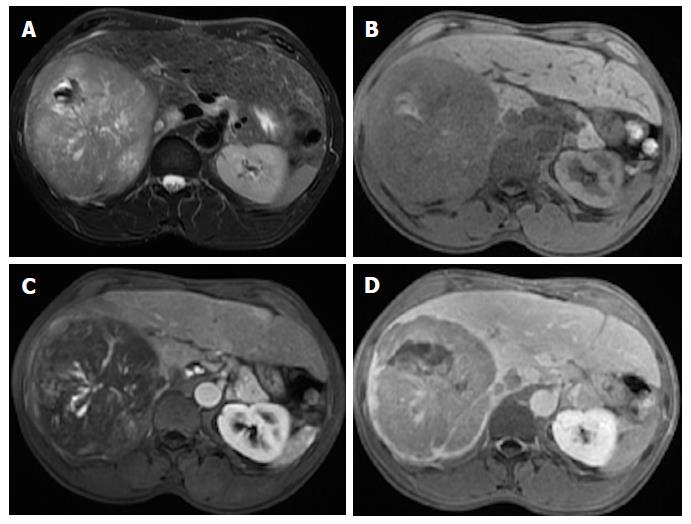
Mosaic architecture refers to the presence of randomly distributed internal nodules, or compartments, differing in enhancement, intensity, shape and size and often separated by fibrous septations[12,15]. Among high-risk patients, most lesions with mosaic architecture represent HCCs; this feature being more characteristic of large HCCs. Although heterogeneity is a common characteristic of many liver lesions, mosaic architecture is extremely unusual in other tumors[7].
Care must be taken, however, to differentiate mosaic architecture from intralesional necrosis, which manifests as hyperintensity on T2-WI[38]. In addition, the incremental value of this feature relating to HCC diagnosis may be modest because it is uncommon in small HCCs. Some authors have studied the accuracy of this finding and have reported a sensitivity of up to 63% (in studies with limited population), and more recently of up to 31% using conventional MR sequences[39,40,41]. Nonetheless, this feature may be helpful for the diagnosis of large hypovascular HCCs in which arterial hyperenhancement is often more difficult to appreciate (Figure 13) and is also being recognized for its prognostic value[38].
The presence of intralesional or peri-lesional hemorrhage in the absence of previous biopsy, intervention or trauma is also an ancillary feature favoring HCC, as it is associated with HCC expansion[41]. In MRI, blood products usually manifest as areas with predominantly high signal intensity on T1-WI and heterogeneous, and predominantly low signal intensity on T2-WI. Due to T2* shortening, there may be signal loss on the second echo of a dual-echo gradient-echo sequence[15] (Figure 14). Chen et al[40] reported a sensitivity of 64% for intralesional hemorrhage in HCC diagnosis.
Gadolinium-enhanced MR imaging has become the non-invasive method of choice for HCC diagnosis among patients at risk. Arterial wash-in and portal or delayed-phase washout allows for a precise diagnosis, discarding the need for histologic confirmation. However, the diagnosis may be challenging in the absence of typical dynamic features. This is even more important when early diagnosis is critical in the management of HCC patients. In this setting, ancillary imaging features may provide additional information, which can be used for further lesional characterization, and hence increase diagnostic sensitivity. These include non-specific features suggesting malignancy (mild-moderate T2 hyperintensity, restricted diffusion, lesional fat sparing, lesional iron sparing, a diameter increase less than threshold growth and corona enhancement) and HCC-specific features suggesting malignancy (intra-lesional fat, nodule-in-nodule architecture, mosaic architecture and the presence of blood products). Recognition of these features is extremely important for practicing radiologists.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Guan YS, Qin JM S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 514] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Ramalho M, Matos AP, AlObaidy M, Velloni F, Altun E, Semelka RC. Magnetic resonance imaging of the cirrhotic liver: diagnosis of hepatocellular carcinoma and evaluation of response to treatment - Part 1. Radiol Bras. 2017;50:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 5. | Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 8. | Watanabe A, Ramalho M, AlObaidy M, Kim HJ, Velloni FG, Semelka RC. Magnetic resonance imaging of the cirrhotic liver: An update. World J Hepatol. 2015;7:468-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6574] [Article Influence: 469.6] [Reference Citation Analysis (1)] |
| 11. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Niendorf E, Spilseth B, Wang X, Taylor A. Contrast Enhanced MRI in the Diagnosis of HCC. Diagnostics (Basel). 2015;5:383-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Khan AS, Hussain HK, Johnson TD, Weadock WJ, Pelletier SJ, Marrero JA. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J Magn Reson Imaging. 2010;32:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Rimola J, Forner A, Tremosini S, Reig M, Vilana R, Bianchi L, Rodríguez-Lope C, Solé M, Ayuso C, Bruix J. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol. 2012;56:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | An C, Rakhmonova G, Choi JY, Kim MJ. Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm. Clin Mol Hepatol. 2016;22:296-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Zhang YD, Zhu FP, Xu X, Wang Q, Wu CJ, Liu XS, Shi HB. Liver Imaging Reporting and Data System:: Substantial Discordance Between CT and MR for Imaging Classification of Hepatic Nodules. Acad Radiol. 2016;23:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR. Differences in Liver Imaging and Reporting Data System Categorization Between MRI and CT. AJR Am J Roentgenol. 2016;206:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Rhee H, Kim MJ, Park YN, Choi JS, Kim KS. Gadoxetic acid-enhanced MRI findings of early hepatocellular carcinoma as defined by new histologic criteria. J Magn Reson Imaging. 2012;35:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Ouedraogo W, Tran-Van Nhieu J, Baranes L, Lin SJ, Decaens T, Laurent A, Djabbari M, Pigneur F, Duvoux C, Kobeiter H. Evaluation of noninvasive diagnostic criteria for hepatocellular carcinoma on pretransplant MRI (2010): correlation between MR imaging features and histological features on liver specimen. J Radiol. 2011;92:688-700. [PubMed] |
