Published online Mar 26, 2017. doi: 10.4330/wjc.v9.i3.230
Peer-review started: November 2, 2016
First decision: December 1, 2016
Revised: December 14, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 26, 2017
Processing time: 150 Days and 15.7 Hours
Pulmonary vein isolation by point-by-point radiofrequency catheter ablation constitutes the cornerstone of catheter ablation strategies for the treatment of atrial fibrillation. However, despite advances in pulmonary vein isolation ablation strategies, long-term success rates after ablation remain suboptimal, which highlights the need to develop techniques to achieve more durable lesions. Strategies proposed to improve the durability of pulmonary vein isolation can be divided into two groups: Those addressed to improving the quality of the lesion and those that optimize the detection of acute PV reconnection during the ablation procedure. This manuscript reviews the role and potential benefits of these techniques according to current clinical evidence.
Core tip: Results of pulmonary vein isolation remains suboptimal in terms of long-term outcomes. Improving lesion durability could reduce atrial fibrillation recurrence rate after pulmonary vein isolation. This manuscript reviews current techniques proposed in order to achieve more durable pulmonary vein isolation by point-by-point radiofrequency ablation. The role and potential benefits of these techniques are discussed according to current clinical evidence. Furthermore a stepwise approach to achieve permanent pulmonary vein isolation is proposed.
- Citation: Pedrote A, Acosta J, Jáuregui-Garrido B, Frutos-López M, Arana-Rueda E. Paroxysmal atrial fibrillation ablation: Achieving permanent pulmonary vein isolation by point-by-point radiofrequency lesions. World J Cardiol 2017; 9(3): 230-240
- URL: https://www.wjgnet.com/1949-8462/full/v9/i3/230.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i3.230
Atrial fibrillation (AF) is one of the major causes of stroke, heart failure, and cardiovascular morbidity worldwide[1]. Since Haïssaguerre et al[2] identified the pulmonary veins (PVs) as triggers capable of initiating AF paroxysms, radiofrequency (RF) catheter ablation through pulmonary vein isolation (PVI) has been developed and now constitutes the cornerstone of catheter ablation strategies for the treatment of AF[3]. Current indications for PVI include symptomatic paroxysmal or persistent AF, in general as second-line treatment after failure of or intolerance to antiarrhythmic drug therapy, but also as first-line therapy in selected cases[4].
According to the most recent consensus statement on catheter ablation of AF[3], the technique for achieving PVI should target a wide area around the PVs, called the antrum, with complete electrical isolation as the endpoint of the procedure. However, despite advances in PVI ablation strategies, long-term success rates after ablation remain suboptimal, which has led to the development of new techniques to achieve more durable lesions.
In the majority of patients with AF recurrence, an electrical reconnection between the PV and LA can be observed[5-7]. The probability of AF recurrence during follow-up after a PVI procedure has been linked with the presence of gaps, defined as poor isolation areas between the PV and LA, due to suboptimal RF lesions[8]. A recent meta-analysis of 11 studies[9] including 683 patients showed that 85.5% of patients with AF recurrence had at least one PV reconnected, opposed to 58.6% of those without AF recurrence. Although not fully established, it has been suggested that the biological mechanism underlying PV reconnection may be related to the recovery of tissue conduction after a transient phase of reversible tissue injury with inflammation and edema[10]. Therefore, achievement of permanent PV isolation should be considered the main goal of current AF ablation approaches in order to avoid recurrences.
The reasons for long-term failure of AF ablation are largely based on a suboptimal ability to effectuate a durable transmural lesion using the contemporary ablation toolset. While electrical PVI may be achieved acutely, the combination of inadequate electrode-tissue contact, insufficient power delivery, and tissue edema may prevent RF-induced heating of myocardium to lethal temperatures. With time, as the acute effects of RF energy resolve, the transient injury induced at the time of index ablation recovers, revealing gaps in the initial line of ablation and allowing PV triggers to excite the adjacent LA and induce AF[6,10]. Several techniques to improve the durability of PVI have been proposed, and can be divided into two groups: Those addressed to improving the quality of the lesion and those that optimize the detection of acute PV reconnection during the ablation procedure.
The use of irrigated catheters for PVI was associated with a dramatic decrease in PV reconnection rate[11]. However, even when irrigated catheters are used, the recurrence rate after a single PVI procedure remains high (30%-35%)[12]. Further strategies are required in order to improve long-term durability of the lesions obtained with this type of catheters.
Efficient catheter contact can be facilitated through the use of non-steerable and steerable sheaths that allow easy maneuverability, access, and contact to target sites. Piorkowski et al[13] compared the use of steerable sheaths with the use of non-steerable sheaths during AF ablations in a prospective randomized trial. Although the rate of acute PVI and total RF application time did not differ between the study groups, single procedure success was significantly higher in patients treated with a steerable sheath (76% vs 53% at 6 mo). The difference persisted at 12 mo (75.7% success) after a single AF catheter ablation procedure using steerable sheath[14]. Therefore, use of a steerable sheath may help to improve the maneuverability of the ablation catheter, catheter stability, and tissue contact. This could potentially reduce recurrence through the enhancement of lesion continuity and transmurality.
In a multicenter trial, Di Biase et al[15] randomized 257 consecutive patients undergoing a first AF ablation procedure to general anesthesia or conscious sedation. During follow-up (mean 17 ± 8 mo), fewer patients randomized to conscious sedation were free of atrial arrhythmias while off antiarrhythmic drugs (69% vs 88% of patients randomized to general anesthesia). In their study, all patients with recurrence had a second procedure. Interestingly, 42% of PVs in the conscious sedation arm at the repeat procedure had recovered PV conduction, compared with 19% in the general anesthesia group[15]. Better and more stable tissue-catheter contact due to controlled breathing patterns and elimination of patient movements may explain this finding.
Contact force (CF) sensing is a novel technology used to assess the degree of catheter-tissue contact through a sensor at the distal tip of the ablation catheter. Studies based on animal models have shown that catheter-tissue CF is directly correlated with lesion size, and that excessive CF (> 50 g) could even provoke steam pops[16,17]. The concept of force-time integral (FTI) has also been proposed as a major factor in RF lesion size[18]. Shah et al[18] calculated the FTI by measuring the area under the CF curve beyond 60 s and found a linear correlation with lesion size during RF ablation. Despite similar power and peak CF values, lesions were larger with constant contact and smaller with intermittent contact.
Several studies have assessed the relationship between CF and lesion transmurality by means of electrogram analysis, cardiac imaging, and histopathology. Squara et al assessed the CF and FTI needed to create effective transmural lesions during AF ablation by analyzing bipolar electrograms before, during, and after RF application. Based on post-ablation changes in electrogram characteristics, they identified a cutoff FTI of > 392 gs to predict transmurality with 89% sensitivity and 93% specificity[19]. Two cardiac MRI studies have demonstrated a direct correlation of CF and FTI with lesion transmurality. In the first study, Sohns et al[20] performed contrast-enhanced cardiac MRI in patients treated with AF ablation using CF catheters. They found a correlation between regions where higher FTI (> 1200 g) was maintained during ablation and those showing increased late gadolinium enhancement on MRI at 3 mo after ablation[20]. In the second study, Andreu et al performed cardiac MRI at 3 mo after PVI ablation to assess CF thresholds required to create permanent lesions using a dragging catheter (as opposed to a point-by-point lesion delivery) technique[21]. They reported that PV segments where MRI gaps were seen had lower maximal CF values, compared to segments without gaps, and a CF threshold of > 12 g predicted the formation of a complete PV lesion with 94% specificity and 91% positive predictive value.
Results from a recent study question the correlation between CF and chronic lesion formation. Williams et al[22] placed linear intercaval right atrial lesions in eight pigs using high (> 20 g) or low (< 10 g) CF, intentionally leaving a gap between segments. Voltage maps and cardiac MRI were performed before, immediately after, and 2 mo after ablation. The authors found that tissue edema was greater in the acute post-ablation setting with high CF, but there was no difference in chronic lesion size or volume by voltage mapping or cardiac MRI between high vs low CF regions at 2 mo. Their results suggest that a transmural lesion can be created whenever continuous tissue-catheter contact is achieved (independently of the CF value) and adequate power is delivered with a stable catheter position throughout the lesion.
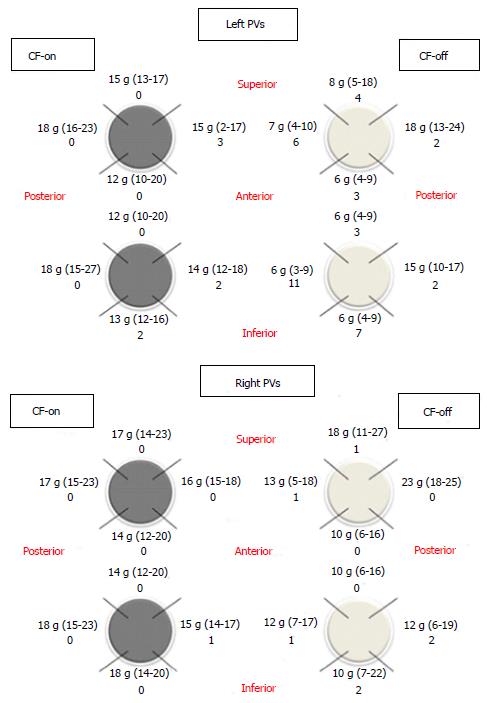
Obtaining adequate CF can be difficult in certain portions of the LA, and certain LA regions may require less CF to achieve transmurality with RF ablation. This may explain the observation that PV reconnection tends to recur at specific regions in the LA. For example, Schluermann et al[23] reported lower CF obtained in left PVs than in right PVs and found the lowest values in the anterior segments, where the ridge between the left upper PV and the LA appendage represents anespecially challenging region for obtaining appropriate CF. Consistently with these data, our group observed that when operators were blinded to CF, the lowest CF values were recorded at the anterior segments of left PVs[24] (Figure 1).
On the other hand, given the differences in LA wall thickness, the amount of CF needed to achieve transmural lesions may vary in different portions of the LA. Sotomi et al[25] showed that higher CF may be necessary in certain regions such as the inferior right PV and posterior-superior right PV regions (22 g CF), while other areas such as the posterior-inferior right PV region may require only 10 g CF to assure acute PVI. Knowledge of CF requirements in various regions of the LA can improve safety during ablation by allowing the operator to control RF power based on CF to prevent steam pops without compromising lesion durability.
Several studies (Table 1) have assessed the role of CF technology in short and long-term ablation outcomes.
| Study | n | Type of study | CF catheter | Control catheter | Follow-up (mo) | Findings |
| Andrade et al[55], 2014 | 75 | Prospective observational | Thermocool SmarTouch | Navistar Thermocool | 13.3 | CF reduced dormant conduction (16% vs 52%) and improved long-term arrhythmia-free survival (88% vs 66%) |
| Kimura et al[31], 2014 | 38 | Randomized controlled trial | Thermocool SmarTouch | Thermocool SmarTouch (blinded operador) | 6 | CF reduced procedure time and additional touch-up ablation |
| Marijon et al[61], 2014 | 60 | Prospective observational | Thermocool SmarTouch | EZ Steer Thermocool | 12 | CF reduced AF recurrence at 12 mo (10.5% vs 35.9%) |
| Shurrab et al[33], 2015 | 42 | Observational | Thermocool SmarTouch | Navistar Thermocool | 2.5 | CF reduced reconnection rate at 30 min postablation |
| TOCCASTAR, 2015 | 300 | Randomized controlled trial | Tacticath | Thermocool Navistar | 12 | No differences in arrhythmia-free survival |
| Pedrote et al[24], 2016 | 50 | Randomized controlled trial | Thermocool SmarTouch | Thermocool SmarTouch (blinded operador) | 12 | CF reduced PV gaps (20% vs 68%). No benefits in arrhythmia-free survival |
| Ullah et al[34], 2016 | 117 | Randomized controlled trial | Thermocool SmarTouch | Thermocool SmarTouch (blinded operador) | 12 | CF reduced acute reconnections (22% vs 32%). No benefits in arrhythmia-free survival |
The TOCCATA study was the first multicenter, prospective study to demonstrate the safety of CF-sensing catheters (Tacticath, Endosense) for ablation of cardiac arrhythmias[26]. The study included 34 patients undergoing PVI for paroxysmal AF and showed that low CF was associated with higher rates of AF recurrence[26]. Specifically, all patients treated with a CF < 10 g experienced AF recurrences, whereas 80% of the patients treated with an average CF > 20 g remained free from AF recurrence at 12 mo[26].
In order to demonstrate the correlation between CF parameters during initial procedure and PV reconnection, the EFFICAS-I study of PVI using CF-sensing catheters assessed the incidence of isolation gaps at 3-mo follow-up (Tacticath, Endosense)[27]. Interestingly, operators were blinded to CF information during the initial procedure. Isolation gap sites correlated with lower minimum CF and FTI during the initial ablation, and the authors proposed an optimal CF target of 20 g with minimum FTI of 400 gs. These cut-off values were prospectively tested in the EFFICAS-II study, which showed that 85% of PVs treated within the proposed CF guidelines were chronically isolated, suggesting a more durable PVI[28].
The SMART-AF trial, a prospective, multicenter, non-randomized single-arm study, examined the efficacy and safety of AF ablation using a SmartTouch CF-sensing catheter[29]. Only 2.5% of the 172 patients included had severe complications, suggesting that safety was not inferior to non-CF-sensing catheters. On the other hand, CF-sensing ablation that remained within target range > 80% of the time resulted in superior 1-year ablation success (81% of patients free from AF recurrence vs 66%, P = 0.005)[29].
The TOCCASTAR study was a prospective, multicenter, randomized clinical trial that compared AF ablation with CF (TactiCath) vs non-CF (ThermoCool Navistar) catheters in 300 patients[30]. Achieving optimal CF resulted in higher rates of acute PVI and no differences were observed in long-term success (freedom from AF or atrial tachycardia recurrence at 12 mo, excluding 3-mo blanking period).
Kimura et al[31] compared acute bidirectional block after PVI in 38 patients randomized to non-CF guided vs CF-guided (target CF 10-20 g) ablation using Thermocool Smart Touch catheter. This study showed that CF-guided PVI reduces procedure time and the need for additional touch-up ablation. Furthermore, a nonsignificant trend towards lower AF recurrence rate at 6 mo post-PVI was observed in the CF-guided group.
Two large meta-analyses have compared AF ablation with CF vs non-CF catheters. Afzal et al[32] examined data on 1148 patients in 9 studies and found that the use of CF-sensing technology reduced AF recurrence 37% overall at a median 12 mo of follow-up. Those treated with CF catheters also had reduced RF ablation duration, although no significant difference was seen in total procedure length or fluoroscopic exposure, compared to non-CF catheters. Shurrab et al[33] subsequently published another meta-analysis, which included 1428 patients from 11 studies (an overlap of 6 studies from the previous meta-analysis). They found a similar 38% overall reduction in AF recurrence at long-term follow-up. However, in addition to reduced RF ablation time, overall procedure length and fluoroscopic exposure duration were significantly lower in patients treated with CF technology. This meta-analysis also demonstrated a non-significant trend toward lower complication rates in the CF group.
It should be noted that the studies mentioned above assessed the impact of CF parameters in PVI performed with a circular catheter inside the PV. The use of circular catheters allows continuous recording of the electrical signal inside the PV, which can condition the endpoint of the procedure and prevents “naïve” assessment of the potential benefit of CF monitoring. In order to test the benefits of CF monitoring in PVI with an exclusively anatomic approach (blinded to the PV catheter), our group conducted a randomized, controlled study in which 50 patients with paroxysmal atrial fibrillation were randomized into CF-on (CF > 10 g) or CF-off (CF blinded; n = 25) groups. In the CF-on group, there was a reduction in the PV gaps at the expense of the left PVs and shortening of the procedure and radioscopy times. This confirms the benefits of operator monitoring and control of a mean CF > 10 g during PVI[24]. However, at 12 mo the AF recurrence rate was similar in both groups[24]. Consistent with these data, a larger study by Ullah et al[34] using the same methodology showed that access to CF data during the procedure was associated with reduced acute PV reconnection, although no benefit was observed in terms of 1-year success rate. These results suggest that CF monitoring during PVI may not impact long-term clinical outcome because it is only one of multiple factors that determine lesion durability.
As has been explained, previously described endpoints (CF and FTI) do not take the power used during RF application into account. In order to resolve this limitation, the ablation index has been proposed as a marker of ablation lesion quality that incorporates CF, ablation time, and RF power in a weighted formula (the greater the impact of power over CF, the greater the impact on the initial phase of ablation). A recent study by Das et al[35] showed that the minimum ablation index was an independent predictor of conduction recovery after PVI. Furthermore, in this study, higher ablation index values were required to prevent reconnection of anterior/roof segments, compared to posterior/inferior segments[35].
The EFFICAS-II study demonstrated that lesion contiguity is an essential component of effective PVI. The analysis of the contiguity index revealed that even with effective use of optimized CF, 15% of PVs were reconnected after ablation due to non-contiguity between point-by-point lesions along ablation line[28]. Consistent with these data, Park et al[36] showed that acutely durable PVI can be achieved in CF-guided ablation when RF lesions are delivered with a mean CF > 10 g and an inter-lesion distance < 5 mm.
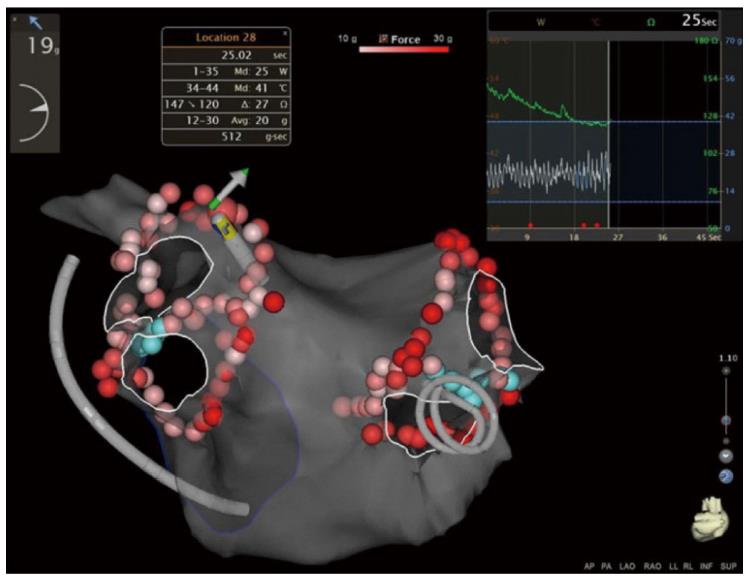
A novel automated technology for tagging ablation lesions (VisiTag module) allows real-time assessment of catheter stability, contact force, power, and impedance drop during radiofrequency applications (Figure 2). This technology improves lesion efficiency and reduces the number of ineffective applications[37]. Catheter stability tracking during PVI is essential in order to achieve appropriate lesion contiguity. Okumura et al[38] reported that a strict stability setting (3-mm distance limit for at least 10 s) for VisiTag reduced acute PV reconnection, although no benefit was observed in mid-term outcomes.
Circumferential PVI guided by nonfluoroscopic electroanatomic mapping systems, without confirmation of electrical isolation with a circular mapping catheter, has been shown to be ineffective in achieving long-term arrhythmia control[39]. Additionally, a randomized study comparing PVI guided by circular mapping catheter vs PVI using only RF catheter showed that the use of circular mapping catheter is associated with better acute results and lower recurrence rates[40]. Therefore, electroanatomic mapping-guided circumferential PV ablation without use of the circular mapping catheter has been demonstrated to be less reliable to achieve PVI and significantly less effective than circular mapping catheter-guided PVI in terms of arrhythmia-free survival.
The identification of dormant tissue that has been rendered unexcitable by “stunning” or edema is a significant challenge that may potentially increase risk of AF recurrence. The detection of such “dormant conduction” during the initial ablation procedure may therefore help identify PVs with the potential to reconnect after the index procedure, and targeted ablation at these sites may reduce the risk of recurrent AF. Adenosine has been shown to effectively uncover dormant conduction. Following ablation, adenosine selectively hyperpolarizes PV cells by increasing inward rectifier potassium current, thereby restoring excitability of inactivated voltage-dependent Na+ (INa) and reestablishing conduction in dormant PVs[41]. Multiple studies have shown that adenosine is clinically useful in identifying PV reconnection, as well as cavotricuspid isthmus reconnection[42]. An early study reported that adenosine induced reconnection in 25% of PVs immediately after successful isolation[43]. Tritto et al[44] further demonstrated that delivering additional RF lesions at electrical gap sites elicited by adenosine definitively eliminated recovery of PV reconnection in all cases. Subsequent studies have shown that AF recurrence after PV isolation could be reduced by delivering additional ablation lesions to eliminate adenosine-induced dormant PV conduction[45-47]. Other studies[48,49] did not confirm the usefulness of adenosine in AF recurrence after PVI, fueling a need for randomized trials.
Two randomized trials (ADVICE and UNDER-ATP) have assessed whether elimination of dormant PV conduction after PVI is better than conventional PVI in terms of arrhythmia-free survival. The ADVICE study showed that the use of adenosine to identify and target areas of dormant conduction significantly improved long-term arrhythmia-free survival, compared to PVI alone[50]. In contrast, the UNDER-ATP trial found no significant reduction in arrhythmia-free survival by ATP-guided PVI, compared with conventional PVI[51]. The discrepancy between ADVICE and UNDER-ATP trials may be due to differences in the rate of dormant conduction, around 50% in the ADVICE trial and 28% in UNDER-ATP. This suggests that the benefit of using adenosine after PVI depends on how frequently dormant conduction is observed; which is highly affected by the ablation procedural method.
Entrance and exit block confirmed by the absence of PV potentials and by pacing inside the PVs is a common procedural endpoint of encircling PVI. However, it has been suggested that pacing along the ablation line may identify latent spots of PV-LA antrum connection gaps not detected by circular mapping catheters. Steven et al[52] showed that more RF ablation energy was required to achieve loss of pace capture along the ablation line than for entrance block into the PVs, suggesting that reaching the endpoint of loss of capture along the ablation line may be associated with more durable lesions. Consistent with this hypothesis, a randomized study confirmed that the use of pacing to ensure an unexcitable gap along the ablation line improved success rates at 12 mo post-PVI, compared to reliance on bidirectional block alone (83% vs 52%, respectively)[53]. However, it should be noted that adenosine was not used to identify dormant conduction after PVI in this study. In contrast to these findings, two recent studies showed that although PVI followed by the pace and ablate method reduced dormant PV conduction unmasked by adenosine, there was no difference in 1-year AF recurrence, compared to adenosine-guided ablation[54,55].
Although the available results suggest that both techniques achieve similar long-term outcomes, the potential effect on recurrence rates of combining pace and ablate with adenosine-guided PVI remains unknown. A recent study by Kogawa et al[56] showed that sites with adenosine-induced dormant PV reconnection did not match the excitable gaps identified by pacing, suggesting a difference in the underlying mechanism to elucidate potential PV-antrum gaps. Thus, the authors proposed that an adenosine provocation test followed by pace and ablate method could be useful in reducing AF recurrence. Further prospective and randomized studies are required to confirm this hypothesis.
It should be noted that a variable proportion of patients may have AF recurrence despite persistent PVI. This could be due to the existence of non-PV triggers[57]. Typically, these non-PV triggers are located in specific regions such as the crista terminalis, the superior vena cava, the Eustachian ridge, the fossa ovalis, the left atrial appendage, the inferior mitral annulus and the coronary sinus. Empirical ablation of these common origins of triggers is not recommended. However, once a trigger is identified, it should be eliminated in order to achieve better outcomes[58].
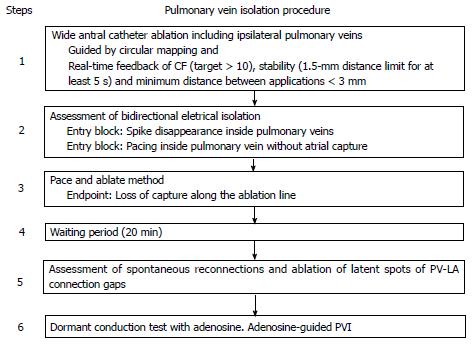
Based on our own experience, we propose the following step-wise approach to achieve permanent PVI (Figure 3). Our unit adopted this strategy two years ago, with good arrhythmia-free survival at 12 mo (84%), a very low complication rate (1%), and no increase in procedure time[24,59].
The implementation of non-fluoroscopic navigation systems in the electrophysiology laboratory has improved anatomic definition of cardiac structures. However, the increased complexity of ablation procedures demands better intra-procedural anatomic definition and improved accuracy in catheter positioning. Novel non-fluoroscopic systems have been proposed for catheter guidance during PVI procedures. In animal studies, Ranjan et al[60] showed the feasibility of catheter tracking, electrogram recording, and RF energy delivery in a real-time MRI environment. Intra-procedural MRI allowed real-time visualization of lesion formation and tissue characterization, which could permit the assessment of lesion depth and transmurality. Furthermore, their work demonstrates the utility of MRI-guided PVI to identify gaps intra-procedurally and guide catheter positioning to target them. However, this proof-of-concept has not been tested in humans. In order to use this technology in clinical settings, several technical challenges must be overcome to obtain better signals and develop more maneuverable and easily visible catheters. However, this promising technology will provide considerable benefits by delivering accurate anatomic definition and monitoring of RF lesions.
PVI is the cornerstone of catheter-based therapies for AF. PV reconnection after PVI represents the main limitation of AF ablation techniques. Efforts should be made to develop strategies that achieve more durable lesions. Current techniques associated with better acute (and probably long-term) outcomes include antral PVI guided by circular mapping catheters, the use of CF catheters, lesion contiguity, and the assessment of dormant PV conduction by adenosine and/or pace and ablate. Finally, a subset of patients may still have AF recurrences despite persistent PVI, due to the presence of non-PV triggers. Efforts should be made in order to individualize the treatment according to each patient’s specific mechanism of recurrence (drivers, rotors, focal activity…).
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chei CL, Ciconte G, den Uil CA S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5167] [Cited by in RCA: 4884] [Article Influence: 542.7] [Reference Citation Analysis (0)] |
| 2. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5397] [Article Influence: 199.9] [Reference Citation Analysis (0)] |
| 3. | Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Davies DW, DiMarco J. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1073] [Cited by in RCA: 1162] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 4. | Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | Callans DJ, Gerstenfeld EP, Dixit S, Zado E, Vanderhoff M, Ren JF, Marchlinski FE. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 639] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 7. | Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Kowalski M, Grimes MM, Perez FJ, Kenigsberg DN, Koneru J, Kasirajan V, Wood MA, Ellenbogen KA. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol. 2012;59:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 912] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 12. | Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016;374:2235-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1436] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 13. | Piorkowski C, Eitel C, Rolf S, Bode K, Sommer P, Gaspar T, Kircher S, Wetzel U, Parwani AS, Boldt LH. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol. 2011;4:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Arya A, Hindricks G, Sommer P, Huo Y, Bollmann A, Gaspar T, Bode K, Husser D, Kottkamp H, Piorkowski C. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2010;12:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Di Biase L, Conti S, Mohanty P, Bai R, Sanchez J, Walton D, John A, Santangeli P, Elayi CS, Beheiry S. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011;8:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, Ikeda A, Pitha JV, Sharma T, Lazzara R. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 17. | Thiagalingam A, D’Avila A, Foley L, Guerrero JL, Lambert H, Leo G, Ruskin JN, Reddy VY. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol. 2010;21:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol. 2010;21:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace. 2014;16:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Sohns C, Karim R, Harrison J, Arujuna A, Linton N, Sennett R, Lambert H, Leo G, Williams S, Razavi R. Quantitative magnetic resonance imaging analysis of the relationship between contact force and left atrial scar formation after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Andreu D, Gomez-Pulido F, Calvo M, Carlosena-Remírez A, Bisbal F, Borràs R, Benito E, Guasch E, Prat-Gonzalez S, Perea RJ. Contact force threshold for permanent lesion formation in atrial fibrillation ablation: A cardiac magnetic resonance-based study to detect ablation gaps. Heart Rhythm. 2016;13:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Williams SE, Harrison J, Chubb H, Bloch L, Andersen NP, Dam H, Karim R, Whitaker J, Gill J, Cooklin M. The Effect of Contact Force in Atrial Radiofrequency Ablation: Electroanatomical, Cardiovascular Magnetic Resonance, and Histological Assessment in a Chronic Porcine Model. JACC: Clinical Electrophysiology. 2015;421. |
| 23. | Schluermann F, Krauss T, Biermann J, Hartmann M, Trolese L, Pache G, Bode C, Asbach S. In vivo contact force measurements and correlation with left atrial anatomy during catheter ablation of atrial fibrillation. Europace. 2015;17:1526-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Pedrote A, Arana-Rueda E, Arce-León A, Acosta J, Gómez-Pulido F, Martos-Maine JL, Frutos-López M, Sánchez-Brotons J, García-Riesco L. Impact of Contact Force Monitoring in Acute Pulmonary Vein Isolation Using an Anatomic Approach. A Randomized Study. Pacing Clin Electrophysiol. 2016;39:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Sotomi Y, Kikkawa T, Inoue K, Tanaka K, Toyoshima Y, Oka T, Tanaka N, Nozato Y, Orihara Y, Iwakura K. Regional difference of optimal contact force to prevent acute pulmonary vein reconnection during radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, Kautzner J, Herrera C, Hindricks G, Jaïs P. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm. 2012;9:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, Lambert H, Yulzari A, Wissner E, Kuck KH. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 28. | Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R, Skoda J, Wichterle D, Wissner E, Yulzari A. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 29. | Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer DL. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 30. | Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque JP, Kautzner J, Shah D, Michaud G, Wharton M, Harari D. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015;132:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 31. | Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, Ishida Y, Kinjo T, Tomita H, Okumura K. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm. 2014;11:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Afzal MR, Chatta J, Samanta A, Waheed S, Mahmoudi M, Vukas R, Gunda S, Reddy M, Dawn B, Lakkireddy D. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm. 2015;12:1990-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Shurrab M, Di Biase L, Briceno DF, Kaoutskaia A, Haj-Yahia S, Newman D, Lashevsky I, Nakagawa H, Crystal E. Impact of Contact Force Technology on Atrial Fibrillation Ablation: A Meta-Analysis. J Am Heart Assoc. 2015;4:e002476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Ullah W, McLean A, Tayebjee MH, Gupta D, Ginks MR, Haywood GA, O’Neill M, Lambiase PD, Earley MJ, Schilling RJ. Randomized trial comparing pulmonary vein isolation using the SmartTouch catheter with or without real-time contact force data. Heart Rhythm. 2016;13:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJ, Waktare JE, Todd DM, Hall MC, Snowdon RL. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2016; May 31; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Park CI, Lehrmann H, Keyl C, Weber R, Schiebeling J, Allgeier J, Schurr P, Shah A, Neumann FJ, Arentz T. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol. 2014;25:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Anter E, Tschabrunn CM, Contreras-Valdes FM, Buxton AE, Josephson ME. Radiofrequency ablation annotation algorithm reduces the incidence of linear gaps and reconnection after pulmonary vein isolation. Heart Rhythm. 2014;11:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Okumura Y, Watanabe I, Iso K, Nagashima K, Sonoda K, Sasaki N, Kogawa R, Takahashi K, Ohkubo K, Nakai T. Clinical utility of automated ablation lesion tagging based on catheter stability information (VisiTag Module of the CARTO 3 System) with contact force-time integral during pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol. 2016;47:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Kanagaratnam L, Tomassoni G, Schweikert R, Pavia S, Bash D, Beheiry S, Lesh M, Niebauer M, Saliba W, Chung M. Empirical pulmonary vein isolation in patients with chronic atrial fibrillation using a three-dimensional nonfluoroscopic mapping system: long-term follow-up. Pacing Clin Electrophysiol. 2001;24:1774-1779. [PubMed] |
| 40. | Tamborero D, Mont L, Berruezo A, Guasch E, Rios J, Nadal M, Matiello M, Andreu D, Sitges M, Brugada J. Circumferential pulmonary vein ablation: does use of a circular mapping catheter improve results? A prospective randomized study. Heart Rhythm. 2010;7:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Datino T, Macle L, Qi XY, Maguy A, Comtois P, Chartier D, Guerra PG, Arenal A, Fernández-Avilés F, Nattel S. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010;121:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 42. | Morales GX, Macle L, Khairy P, Charnigo R, Davidson E, Thal S, Ching CK, Lellouche N, Whitbeck M, Delisle B. Adenosine testing in atrial flutter ablation: unmasking of dormant conduction across the cavotricuspid isthmus and risk of recurrence. J Cardiovasc Electrophysiol. 2013;24:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, Haissaguerre M. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Tritto M, De Ponti R, Salerno-Uriarte JA, Spadacini G, Marazzi R, Moretti P, Lanzotti M. Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. Eur Heart J. 2004;25:2155-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Matsuo S, Yamane T, Date T, Inada K, Kanzaki Y, Tokuda M, Shibayama K, Miyanaga S, Miyazaki H, Sugimoto K. Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. J Cardiovasc Electrophysiol. 2007;18:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Datino T, Macle L, Chartier D, Comtois P, Khairy P, Guerra PG, Fernandez-Aviles F, Nattel S. Differential effectiveness of pharmacological strategies to reveal dormant pulmonary vein conduction: a clinical-experimental correlation. Heart Rhythm. 2011;8:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Matsuo S, Yamane T, Date T, Lellouche N, Tokutake K, Hioki M, Ito K, Narui R, Tanigawa S, Nakane T. Dormant pulmonary vein conduction induced by adenosine in patients with atrial fibrillation who underwent catheter ablation. Am Heart J. 2011;161:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Gula LJ, Massel D, Leong-Sit P, Gray C, Fox DJ, Segal OR, Krahn AD, Yee R, Klein GJ, Skanes AC. Does adenosine response predict clinical recurrence of atrial fibrillation after pulmonary vein isolation? J Cardiovasc Electrophysiol. 2011;22:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Miyazaki S, Kuwahara T, Kobori A, Takahashi Y, Takei A, Sato A, Isobe M, Takahashi A. Impact of adenosine-provoked acute dormant pulmonary vein conduction on recurrence of atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, Arentz T, Deisenhofer I, Veenhuyzen G, Scavée C. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015;386:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 51. | Kobori A, Shizuta S, Inoue K, Kaitani K, Morimoto T, Nakazawa Y, Ozawa T, Kurotobi T, Morishima I, Miura F. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate (UNDER-ATP) trial. Eur Heart J. 2015;36:3276-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Steven D, Reddy VY, Inada K, Roberts-Thomson KC, Seiler J, Stevenson WG, Michaud GF. Loss of pace capture on the ablation line: a new marker for complete radiofrequency lesions to achieve pulmonary vein isolation. Heart Rhythm. 2010;7:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Steven D, Sultan A, Reddy V, Luker J, Altenburg M, Hoffmann B, Rostock T, Servatius H, Stevenson WG, Willems S. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol. 2013;62:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Okumura Y, Watanabe I, Nagashima K, Sonoda K, Mano H, Sasaki N, Kogawa R, Takahashi K, Iso K, Ohkubo K. The effects of standard electrical PV isolation vs. “pace and ablate” on ATP-provoked PV reconnections. J Interv Card Electrophysiol. 2014;40:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Andrade JG, Pollak SJ, Monir G, Khairy P, Dubuc M, Roy D, Talajic M, Deyell M, Rivard L, Thibault B. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation--a prospective study. Circ Arrhythm Electrophysiol. 2013;6:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Kogawa R, Okumura Y, Watanabe I, Sonoda K, Sasaki N, Takahashi K, Iso K, Nagashima K, Ohkubo K, Nakai T. Difference Between Dormant Conduction Sites Revealed by Adenosine Triphosphate Provocation and Unipolar Pace-Capture Sites Along the Ablation Line After Pulmonary Vein Isolation. Int Heart J. 2016;57:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Dukkipati SR, Neuzil P, Kautzner J, Petru J, Wichterle D, Skoda J, Cihak R, Peichl P, Dello Russo A, Pelargonio G. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: multicenter results of pulmonary vein remapping studies. Heart Rhythm. 2012;9:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 58. | Hsu LF, Jaïs P, Keane D, Wharton JM, Deisenhofer I, Hocini M, Shah DC, Sanders P, Scavée C, Weerasooriya R. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Arana-Rueda E, Pedrote A, García-Riesco L, Arce-León A, Gómez-Pulido F, Durán-Guerrero JM, Fernández-Cisnal A, Frutos-López M, Sánchez-Brotons JA. Reverse atrial remodeling following pulmonary vein isolation: the importance of the body mass index. Pacing Clin Electrophysiol. 2015;38:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Ranjan R, Kholmovski EG, Blauer J, Vijayakumar S, Volland NA, Salama ME, Parker DL, MacLeod R, Marrouche NF. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ Arrhythm Electrophysiol. 2012;5:1130-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Marijon E, Fazaa S, Narayanan K, Guy-Moyat B, Bouzeman A, Providencia R, Treguer F, Combes N, Bortone A, Boveda S. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J Cardiovasc Electrophysiol. 2014;25:130-137 [PMID 24433324 DOI 10.1111/jce.12303]. |