Published online Jun 26, 2015. doi: 10.4330/wjc.v7.i6.344
Peer-review started: September 11, 2014
First decision: September 28, 2014
Revised: February 12, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: June 26, 2015
Processing time: 287 Days and 12 Hours
AIM: To evaluate the prognostic value of electrophysiological stimulation (EPS) in the risk stratification for tachyarrhythmic events and sudden cardiac death (SCD).
METHODS: We conducted a prospective cohort study and analyzed the long-term follow-up of 265 consecutive patients who underwent programmed ventricular stimulation at the Luzerner Kantonsspital (Lucerne, Switzerland) between October 2003 and April 2012. Patients underwent EPS for SCD risk evaluation because of structural or functional heart disease and/or electrical conduction abnormality and/or after syncope/cardiac arrest. EPS was considered abnormal, if a sustained ventricular tachycardia (VT) was inducible. The primary endpoint of the study was SCD or, in implanted patients, adequate ICD-activation.
RESULTS: During EPS, sustained VT was induced in 125 patients (47.2%) and non-sustained VT in 60 patients (22.6%); in 80 patients (30.2%) no arrhythmia could be induced. In our cohort, 153 patients (57.7%) underwent ICD implantation after the EPS. During follow-up (mean duration 4.8 ± 2.3 years), a primary endpoint event occurred in 49 patients (18.5%). The area under the receiver operating characteristic curve (AUROC) was 0.593 (95%CI: 0.515-0.670) for a left ventricular ejection fraction (LVEF) < 35% and 0.636 (95%CI: 0.563-0.709) for inducible sustained VT during EPS. The AUROC of EPS was higher in the subgroup of patients with LVEF ≥ 35% (0.681, 95%CI: 0.578-0.785). Cox regression analysis showed that both, sustained VT during EPS (HR: 2.26, 95%CI: 1.22-4.19, P = 0.009) and LVEF < 35% (HR: 2.00, 95%CI: 1.13-3.54, P = 0.018) were independent predictors of primary endpoint events.
CONCLUSION: EPS provides a benefit in risk stratification for future tachyarrhythmic events and SCD and should especially be considered in patients with LVEF ≥ 35%.
Core tip: In our long-term prospective cohort study we could reveal several important findings about the prognostic value of programmed ventricular stimulation for risk stratification of sudden cardiac death (SCD). First, in a mixed population with different cardiac pathologies inducible sustained ventricular tachyarrhythmia during electrophysiological stimulation (EPS) identified those at higher risk for SCD or appropriate implantable cardioverter defibrillators (ICD) activation. Second, left ventricular ejection fraction (LVEF) < 35% was another independent predictor of SCD surrogate. Third, in patients with LVEF > 35% negative EPS had a high negative predictive value for SCD and ICD activation.
- Citation: Hilfiker G, Schoenenberger AW, Erne P, Kobza R. Utility of electrophysiological studies to predict arrhythmic events. World J Cardiol 2015; 7(6): 344-350
- URL: https://www.wjgnet.com/1949-8462/full/v7/i6/344.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i6.344
Implantable cardioverter defibrillators (ICD) are an established therapy for primary and secondary sudden cardiac death (SCD) prevention. Randomized trials have shown a significant mortality reduction in implanted patients at high risk for SCD[1-4]. For ICD therapy guidance, evaluation of SCD risk is crucial. Guidelines recommend various non-invasive techniques to recognize patients at higher risk for life-threatening arrhythmias[5]. Factors associated with a significantly increased risk of ventricular tachyarrhythmia include increased resting heart rate, wide QRS, presence of late potentials, presence of heart failure, or lower left ventricular ejection fraction[6-10]. However, currently available methods for SCD risk estimation are still imprecise. Therefore, many patients, who received an ICD, are not going to suffer from a future arrhythmic event and do not benefit from ICD. On the other hand, many patients, who are not recognized at high risk, die from SCD and numbers of SCD victims are still highest in these patients with normal left ventricular ejection fraction (LVEF)[11,12]. It remains an ongoing challenge to predict an individual patient’s risk.
Currently, the electrophysiological study (EPS) is widely used for risk stratification and several randomized trials suggest a significant predictive value of this examination. However, many previous studies focused on the predictive value in one subgroup of patients with a specific cardiac pathology. Most data are available from post myocardial infarction patients in whom inducible ventricular tachycardias (VT) or ventricular fibrillation (VF) during EPS indicate higher risk for future arrhythmic events[13]. An improved risk stratification with EPS has especially been shown in patients who were preselected as a high risk population based on previous non-invasive tests[14,15]. Other studies provided a prognostic value of EPS in patients with Brugada syndrome, hypertrophic or dilated cardiomyopathy[16-18]. The discussion about the general prognostic value of electrophysiological testing is ongoing. We therefore performed an induction study of VT or VF by programmed electrical stimulation either for primary prevention of SCD, after documented VT or in SCD survivors. The usefulness of EPS for the prediction of future arrhythmic events was evaluated in a prospective long-term follow-up in patients with different cardiac pathologies.
This prospective cohort study evaluated all patients who were examined by EPS at the Luzerner Kantonsspital (Lucerne, Switzerland) between October 2003 and April 2012. Patients underwent EPS for SCD risk evaluation because of structural or functional heart disease and/or electrical conduction abnormality and/or after syncope/cardiac arrest. The study population therefore embraced patients with coronary artery disease (CAD), dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, impaired LVEF, Brugada or Long QT syndrome, and other rare cardiac diseases (e.g., valvular heart disease, tetralogy of Fallot, variant angina). Some patients who underwent EPS did not have cardiac disease but were assessed because of unclear syncope or family history of cardiomyopathy. Patients who did not provide written informed consent were excluded. The study complies with the Declaration of Helsinki and was approved by the local ethics committee.
All participating patients were evaluated at baseline. Patient history was recorded including cardiovascular risk factors, underlying heart disease, and medication. Electrocardiogram (ECG) was recorded in all patients. LVEF was measured with transthoracic echocardiography in all patients and was determined on two- and four-chamber views using the modified biplane Simpson method.
EPS was part of the baseline evaluation and was performed according to the ACC/AHA/ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death[19]. The ventricular arrhythmia induction protocol during EPS included programmed stimulation at three basic cycle lengths (600, 450, and 350 ms) and up to three extrastimuli with a minimum coupling interval of 180 ms. A third extrastimulus was introduced during a basic drive cycle length of minimal 500 ms after completion of programmed ventricular stimulation with 1 and 2 extrastimuli during paced cycle lengths of 600, 450, and 350 ms[20]. EPS was considered abnormal, if a sustained VT was inducible.
Patients with ICD were regularly followed-up at Luzerner Kantonsspital in six-month or yearly intervals. In addition, they were followed-up immediately, if shocks occurred. The clinical course and the numbers of appropriate and inappropriate ICD therapies were protocoled at each follow-up visit. In patients who had no ICD, follow-up was obtained from several sources: first, medical records at Luzerner Kantonsspital were studied, if available (i.e., in patients who were re-admitted after the EPS); second, the patients and/or their general practitioner were contacted by phone and interviewed using a structured protocol. In all patients who died, additional information on the circumstances of death was collected. Death was classified as non-cardiac or cardiac. Among cardiac deaths, SCD was defined according to the Hinkle-Thaler method[21].
The primary endpoint of this study was SCD or, in implanted patients, adequate ICD activation [shock or antitachycardia pacing (ATP)]. The secondary endpoint was SCD or adequate ICD shock. For the secondary endpoint, events with ATP were not counted. If a patient experienced more than one endpoint event (e.g., ICD shock in a patient who later died from SCD), only the first endpoint event was counted.
We descriptively analyzed baseline characteristics. We then calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the receiver operating characteristic curve (AUROC) with its 95%CI of the EPS for the prediction of both endpoints[22]. The diagnostic accuracy of the EPS was compared to that of the LVEF. For this purpose, LVEF was dichotomized at 35% (< 35% indicating higher risk vs≥ 35% indicating lower risk). We also performed a Cox regression analysis with age, sex, EPS and LVEF as independent variables. Kaplan-Meier survivor functions were generated to illustrate the ability to stratify the risk of both dichotomized predictors separately[23]. Calculations were done for all study participants together and, in a sensitivity analysis, repeated separate for participants with CAD and DCM. Data were analyzed using Stata 11.2 (StataCorp LP, College Station, TX, United States).
Overall, 289 patients underwent EPS during the study period. Twenty-four patients (8.3%) were lost to follow-up, resulting in 265 patients who were analyzed. Table 1 shows the baseline characteristics of study participants. Mean age was 57.4 ± 10.7 years with a maximum range from 21.8 to 76.7 years. Most participants were male. CAD was present in a majority of patients.
| Characteristic | All study participants n = 265 | Participants without primary endpoint event1n = 216 | Participants with primary endpoint event1n = 49 | P value2 |
| Age, mean ± SD, yr | 57.4 ± 10.7 | 57.5 ± 10.6 | 57.2 ± 11.3 | 0.848 |
| Male sex | 230 (86.8%) | 185 (85.6%) | 45 (91.8%) | 0.350 |
| Cardiovascular risk factors | ||||
| Hypertension | 152 (57.4%) | 124 (57.4%) | 28 (57.1%) | 0.973 |
| Dyslipidemia | 161 (60.8%) | 131 (60.6%) | 30 (61.2%) | 0.941 |
| Diabetes mellitus | 60 (22.6%) | 49 (22.7%) | 11 (22.4%) | 0.972 |
| Smoking3 | 154 (58.1%) | 121 (56.0%) | 33 (67.3%) | 0.147 |
| Family history of CAD | 81 (30.6%) | 65 (30.1%) | 16 (32.7%) | 0.725 |
| Cardiac disease | ||||
| CAD | 152 (57.4%) | 120 (55.6%) | 32 (65.3%) | 0.213 |
| DCM | 58 (21.9%) | 45 (20.8%) | 13 (26.5%) | 0.384 |
| HCMObstructiveNon-obstructive | 1 (0.4%)3 (1.1%) | 1 (0.5%)2 (0.9%) | 0 (0.0%)1 (2.0%) | 1.0000.460 |
| Brugada syndrome | 2 (0.8%) | 2 (0.9%) | 0 (0.0%) | 1.000 |
| Long QT | 3 (1.1%) | 3 (1.4%) | 0 (0.0%) | 1.000 |
| Other cardiac disease | 30 (11.3%) | 26 (12.0%) | 4 (8.2%) | 0.618 |
| Echocardiography | ||||
| LVEF, mean ± SD | 41.1% ± 15.9% | 42.8% ± 16.2% | 33.6% ± 11.7% | < 0.001 |
| LVEF < 35% | 106 (40.0%) | 79 (36.6%) | 27 (55.1%) | 0.017 |
| EPS | ||||
| Induction of sustained VT | 125 (47.2%) | 91 (42.1%) | 34 (69.4%) | 0.001 |
| Induction of non-sustained VT | 60 (22.6%) | 53 (24.5%) | 7 (14.3%) | 0.122 |
| No VT induction | 80 (30.2%) | 72 (33.3%) | 8 (16.3%) | 0.019 |
The EPS was performed for primary prevention in 209 patients (78.9%). In 56 patients (21.1%) the indication was secondary prevention: twenty-nine cardiac arrest survivors (10.9%), and 27 patients (10.2%) who had documented VT/VF on previous ECGs. During EPS, sustained VT was induced in 125 patients (47.2%) and non-sustained VT in 60 patients (22.6%). In 80 patients (30.2%) no arrhythmia could be induced.
In our cohort, 153 patients (57.7%) underwent ICD implantation after the EPS, and 112 patients (42.3%) received no ICD. Patients were selected for device implantation according to the specific ACC/AHA/ESC guidelines for the underlying heart disease. The decision to implant an ICD was influenced by the result of the EPS if recommended in the guidelines. Antiarrhythmic medication consisted of beta blockers in 214 patients (80.8%), amiodarone in 38 patients (14.3%), and digoxin in 37 patients (14.0%).
The mean duration of the follow-up was 4.8 ± 2.3 years (interquartile range 3.1-6.2 years, maximum range 0.2-9.2 years). During follow-up, 28 patients (10.6%) died, 12 of them due to non-cardiac causes. Among the 16 patients with a cardiac cause of death, SCD occurred in 8 patients. A primary endpoint event occurred in 49 patients (18.5%) with a mean time interval since the EPS of 947 ± 778 d (maximum range 16-3050 d). Table 1 shows baseline characteristics separate for patients with and without primary endpoint event. There were no significant differences between the two groups, except for LVEF which was lower in patients with primary endpoint event, and the findings during EPS that found more sustained VTs in patients with primary endpoint event. A secondary endpoint event occurred in 33 patients (12.5%) with a mean time interval since the EPS of 997 ± 761 d (maximum range 63-2709 d).
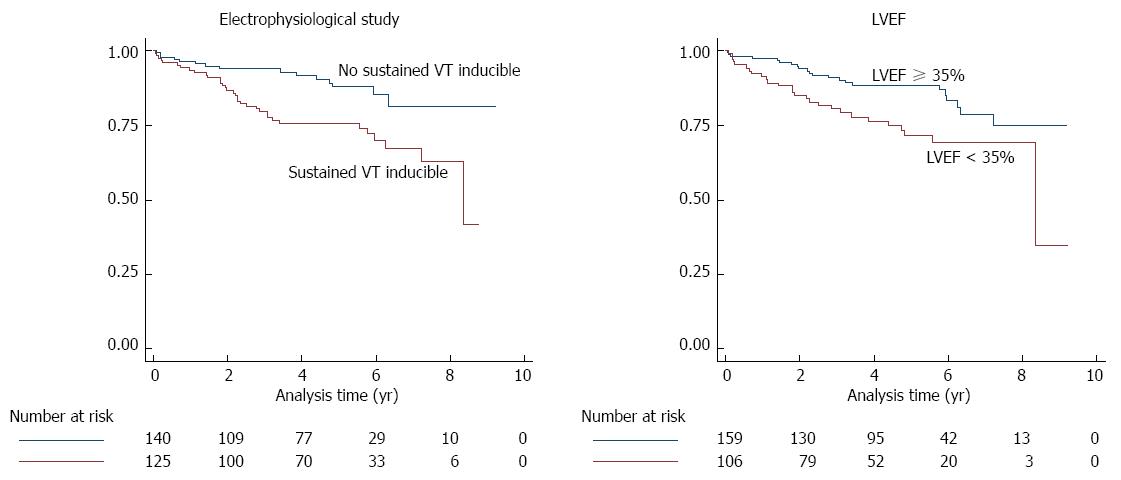
Table 2 shows the diagnostic accuracy of the EPS and the LVEF for primary and secondary endpoint. The AUROCs of EPS and of LVEF did not significantly differ (P = 0.427 for primary endpoint, and P = 0.676 for secondary endpoint). There was a non-significant trend for a higher AUROC of the EPS in the subgroup of patients with an LVEF ≥ 35% as compared to the subgroup of patients with LVEF < 35% (P = 0.156 for primary endpoint, and P = 0.113 for secondary endpoint). Cox regression analysis showed that both, sustained VT during EPS (HR: 2.26, 95%CI: 1.22-4.19, P = 0.009) and LVEF < 35% (HR: 2.00, 95%CI: 1.13-3.54, P = 0.018) were independent predictors of primary endpoint events. Kaplan-Meier survivor functions of EPS and LVEF for the primary endpoint are shown in Figure 1.
| Predictor variable | Sensitivity | Specificity | PPV | NPV | AUROC (95%CI) |
| Primary endpoint | |||||
| Sustained VT during EPS | |||||
| All study participants (n = 265) | 69.4% | 57.9% | 27.2% | 89.3% | 0.636 (0.563-0.709) |
| Subgroup of study participants with LVEF < 35% (n = 106) | 66.7% | 48.1% | 30.5% | 80.9% | 0.574 (0.468-0.680) |
| Subgroup of study participants with LVEF ≥ 35% (n = 159) | 72.7% | 63.5% | 24.2% | 93.5% | 0.681 (0.578-0.785) |
| LVEF < 35% | 55.1% | 63.4% | 25.5% | 86.1% | 0.593 (0.515-0.670) |
| Secondary endpoint | |||||
| Sustained VT during EPS | |||||
| All study participants (n = 265) | 66.7% | 55.6% | 17.6% | 92.1% | 0.611 (0.524-0.699) |
| Subgroup of study participants with LVEF < 35% (n = 106) | 61.1% | 45.5% | 18.6% | 85.1% | 0.533 (0.406-0.660) |
| Subgroup of study participants with LVEF ≥ 35% (n = 159) | 73.3% | 61.8% | 16.7% | 95.7% | 0.676 (0.553-0.798) |
| LVEF < 35% | 54.5% | 62.1% | 17.0% | 90.6% | 0.583 (0.491-0.675) |
Main analysis were repeated separate for participants with CAD and DCM. Among the 152 CAD patients, a primary endpoint event occurred in 32 patients (21.1%). The AUROCs of the EPS (0.604, 95%CI: 0.516-0.693) and of LVEF (0.606, 95%CI: 0.509-0.704) were similar to the overall study population and did not significantly differ (P = 0.975). Among the 58 patients with DCM, a primary endpoint event occurred in 13 patients (22.4%). Due to the low numbers of patients, the AUROC of the EPS (0.625, 95%CI: 0.469-0.781) had a broad 95%CI. The AUROC of LVEF (0.425, 95%CI: 0.268-0.582) was low (P = 0.105 as compared to the AUROC of the EPS).
This long-term prospective cohort study revealed several important findings. First, in a mixed population with different cardiac pathologies inducible sustained ventricular tachyarrhythmia during EPS identified those at higher risk for a SCD surrogate, defined either as appropriate ICD activations and/or as documented SCD. Second, LVEF < 35% was another independent predictor of primary endpoint events. Third, in patients with LVEF > 35% negative EPS had a high negative predictive value for the primary and for the secondary endpoint.
Electrophysiologic testing of ventricular tachycardia was introduced 1972[24]. Amongst others, programmed ventricular stimulation was used to assess the efficacy of antiarrhythmic drugs for suppression of inducible ventricular arrhythmias or the efficacy of antitachycardia surgery[25]. With the availability of ICDs EPS has become an important test for risk stratification to predict SCD[19]. The prognostic value of EPS is based on the assumption that patients with inducible ventricular tachyarrhythmias should have a high likelihood of spontaneous arrhythmic events and that non-inducible patients should be at low risk[26]. In the current guidelines electrophysiologic testing has a class I recommendation for diagnostic evaluation of patient with remote myocardial infaction with symptoms suggestive of ventricular arrhythmias, including palpitations, presyncope and syncope. Another class I indication is syncope of unkown cause with impaired LV function or structural heart disease[19]. In nonischemic DCM electrophysiologic testing is not recommended for risk stratificiation. However, in a recent study of Gatzoulis et al[18] inducibility of VT/VF in patients with idiopathic dilated cardiomyopathy was associated with an increased likelihood of subsequent ICD activation and SCD surrogate. In the present study we have shown, that EPS is useful for the prediction of future arrhythmic events in a collective of patients with different cardiac pathologies. It is well established that the predictive accuracy of LVEF for lifethreatening arrhythmic events is limited[26]. However ICD-Implantation guided by LVEF alone lacks of specificity because in progressive heart failure unexpected SCD accounted for only 30% of deaths while many were due to progressive pump failure not preventable by an ICD.
According to our findings, the additional use of electrophysiologic testing in patients with LVEF < 35% is disputable as the PPV is only improved from 25.5% to 30.5% in cases with a positive EPS. This might not influence the further clinical management of the patient because a 25.5% risk of a future tachyarrhythmic event or SCD already seems to justify ICD implantation. However in patients with LVEF > 35% and a negative EPS the NPV was improved from 86.1% to 93.5%. This means that the risk estimation for future arrhythmic events during the follow-up period of 4.8 ± 2.3 years is reduced from 13.9% to 6.5%. In addition a positive EPS still exhibits a PPV of 24.2% in patients with LVEF > 35%.
Similar results were found in the MUSTT trial[27]. Both, low ejection fraction and inducible sustained ventricular tachycardia during EPS, identified patients at increased mortality risk. Inducible tachyarrhythmias identified patients for whom death was significantly more likely to be arrhythmic and this was observed especially if ejection fraction was higher than or equal to 30%. Due to our findings, invasive testing should especially be considered in this group of higher to normal LVEF, as it might prevent implantation of ICD in patient who won’t benefit.
This study has some limitations. First, the findings of this study are from a single center. Therefore, generalizability is limited and confirmation in an independent sample is of importance. Second, we included patients who underwent EPS for primary prevention as well as for secondary prevention. NPV and PPV of sustained ventricular tachycardia during EPS might differ in these subgroups, however the study population is too small to perform an independent statistical analysis.
EPS provides a benefit in risk stratification for future tachyarrhythmic events and SCD and should especially be considered in patients with LVEF > 35%.
Patients with preexisting cardiac disease are at higher risk for future cardiac arrhythmias, potentially leading to sudden cardiac death (SCD). In the study the authors evaluated the prognostic value of electrophysiological stimulation (EPS) in the risk stratification for future cardiac arrhythmias.
Guidelines recommend various non-invasive techniques to recognize patients at higher risk for life-threatening arrhythmias. Currently, the electrophysiological study (EPS) is widely used for risk stratification and several randomized trials suggest a significant predictive value of this examination.
The authors found that in a mixed population with different cardiac pathologies inducible sustained ventricular tachyarrhythmia during EPS identified those at higher risk for SCD or appropriate activation of implantable cardioverter defibrillator (ICD). Furthermore left ventricular ejection fraction (LVEF) < 35% was another independent predictor of SCD surrogate. In patients with LVEF > 35% negative EPS had a high negative predictive value for SCD and ICD activation.
EPS provides a benefit in risk stratification for future tachyarrhythmic events and SCD and should especially be considered in patients with LVEF > 35%.
The authors have prospectively evaluated the role of programmed ventricular stimulation in the risk stratification of tachyarrhythmic events and sudden cardiac death in a large patient population; the possibility to optimize selection of patients undergoing ICD implantation is very relevant and evidence-based conclusions would be of great clinical importance.
P- Reviewer: Bonanno C, Biyik I, Carbucicchio C, Dizon JM, Lee TM S- Editor: Gong XM L- Editor: A E- Editor: Zhang DN
| 1. | Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940. |
| 2. | Vriesendorp PA, Schinkel AF, Van Cleemput J, Willems R, Jordaens LJ, Theuns DA, van Slegtenhorst MA, de Ravel TJ, ten Cate FJ, Michels M. Implantable cardioverter-defibrillators in hypertrophic cardiomyopathy: patient outcomes, rate of appropriate and inappropriate interventions, and complications. Am Heart J. 2013;166:496-502. |
| 3. | Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158. |
| 4. | Ezekowitz JA, Armstrong PW, McAlister FA. Implantable cardioverter defibrillators in primary and secondary prevention: a systematic review of randomized, controlled trials. Ann Intern Med. 2003;138:445-452. |
| 5. | Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385-e484. |
| 6. | Lelakowski J, Piekarz J, Rydlewska A, Majewski J, Senderek T, Ząbek A, Małecka B. Factors predisposing to ventricular tachyarrhythmia leading to appropriate ICD intervention in patients with coronary artery disease or non-ischaemic dilated cardiomyopathy. Kardiol Pol. 2012;70:1264-1275. |
| 7. | Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6-75. |
| 8. | Schoenenberger AW, Erne P, Ammann S, Gillmann G, Kobza R, Stuck AE. Prediction of arrhythmic events after myocardial infarction based on signal-averaged electrocardiogram and ejection fraction. Pacing Clin Electrophysiol. 2008;31:221-228. |
| 9. | Schoenenberger AW, Kobza R, Jamshidi P, Zuber M, Abbate A, Stuck AE, Pfisterer M, Erne P. Sudden cardiac death in patients with silent myocardial ischemia after myocardial infarction (from the Swiss Interventional Study on Silent Ischemia Type II [SWISSI II]). Am J Cardiol. 2009;104:158-163. |
| 10. | Schoenenberger AW, Schär O, Kobza R, Erne P. Prediction of arrhythmic events by Wedensky modulation in patients with coronary artery disease. Swiss Med Wkly. 2014;144:w13929. |
| 11. | Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204-1209. |
| 12. | Mäkikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762-769. |
| 13. | Bourke JP, Richards DA, Ross DL, Wallace EM, McGuire MA, Uther JB. Routine programmed electrical stimulation in survivors of acute myocardial infarction for prediction of spontaneous ventricular tachyarrhythmias during follow-up: results, optimal stimulation protocol and cost-effective screening. J Am Coll Cardiol. 1991;18:780-788. |
| 14. | Bailey JJ, Berson AS, Handelsman H, Hodges M. Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol. 2001;38:1902-1911. |
| 15. | Schmitt C, Barthel P, Ndrepepa G, Schreieck J, Plewan A, Schömig A, Schmidt G. Value of programmed ventricular stimulation for prophylactic internal cardioverter-defibrillator implantation in postinfarction patients preselected by noninvasive risk stratifiers. J Am Coll Cardiol. 2001;37:1901-1907. |
| 16. | Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003;108:3092-3096. |
| 17. | Fananapazir L, Chang AC, Epstein SE, McAreavey D. Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter, hemodynamic, and electrophysiological findings. Circulation. 1992;86:730-740. |
| 18. | Gatzoulis KA, Vouliotis AI, Tsiachris D, Salourou M, Archontakis S, Dilaveris P, Gialernios T, Arsenos P, Karystinos G, Sideris S. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2013;6:504-512. |
| 19. | Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746-837. |
| 20. | Martínez-Rubio A, Kuschyk J, Sierra G, Breithardt G, Borggrefe M. Programmed ventricular stimulation: influence of early versus late introduction of a third extrastimulus, a randomized, prospective study. Europace. 2002;4:77-85. |
| 21. | Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457-464. |
| 22. | Pregibon D. Logistic regression diagnostics. Ann Statist. 1981;9:705-724. |
| 23. | Lee ET, Wang JW. Statistical methods for survival data analysis, wiley series in probability and statistics. 3rd ed. Hoboken: Wiley-Interscience 2003; . |
| 24. | Wellens HJ, Schuilenburg RM, Durrer D. Electrical stimulation of the heart in patients with ventricular tachycardia. Circulation. 1972;46:216-226. |
| 25. | Horowitz LN, Josephson ME, Farshidi A, Spielman SR, Michelson EL, Greenspan AM. Recurrent sustained ventricular tachycardia 3. Role of the electrophysiologic study in selection of antiarrhythmic regimens. Circulation. 1978;58:986-997. |
| 26. | Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34:1964-1971. |
| 27. | Klein HU, Reek S. The MUSTT study: evaluating testing and treatment. J Interv Card Electrophysiol. 2000;4 Suppl 1:45-50. |