Published online Jul 26, 2015. doi: 10.4330/wjc.v7.i7.410
Peer-review started: March 4, 2015
First decision: March 20, 2015
Revised: April 14, 2015
Accepted: April 28, 2015
Article in press: April 30, 2015
Published online: July 26, 2015
Processing time: 152 Days and 22.6 Hours
Duchenne and Becker muscular dystrophy (DMD/BMD) are X-linked muscular diseases responsible for over 80% of all muscular dystrophies. Cardiac disease is a common manifestation, not necessarily related to the degree of skeletal myopathy; it may be the predominant manifestation with or without any other evidence of muscular disease. Death is usually due to ventricular dysfunction, heart block or malignant arrhythmias. Not only DMD/BMD patients, but also female carriers may present cardiac involvement. Clinically overt heart failure in dystrophinopathies may be delayed or absent, due to relative physical inactivity. The commonest electrocardiographic findings include conduction defects, arrhythmias (supraventricular or ventricular), hypertrophy and evidence of myocardial necrosis. Echocardiography can assess a marked variability of left ventricular dysfunction, independently of age of onset or mutation groups. Cardiovascular magnetic resonance (CMR) has documented a pattern of epicardial fibrosis in both dystrophinopathies’ patients and carriers that can be observed even if overt muscular disease is absent. Recently, new CMR techniques, such as postcontrast myocardial T1 mapping, have been used in Duchenne muscular dystrophy to detect diffuse myocardial fibrosis. A combined approach using clinical assessment and CMR evaluation may motivate early cardioprotective treatment in both patients and asymptomatic carriers and delay the development of serious cardiac complications.
Core tip: Duchenne and Becker muscular dystrophy are the commonest X-linked muscular diseases. Death is usually due to cardiac disease including ventricular dysfunction, heart block or malignant arrhythmias. Female carriers may also present cardiac involvement. Overt heart failure may be delayed or absent. Electrocardiography findings include conduction defects, arrhythmias and myocardial necrosis. Echocardiography assesses a marked variability of left ventricular dysfunction. Epicardial fibrosis in both patients and carriers has been documented by Cardiovascular Magnetic Resonance (CMR), even if overt muscular disease is absent. A combined approach using clinical and CMR assessment may motivate early cardioprotective treatment and delay serious cardiac complications.
- Citation: Mavrogeni S, Markousis-Mavrogenis G, Papavasiliou A, Kolovou G. Cardiac involvement in Duchenne and Becker muscular dystrophy. World J Cardiol 2015; 7(7): 410-414
- URL: https://www.wjgnet.com/1949-8462/full/v7/i7/410.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i7.410
Duchenne and Becker muscular dystrophy (DMD/BMD) includes a group of X-linked muscular diseases responsible for over 80% of all cases of muscular dystrophy[1]. The incidence of DMD is 1 in 3500 male newborns with a prevalence of 6 in 100000 males[1] and is characterized by weakness of leg, pelvic and shoulder girdle muscles starting in early childhood. BMD is a milder variant of dystro-phinopathy with a better prognosis. The incidence of BMD is 1 in 18450 males and prevalence 2.4 per 100000 in the general population[2,3]. First symptoms appear between ages of 3-21 years with a mean age of onset at 11 years. Age at death is at 21-89 years with an average age of about 45 years[4-7].
In DMD, boys are diagnosed as toddlers and most are wheelchair bound by age 15. Death usually occurs at the age of 20 years, due to respiratory complications or cardiomyopathy. Currently more patients survive until the age of 30 years, due to home ventilation and corticosteroids, which can prolong ambulation by 2-3 years, reduce risk of scoliosis and postpone pulmonary and heart failure after the age of 20 years[1]. Despite this documented efficacy, more than 25% of DMD boys are not treated with corticosteroids, either due to side-effects or lack of response[1]. In BMD, the disease is milder and more heterogenous, compared to DMD. Muscle weakness often is first noticed in adolescence or young adulthood. Cardiac involvement in BMD may precede the skeletal muscle decline, with death due to cardiomyopathy often occurring before the age 60 years[1].
Mutations leading in the absence of a functional dystrophin protein cause DMD, whereas mutations leading in a reduced amount or shortened dystrophin protein cause BMD[8,9]. Dystrophin is a large (427 kDa) subsarcolemmal protein that represents a physical link between the intracellular actin cytoskeleton and the extracellular matrix[10]. Loss or abnormal dystrophin destabilizes the sarcolemma, making the muscle fibers susceptible to contraction injury[11]. The repeated episodes of necrosis followed by regeneration finally lead to replacement of muscles by fat and connective tissue that is clinically manifested as progressive muscle weakness[10]. Dystrophin is also a scaffold protein that localizes other proteins to the sarcolemma and forms a highly organized multimeric dystrophin-associated glycoprotein complex (DGC)[10]. Dystrophin deficiency disrupts the DGC, resulting in downregulation and/or mislocalization of the dystrophin-associated proteins.
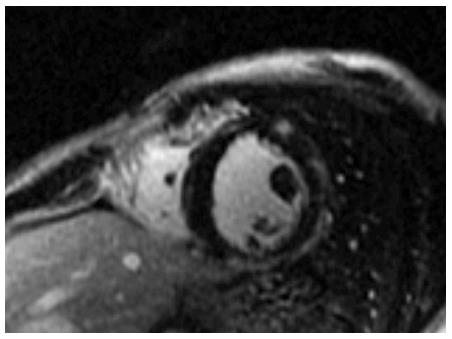
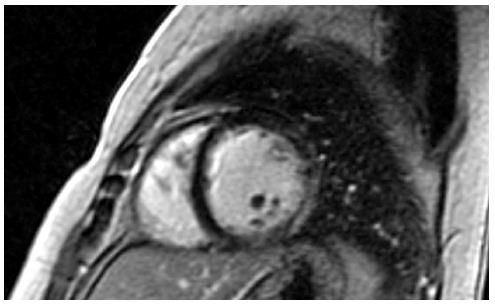
Cardiac disease in DMD is progressive and finally leads to ventricular dysfunction, usually accompanied by ventricular dilation[12]. Pathology examination during the late stages of the disease shows cardiomyocytes’ hypertrophy, atrophy and fibrosis[13-15]. Fibrosis of the left ventricle in DMD, BMD and DMD/BMD carriers has been observed at autopsy[13-15] and after evaluation with cardiovascular magnetic resonance (CMR) using late gadolinium enhancement (LGE)[16-19] (Figures 1 and 2).
The majority of DMD after the third decade of their age have established cardiomyopathy[20]. Although clinically overt heart failure may be delayed or absent, due to relative physical inactivity, cardiomyopathy is the leading cause of death in DMD and myocardial damage precedes decline in left ventricular systolic function. Neither the age of onset nor the severity of cardiomyopathy was correlated with the type of mutation[21]. It was recently documented that in DMD with pre-served ejection fraction, the addition of eplerenone to background ACE inhibitors or ARB attenuates the progressive decline in left ventricular systolic function[22].
Cardiomyopathy is the main clinical complication in patients affected by subclinical or mild BMD. The clinical presentation is usually characterized by early right ventricular dysfunction and is later associated with left ventricular impairment. In mild BMD, myocardial damage may develop because the patients, who are unaware of a possible cardiac disease, can still perform strenuous muscle exercise and, through pressure or volume overload, may induce mechanical stress, which is harmful for dystrophin-deficient myocardial cells[23].
Cardiac disease in female carriers of dystrophinopathies may present with hypertrophy, arrhythmias or dilated cardiomyopathy[24]. The percentage of clinically overt cardiac involvement increases significantly with age, from 15% in carriers < 16 years to 45% in carriers > 16 years. By contrast, significant cardiac disease is unlikely in female carriers < 16 years[25]. In a cross-sectional study of 85 DMD and 44 BMD carriers aged 18-58 years, left ventricular dilatation and dilated cardiomyopathy were assessed in 18% and 8%, respectively[26]. Electrocardiography (ECG) abnormalities were found only in 47% of this population[27]. Another series of 56 adult, female carriers did not present any ECG abnormalities, but ventricular dilatation or hypertrophy was documented in 14% and dilated cardiomyopathy in 7% of them[28]. Nevertheless, severe heart failure may develop in some women necessitating heart transplantation to survive[29,30]. Exercise may unmask left ventricular (LV) systolic dysfunction in female carriers[31]. In a study by our group, CMR documented myocardial fibrosis in the majority of DMD and BMD mother-carriers, although the clinical presentation and the usual noninvasive assessment were mildly abnormal[19]. Therefore, detailed cardiac evaluation, at least once after the teenage years, should be recommended in all female carriers in order to start early cardiac treatment[32].
DMD is associated with increased R/S ratio in the right precordial leads, deep Q waves in the lateral leads, conduction abnormalities and arrhythmias (mainly supraventricular but also ventricular). In a study of 131 DMD, ECG was abnormal in 78.6%. All were in sinus rhythm and the following percentages were found for the main variables studied: short PR interval = 18.3%; abnormal R waves in V1 = 29.7%; abnormal Q waves in V6 = 21.3%; abnormal ventricular repolarization = 54.9%; abnormal QS waves in inferior and/or upper lateral wall = 37.4%; conduction disturbances in right bundle branch = 55.7%; prolonged QTc = 35.8% and wide QRS = 23.6%[33]. According to the study by Petri et al[34], ECG abnormalities were non-progressive in BMD and asymptomatic SVT and NSVT were present in 21% and 14%, respectively. Both ECG and Holter monitoring are necessary for DMD/BMD assessment. Serial clinical evaluation, including routine monitoring of electrocardiograms may detect early cardiomyopathy in DMD/BMD, even if left ventricular function is still preserved[35].
Echocardiography has already documented marked differences in LV function of DMD patients, independently of age of onset or mutation groups[21]. It has also proved a high prevalence of LV dysfunction in DMD, with frequent evidence of systolic ventricular asynchrony, particularly in patients with EF < 35%[36]. New echocardiographic techniques, using transmural strain profile (TMSP), can detect subclinical LV dysfunction in patients with DMD without wall motion abnormalities by conventional echocardiography[37]. The application of myocardial strain imaging in DMD patients was characterized by decreased peak systolic strain of the posterior wall, despite normal standard echocardiographic findings[38]. However, these studies were not universally accepted for the routine assessment of DMD/BMD.
Cardiovascular magnetic resonance (CMR) a non-invasive, non-radiating technique has been proved the most robust tool for detection of early myocardial fibrosis in DMD, BMD and female carriers, using late gadolinium enhancement (LGE). The pathology of cardiomyopathy in dystrophinopathies includes the presence of subepicardial fibrosis in the inferolateral wall[39], similar to that observed in viral myocarditis. The application of CMR in DMD/BMD and female carriers, in addition to the standard monitoring is of great value because: (1) Early start of heart failure treatment may delay the progression of LV dysfunction[22,40]; (2) Myocardial fibrosis, assessed by LGE, may be observed, even if the echocardiographic evaluation remains normal[16-18,40] and can potentially be used as an early sensitive index to start cardioprotective treatment; (3) It can be also applied as a screening tool to detect patients at high risk for ventricular arrhythmias, more advanced disease, adverse LV remodelling and death[41]. An impaired LV systolic function (LV-EF ≤ 45%) and a “transmural” pattern of myocardial fibrosis independently predict the occurrence of adverse cardiac events in DMD/BMD patients. Even in DMD/BMD patients with relatively preserved LV-EF (> 45%), the simple and visually assessable parameter “transmural LGE” is of additive prognostic value[42]; (4) in mutation carriers, CMR revealed a pattern of fibrosis similar to that observed in DMD[36], but without any correlation with genotype-phenotype[43], even in the absence of overt muscular disease; and (5) new CMR techniques, such as postcontrast myocardial T1 mapping, have been used in DMD to detect diffuse myocardial fibrosis. It was documented that post-contrast T1 obtained from the Look-Locker sequences (T1LL) ratio is abnormally shortened in DMD compared with controls, even in DMD patients with otherwise normal CMR study. It is assumed that the application of more aggressive therapy for DMD with shorter T1LL may improve morbidity and mortality in DMD cardiomyopathy[44].
To conclude, heart involvement is common in both DMD/BMD and female carriers. Serial cardiac evaluation, including clinical examination, ECG, Holter monitoring, echocardiographic and CMR study, is the “sine qua non” for this population. Early detection of heart involvement should motivate early cardiac treatment with ACE inhibitors and b-blockers to delay serious cardiac complications.
P- Reviewer: Al-Mohammad A, Albacker T S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177-189. [PubMed] |
| 2. | Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919-928. [PubMed] |
| 3. | Bushby KM, Thambyayah M, Gardner-Medwin D. Prevalence and incidence of Becker muscular dystrophy. Lancet. 1991;337:1022-1024. [PubMed] |
| 4. | Emery AE, Skinner R. Clinical studies in benign (Becker type) X-linked muscular dystrophy. Clin Genet. 1976;10:189-201. [PubMed] |
| 5. | Bradley WG, Jones MZ, Mussini JM, Fawcett PR. Becker-type muscular dystrophy. Muscle Nerve. 1978;1:111-132. [PubMed] |
| 6. | Bushby KM, Gardner-Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. J Neurol. 1993;240:98-104. [PubMed] |
| 7. | Hoogerwaard EM, de Voogt WG, Wilde AA, van der Wouw PA, Bakker E, van Ommen GJ, de Visser M. Evolution of cardiac abnormalities in Becker muscular dystrophy over a 13-year period. J Neurol. 1997;244:657-663. [PubMed] |
| 8. | Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646-650. [PubMed] |
| 9. | Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509-517. [PubMed] |
| 10. | Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291-329. [PubMed] |
| 11. | Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710-3714. [PubMed] |
| 12. | Mazur W, Hor KN, Germann JT, Fleck RJ, Al-Khalidi HR, Wansapura JP, Chung ES, Taylor MD, Jefferies JL, Benson DW. Patterns of left ventricular remodeling in patients with Duchenne Muscular Dystrophy: a cardiac MRI study of ventricular geometry, global function, and strain. Int J Cardiovasc Imaging. 2012;28:99-107. [PubMed] |
| 13. | Moriuchi T, Kagawa N, Mukoyama M, Hizawa K. Autopsy analyses of the muscular dystrophies. Tokushima J Exp Med. 1993;40:83-93. [PubMed] |
| 14. | Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation. 1984;69:33-42. [PubMed] |
| 15. | Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375-386. [PubMed] |
| 16. | Silva MC, Meira ZM, Gurgel Giannetti J, da Silva MM, Campos AF, Barbosa Mde M, Starling Filho GM, Ferreira Rde A, Zatz M, Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49:1874-1879. [PubMed] |
| 17. | Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E, Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25:57-63. [PubMed] |
| 18. | Hor KN, Taylor MD, Al-Khalidi HR, Cripe LH, Raman SV, Jefferies JL, O’Donnell R, Benson DW, Mazur W. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 2013;15:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Mavrogeni S, Bratis K, Papavasiliou A, Skouteli E, Karanasios E, Georgakopoulos D, Kolovou G, Papadopoulos G. CMR detects subclinical cardiomyopathy in mother-carriers of Duchenne and Becker muscular dystrophy. JACC Cardiovasc Imaging. 2013;6:526-528. [PubMed] |
| 20. | McNally EM. New approaches in the therapy of cardiomyopathy in muscular dystrophy. Annu Rev Med. 2007;58:75-88. [PubMed] |
| 21. | Ashwath ML, Jacobs IB, Crowe CA, Ashwath RC, Super DM, Bahler RC. Left ventricular dysfunction in duchenne muscular dystrophy and genotype. Am J Cardiol. 2014;114:284-289. [PubMed] |
| 22. | Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:153-161. [PubMed] |
| 23. | Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, Miorin M, Freda MP, Miorelli M, Mostacciuolo ML, Fasoli G. Myocardial involvement is very frequent among patients affected with subclinical Becker’s muscular dystrophy. Circulation. 1996;94:3168-3175. [PubMed] |
| 24. | Politano L, Nigro V, Nigro G, Petretta VR, Passamano L, Papparella S, Di Somma S, Comi LI. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA. 1996;275:1335-1338. [PubMed] |
| 25. | Nolan MA, Jones OD, Pedersen RL, Johnston HM. Cardiac assessment in childhood carriers of Duchenne and Becker muscular dystrophies. Neuromuscul Disord. 2003;13:129-132. [PubMed] |
| 26. | Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, Van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999;353:2116-2119. [PubMed] |
| 27. | Hoogerwaard EM, van der Wouw PA, Wilde AA, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, van Essen AJ, Leschot NJ, de Visser M. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1999;9:347-351. [PubMed] |
| 28. | Grain L, Cortina-Borja M, Forfar C, Hilton-Jones D, Hopkin J, Burch M. Cardiac abnormalities and skeletal muscle weakness in carriers of Duchenne and Becker muscular dystrophies and controls. Neuromuscul Disord. 2001;11:186-191. [PubMed] |
| 29. | Melacini P, Fanin M, Angelini A, Pegoraro E, Livi U, Danieli GA, Hoffman EP, Thiene G, Dalla Volta S, Angelini C. Cardiac transplantation in a Duchenne muscular dystrophy carrier. Neuromuscul Disord. 1998;8:585-590. [PubMed] |
| 30. | Davies JE, Winokur TS, Aaron MF, Benza RL, Foley BA, Holman WL. Cardiomyopathy in a carrier of Duchenne’s muscular dystrophy. J Heart Lung Transplant. 2001;20:781-784. [PubMed] |
| 31. | Weiss RM, Kerber RE, Jones JK, Stephan CM, Trout CJ, Lindower PD, Staffey KS, Campbell KP, Mathews KD. Exercise-induced left ventricular systolic dysfunction in women heterozygous for dystrophinopathy. J Am Soc Echocardiogr. 2010;23:848-853. [PubMed] |
| 32. | Darras BT, Miller DT, Urion DK. Dystrophinopathies. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle, 1993- 2014; . |
| 33. | Santos MA, Costa Fde A, Travessa AF, Bombig MT, Fonseca FH, Luna Filho B, Mussi A, Souza Dd, Oliveira Ad, Povoa R. [Duchenne muscular dystrophy: electrocardiographic analysis of 131 patients]. Arq Bras Cardiol. 2010;94:620-624. [PubMed] |
| 34. | Petri H, Sveen ML, Thune JJ, Vissing C, Dahlqvist JR, Witting N, Bundgaard H, Køber L, Vissing J. Progression of cardiac involvement in patients with limb-girdle type 2 and Becker muscular dystrophies: a 9-year follow-up study. Int J Cardiol. 2015;182:403-411. [PubMed] |
| 35. | Thomas TO, Jefferies JL, Lorts A, Anderson JB, Gao Z, Benson DW, Hor KN, Cripe LH, Urbina EM. Autonomic dysfunction: a driving force for myocardial fibrosis in young Duchenne muscular dystrophy patients? Pediatr Cardiol. 2015;36:561-568. [PubMed] |
| 36. | Fayssoil A, Nardi O, Orlikowski D, Annane D. Cardiac asynchrony in Duchenne muscular dystrophy. J Clin Monit Comput. 2013;27:587-589. [PubMed] |
| 37. | Yamamoto T, Tanaka H, Matsumoto K, Lee T, Awano H, Yagi M, Imanishi T, Hayashi N, Takeshima Y, Kawai H. Utility of transmural myocardial strain profile for prediction of early left ventricular dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol. 2013;111:902-907. [PubMed] |
| 38. | Mori K, Hayabuchi Y, Inoue M, Suzuki M, Sakata M, Nakagawa R, Kagami S, Tatara K, Hirayama Y, Abe Y. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne’s progressive muscular dystrophy. Echocardiography. 2007;24:598-608. [PubMed] |
| 39. | Mavrogeni S, Papavasiliou A, Skouteli E, Magoutas A, Dangas G. Cardiovascular magnetic resonance imaging evaluation of two families with Becker muscular dystrophy. Neuromuscul Disord. 2010;20:717-719. [PubMed] |
| 40. | Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Bécane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855-857. [PubMed] |
| 41. | Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC, Puchalski MD. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol. 2014;35:1279-1285. [PubMed] |
| 42. | Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;16:81. [PubMed] |
| 43. | Giglio V, Puddu PE, Camastra G, Sbarbati S, Della Sala SW, Ferlini A, Gualandi F, Ricci E, Sciarra F, Ansalone G. Patterns of late gadolinium enhancement in Duchenne muscular dystrophy carriers. J Cardiovasc Magn Reson. 2014;16:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Turkbey EB, Gai N, Lima JA, van der Geest RJ, Wagner KR, Tomaselli GF, Bluemke DA, Nazarian S. Assessment of cardiac involvement in myotonic muscular dystrophy by T1 mapping on magnetic resonance imaging. Heart Rhythm. 2012;9:1691-1697. [PubMed] |