| 21. | Golfieri R, Grazioli L, Orlando E, Dormi A, Lucidi V, Corcioni B, Dettori E, Romanini L, Renzulli M. Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging. 2012;36:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Sofue K, Burke LMB, Nilmini V, Alagiyawanna M, Muir AJ, Choudhury KR, Jaffe TA, Semelka RC, Bashir MR. Liver imaging reporting and data system category 4 observations in MRI: Risk factors predicting upgrade to category 5. J Magn Reson Imaging. 2017;46:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, Lee WJ. Nonhypervascular Hypointense Nodules at Gadoxetic Acid-enhanced MR Imaging in Chronic Liver Disease: Diffusion-weighted Imaging for Characterization. Radiology. 2015;276:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Lim KS. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clin Radiol. 2014;69:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Quaia E, De Paoli L, Pizzolato R, Angileri R, Pantano E, Degrassi F, Ukmar M, Cova MA. Predictors of dysplastic nodule diagnosis in patients with liver cirrhosis on unenhanced and gadobenate dimeglumine-enhanced MRI with dynamic and hepatobiliary phase. AJR Am J Roentgenol. 2013;200:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Wu LM, Xu JR, Lu Q, Hua J, Chen J, Hu J. A pooled analysis of diffusion-weighted imaging in the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Gastroenterol Hepatol. 2013;28:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Liu X, Zou L, Liu F, Zhou Y, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e70896. [PubMed] |
| 28. | Matos AP, Velloni F, Ramalho M, AlObaidy M, Rajapaksha A, Semelka RC. Focal liver lesions: Practical magnetic resonance imaging approach. World J Hepatol. 2015;7:1987-2008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 29. | Park YS, Lee CH, Kim JW, Shin S, Park CM. Differentiation of hepatocellular carcinoma from its various mimickers in liver magnetic resonance imaging: What are the tips when using hepatocyte-specific agents? World J Gastroenterol. 2016;22:284-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 30. | Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhu J, Zhang W, Hu J. Is liver-specific gadoxetic acid-enhanced magnetic resonance imaging a reliable tool for detection of hepatocellular carcinoma in patients with chronic liver disease? Dig Dis Sci. 2013;58:3313-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 32. | Nishie A, Yoshimitsu K, Asayama Y, Irie H, Tajima T, Hirakawa M, Ishigami K, Nakayama T, Kakihara D, Nishihara Y. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol. 2008;190:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Chou CT, Chen RC, Lee CW, Ko CJ, Wu HK, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol. 2012;85:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Sakon M, Nagano H, Nakamori S, Dono K, Umeshita K, Murakami T, Nakamura H, Monden M. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94-99. [PubMed] |
| 35. | Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, Park SH, Yazdi L, Sherman M, Khalili K. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Yu JS, Chung JJ, Kim JH, Kim KW. Fat-containing nodules in the cirrhotic liver: chemical shift MRI features and clinical implications. AJR Am J Roentgenol. 2007;188:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Li M, Xin Y, Fu S, Liu Z, Li Y, Hu B, Chen S, Liang C, Lu L. Corona Enhancement and Mosaic Architecture for Prognosis and Selection Between of Liver Resection Versus Transcatheter Arterial Chemoembolization in Single Hepatocellular Carcinomas >5 cm Without Extrahepatic Metastases: An Imaging-Based Retrospective Study. Medicine (Baltimore). 2016;95:e2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Honda H, Onitsuka H, Murakami J, Kaneko K, Murayama S, Adachi E, Kanematsu T, Sugimachi K, Masuda K. Characteristic findings of hepatocellular carcinoma: an evaluation with comparative study of US, CT, and MRI. Gastrointest Radiol. 1992;17:245-249. [PubMed] |
| 40. | Stevens WR, Gulino SP, Batts KP, Stephens DH, Johnson CD. Mosaic pattern of hepatocellular carcinoma: histologic basis for a characteristic CT appearance. J Comput Assist Tomogr. 1996;20:337-342. [PubMed] |
| 41. | Chen W, DelProposto Z, Liu W, Kassir M, Wang Z, Zhao J, Xie B, Wen Y, Wang J, Hu J. Susceptibility-weighted imaging for the noncontrast evaluation of hepatocellular carcinoma: a prospective study with histopathologic correlation. PLoS One. 2014;9:e98303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |