Published online Sep 26, 2016. doi: 10.4330/wjc.v8.i9.553
Peer-review started: May 16, 2016
First decision: July 5, 2016
Revised: July 15, 2016
Accepted: July 29, 2016
Article in press: August 1, 2016
Published online: September 26, 2016
Processing time: 127 Days and 13.1 Hours
To determine the optimum resting tension (ORT) for in vitro human pulmonary artery (PA) ring preparations.
Pulmonary arteries were dissected from disease free sections of the resected lung in the operating theatre and tissue samples were directly sent to the laboratory in Krebs-Henseleit solution (Krebs). The pulmonary arteries were then cut into 2 mm long rings. PA rings were mounted in 25 mL organ baths or 8 mL myograph chambers containing Krebs compound (37 °C, bubbled with 21% O2: 5% CO2) to measure changes in isometric tension. The resting tension was set at 1-gram force (gf) with vessels being left static to equilibrate for duration of one hour. Baseline contractile reactions to 40 mmol/L KCl were obtained from a resting tension of 1 gf. Contractile reactions to 40 mmol/L KCl were then obtained from stepwise increases in resting tension (1.2, 1.4, 1.6, 1.8 and 2.0 gf).
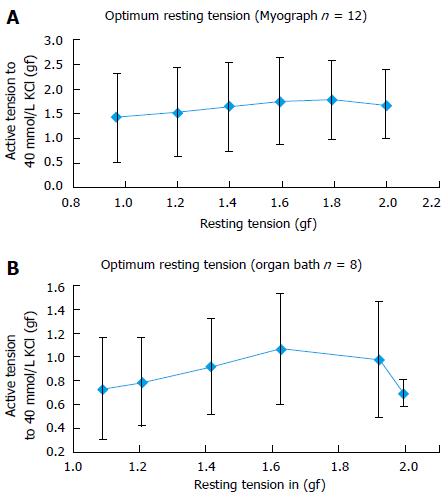
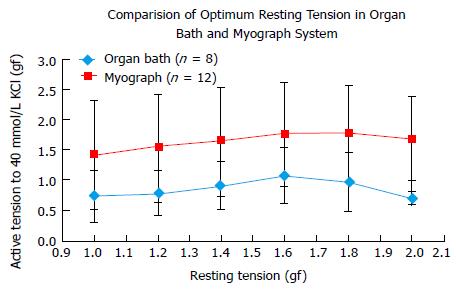
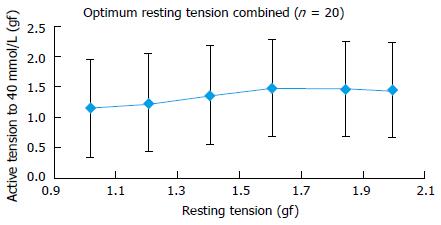
Twenty PA rings of internal diameter between 2-4 mm were prepared from 4 patients. In human PA rings incrementing the tension during rest stance by 0.6 gf, up to 1.6 gf significantly augmented the 40 mmol/L KCl stimulated tension. Further enhancement of active tension by 0.4 gf, up to 2.0 gf mitigate the 40 mmol/L KCl stimulated reaction. Both Myograph and the organ bath demonstrated identical conclusions, supporting that the radial optimal resting tension for human PA ring was 1.61 g.
The radial optimal resting tension in our experiment is 1.61 gf (15.78 mN) for human PA rings.
Core tip: Pulmonary artery (PA) vasoconstriction is an important physiological process to regulate blood flow in the lungs but it also manifests in pathological conditions. Different models have been implemented to assess the baseline molecular and cellular functions of pulmonary ailments. However, a great deal of the research was undertaken on animals with little similarity to human tissue. Isolation of human PA and measurement of pulmonary vascular tension are vital to understand the pathophysiology of human pulmonary vessels. The objective behind this research is to assess the underlying resting tension for undertaking studies of the PA rings in humans.
- Citation: Hussain A, Bennett RT, Chaudhry MA, Qadri SS, Cowen M, Morice AH, Loubani M. Characterization of optimal resting tension in human pulmonary arteries. World J Cardiol 2016; 8(9): 553-558
- URL: https://www.wjgnet.com/1949-8462/full/v8/i9/553.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i9.553
The vascular wall is constituted by three sections or layers; the tunica intima, tunica media, and tunica externa, also otherwise known as the internal, middle and outer layer, respectively[1]. Endothelial cells are located in the intima and play an important role in regulating vascular operations through reacting to neurotransmitters, hormones and vasoactive elements[2]. The endothelium and smooth muscle are the vital components for maintenance of arterial tone and blood pressure directive. The arteries main purpose is to deliver the blood to the organs with high pulse pressure. Arteries are generally classified into conducting arteries, conduit arteries (macrovasculature) and resistance arteries (microvasculature) sourced on size, anatomical position and functionality[3]. Conducting arteries are the largest in size and rich in elastic tissues which support the vessels to expand and recoil to accommodate high changes in blood pressure. The aorta, pulmonary artery (PA) and carotid arteries are the main examples of conducting arteries[4]. Conduit arteries, e.g., femoral, radial and brachial arteries are the subdivisions of conducting arteries, and their role is regulating the flow of blood to particular organs and sections of the body[5]. Conduit arteries advance more through separating into resistance arteries that mainly consist of smooth muscles and are highly innervated by sympathetic nerves. Resistance vessels regulate the blood flow to tissues through constricting or dilating as reaction to sympathetic simulation or dissimulation[6].
Hypoxic pulmonary vasoconstriction (HPV) is a fundamental physiological mechanism to redirect the blood from poorly to better-aerated areas of lungs to optimize the ventilation perfusion matching[7]. Persistent hypoxia leads to increased pulmonary vascular opposition and right ventricular afterload that leads to hypoxic pulmonary hypertension[8]. HPV initially thought to be caused by alveolar hypoxia by means of local lung mechanism but recent advances suggest that PA smooth muscle cells constitute both the sensor and the transducer of the hypoxic signal as well as its contractile effector[9]. A series of experiments performed to explain the phenomenon on macroscopic and microscopic level has been reported although the underlying mechanism is not clear[10-12]. However, vast majority of experiments performed in animals with little data available from humans. Experiments performed on animals are generally inapplicable on humans so we need to adapt new methodologies for use in human to understand the human disease biology.
The objective of this research is assessing the tension in human PA to facilitate future experiments and also to provide a methodology of isolation of PA and their use in studies in the form of arterial rings.
All patients undergoing a lung lobectomy by a Consultant Cardiothoracic Surgeon at Castle Hill Hospital were consented for resected lung tissue to be included in this study prior to their operation at the time of their consent for surgery. Patients under the age of 18 and who cannot give informed consent were excluded from the study. Local research ethics committee and local research and development department approval was obtained for the use of human tissue for this study.
Tissue samples were collected from patients undergoing surgical lung resection for cancer and immediately moved to the laboratory in Krebs-Henseleit solution (consisting of 113.8 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, 25 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 11.4 mmol/L glucose, and 2.4 mmol/L CaCl2 dissolved in distilled water). Pulmonary arteries were dissected from disease free areas of lung resection and after careful removal of adipose and connective tissues cut into 2 mm long rings. The Internal diameter of vessels ranged between 2-4 mm.
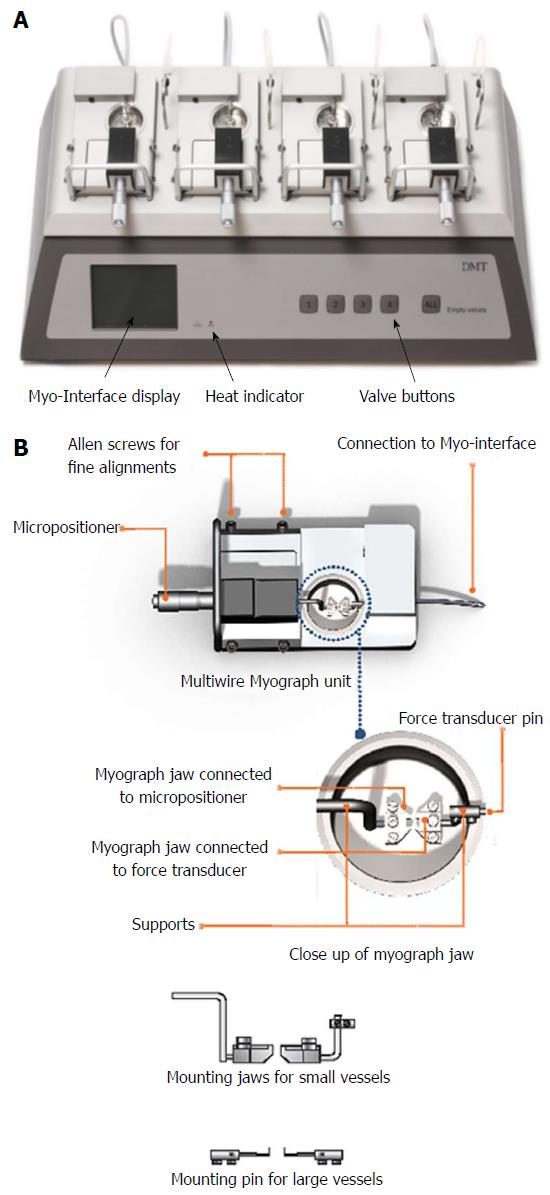
A multiwire myograph system (DMT 620M) and an organ bath system (Radnotti) were used for mounting of PA rings and measurement of ORT. The Multi wire myograph system consists of 4 individual myograph units. Each unit is created with aluminum and has a centralized placement of 8 mL stainless steel chamber (Figure 1A). Pins to support the tissue were placed within the chamber, one end being connected to a force transducer whilst the other connected with a micrometer. PA rings were mounted between the pins. All units were subject to administered gas inflow and suction. Connections for vacuum and gassing, as well as heating are provided in the myograph interface, allowing for all chambers to be smoothly maintained under physiological settings (37 °C, and bubbled with 21% O2: 5% CO2) (Figure 1B). The myograph system was connected to a PC via an amplifier (Power Lab 8/35, AD Instruments) for continuous measurement of isometric tension using data acquisition software (Lab Chart Pro Version 8.0).
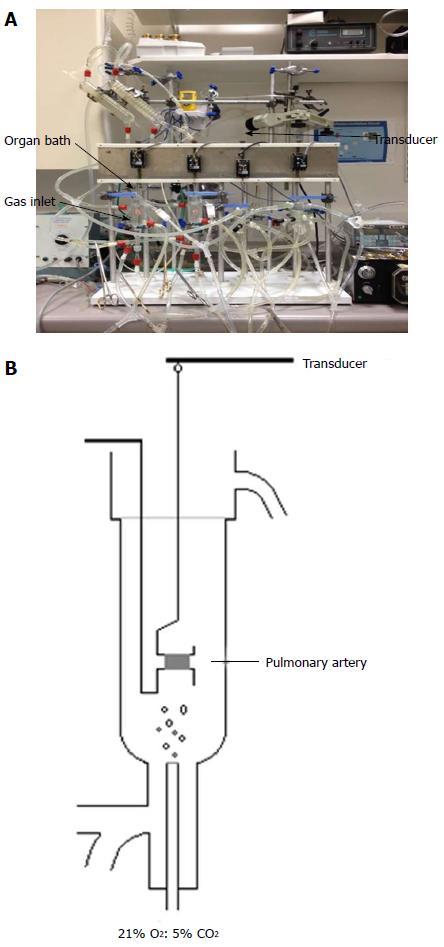
The organ bath system consists of 4 organ baths connected to a gas inlet where gas mixtures can be bubbled through (Figure 2A). Surrounding each bath is a heat exchanger that recreated the physiological temperatures of the human body. Each organ bath contained Krebs’s solution and the PA rings were mounted between two hooks. One hook was fixed and the other connected with a force transducer (Harvard UF1), which was linked to a PC for continuous measurement of isometric tension (Figure 2B).
After mounting of PA rings the resting tension was set at 1 gf and the vessels left to equilibrate under 21% O2: 5% CO2 at 37 °C for 60 min. When a stable resting tension was achieved the vessels were contracted to 40 mmol/L KCl by direct addition to the organ bath. The maximum contraction to KCl was recorded when the contractile response reached a plateau. Active tension was calculated as maximum tension at plateau (gf) - resting tension (gf). Vessels were then washed for 30 min by rapidly replacing the Krebs solution in the chambers with fresh solution three times every five minutes. When a stable resting tension was achieved a repeat reaction with 40 mmol/L KCl was obtained and the vessels again washed before obtaining a third reaction to 40 mmol/L KCl for the purpose of confirming reproducibility in the response. When a reproducible response was obtained the maximum contraction to 40 mmol/L KCl had been established from increasing resting tensions of 1.2, 1.4, 1.6, 1.8 and 2.0 gf with the vessels being washed for 30 min between responses.
At the end of each experiment the integrity of endothelium was confirmed by the addition of 1 umol/L acetylcholine. Rings that did not contract to KCl were excluded from the study.
Five percent of carbon dioxide/balance air (10 lt cylinders) was sourced from BOC Limited. All reagents were obtained from Fischer Scientific and acetylcholine from Sigma Aldrich.
Data are presented as mean ± SD and n represents the number of PA rings used.
Twenty PA rings (internal diameter 2-4 mm) were obtained from 4 patients. Results showed that in human PA rings increasing the basal tension from 1.0 to 1.6 gf significantly augmented the 40 mmol/L KCl induced active tension. Increasing the active tension from 1.6 to 2.0 g mitigate the 40 mmol/L KCl induced response (Figure 3). The myograph and organ bath demonstrated identical conclusions (Figure 4), confirming that the most efficient resting tension for human PA rings is 1.61 gf (Figure 5).
The pulmonary circulation carries deoxygenated blood from right section of heart towards lungs, under which it is subject to oxygenation while carbon dioxide is filtered, thereafter returning the clean blood onto the left section of the heart prepared for dissemination[13] (Figure 6). The pulmonary circulation is in series and reliant not only on the systemic blood flowing to right section of the heart, rather also the outflow from the left section[14]. Therefore, in case of an increment under the left atrium pressure or increase in afterload like in aortic stenosis, greater pressure will be observed in the PA[15]. PA vasoconstriction is an important physiological process to regulate blood flow in lungs but it also results in pathologies. Various models are utilized for assessing the baseline molecular and cellular functions of lung ailments, particularly pulmonary vascular affliction. However, a great deal of researches is undertaken on animals with little similarity to humans. Few centers have the luxury to utilize human tissue to study this phenomenon. Isolation of human PA and measurement of pulmonary vascular tension are vital to understand the pathophysiology of human pulmonary vessels. The objective behind this research is to assess the optimal resting tension for undertaking studies on human PA rings.
Pulmonary artery (PA) vasoconstriction is an important physiological process to regulate blood flow in the lungs but it also manifests in pathological conditions. Isolation of human PA and measurement of pulmonary vascular tension are vital to understand the human pulmonary vessels disease especially pulmonary hypertension.
Further research is required to confirm the conclusion of this research, and also to evaluate if whether the optimum tension varies between various sizes of pulmonary arteries.
The authors yields the base optimal resting tension (ORT) for conducting studies on human PA rings and the ORT measured was 1.61 gf (15.78 mN) for vessels with internal diameter ranged between 2-4 mm.
This study provides a baseline ORT to facilitate future experiments on human PA rings and also provide a methodology of isolation of PA and their use in studies in the form of arterial rings.
Pulmonary hypertension is a hemodynamic state elaborated by defined by a resting mean PA pressure at or above 25 mmHg.
It is a valuable paper for physiology and pathology pulmonary arteries. The system will provide a basic for further research about pulmonary arteries and its-related diseases.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Landesberg G, Tang JM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Levick JR. An introduction to cardiovascular physiology. London: Arnold; New York: Distributed in the United States of America by Oxford University Press 2003; . |
| 2. | Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 352] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | O’Brien SF, McKendrick JD, Radomski MW, Davidge ST, Russell JC. Vascular wall reactivity in conductance and resistance arteries: differential effects of insulin resistance. Can J Physiol Pharmacol. 1998;76:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Pugsley MK, Tabrizchi R. The vascular system. An overview of structure and function. J Pharmacol Toxicol Methods. 2000;44:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Baumbach GL, Heistad DD. Effects of sympathetic stimulation and changes in arterial pressure on segmental resistance of cerebral vessels in rabbits and cats. Circ Res. 1983;52:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Maggiorini M, Mélot C, Pierre S, Pfeiffer F, Greve I, Sartori C, Lepori M, Hauser M, Scherrer U, Naeije R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation. 2001;103:2078-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 324] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest. 2012;122:4218-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Ariyaratnam P, Loubani M, Morice AH. Hypoxic pulmonary vasoconstriction in humans. Biomed Res Int. 2013;2013:623684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Swenson ER. Hypoxic pulmonary vasoconstriction. High Alt Med Biol. 2013;14:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122:932-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Comroe JH. The main functions of the pulmonary circulation. Circulation. 1966;33:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol. 2010;7:648-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Hunt JM, Bethea B, Liu X, Gandjeva A, Mammen PP, Stacher E, Gandjeva MR, Parish E, Perez M, Smith L. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am J Physiol Lung Cell Mol Physiol. 2013;305:L725-L736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |