Published online Jun 26, 2014. doi: 10.4330/wjc.v6.i6.449
Revised: March 14, 2014
Accepted: April 17, 2014
Published online: June 26, 2014
Processing time: 179 Days and 16.6 Hours
While clinical data have suggested that the diabetic heart is more susceptible to ischemic heart disease (IHD), animal data have so far pointed to a lower probability of IHD. Thus, the aim of this present review is to look at these conflicting results and discuss the protective mechanisms that conditioned hyperglycemia may confer to the heart against ischemic injury. Several mechanisms have been proposed to explain the cardioprotective action of high glucose exposure, namely, up-regulation of anti-apoptotic factor Bcl-2, inactivation of pro-apoptotic factor bad, and activation of pro-survival factors such as protein kinase B (Akt), vascular endothelial growth factor (VEGF), hypoxia inducible factor-1α and protein kinase C-ε. Indeed, cytosolic increase in Ca2+ concentration, the mitochondrial permeability transition pore, plays a key role in the genesis of ischemic injury. Previous studies have shown that the diabetic heart decreased Na+/Ca2+ and Na+/H+ exchanger activity and as such it accumulates less Ca2+ in cardiomyocyte, thus preventing cardiac injury and the associated heart dysfunctions. In addition, the expression of VEGF in diabetic animals leads to increased capillary density before myocardial infarction. Despite poor prognostic in the long-term, all these results suggest that diabetes mellitus and consequently hyperglycemia may indeed play a cardioprotective role against myocardial infarction in the short term.
Core tip: Hyperglycemia or diabetes triggers a conditioned state that may protect the heart against ischemic injury and associated detrimental effects. These beneficial effects are present in short term diabetes and/or moderate hyperglycemia. The increase in glucose availability, the preferred energy substrate of the heart in stress condition, is likely to be one of the main cardioprotector mechanisms of hyperglycemia. However, other cardioprotective mechanisms seem to be involved, such as the release of cellular survival factors, ions preventing overload and angiogenesis. A fuller understanding of the mechanisms underlying conditioned hyperglycemia is then critical for the development of effective therapeutic strategies against ischemic heart disease.
- Citation: Malfitano C, de Souza Junior AL, Irigoyen MC. Impact of conditioning hyperglycemic on myocardial infarction rats: Cardiac cell survival factors. World J Cardiol 2014; 6(6): 449-454
- URL: https://www.wjgnet.com/1949-8462/full/v6/i6/449.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i6.449
Diabetes type 1 is a chronic disease characterized by hyperglycemia resulting from genetic and environmental factors. Complications of cardiac function are a leading cause of morbidity and mortality in type 1 diabetic patients[1]. Diabetes induces cardiac dysfunction or diabetic cardiomyopathy, regardless of the presence or absence of vascular disease, coronary artery disease, arteriosclerosis and myocardial infarction[2-4].
In hospital environments, glucose and insulin administration are induced in coronary artery bypass grafting patients. This therapy protects the myocardium and inhibits ischemia-induced adenosine monophosphate-activated protein kinase activation[5]. However, intraoperative insulin resistance is associated with increased risk of complications, regardless of the patient’s diabetic state[6].
The increase in mortality in diabetic patients after myocardial infarction remains controversial. Intensive glucose control is widely used in patients with diabetes mellitus and stress-induced hyperglycemia. In this review study, we found that this strategy increases the risk of hypoglycemia, and dangerously increases catecholamine levels with hemodynamic response. Such significant changes may culminate in serious or even fatal cardiovascular events[7].
Elevated admission glucose levels are common in patients with myocardial infarction and are strongly associated with increased mortality. Mortality of hyperglycemic patients was lower in the 1985 to 2008 period when compared to normoglycemic patients. Efforts to establish optimal treatment for these patients remain warranted[8].
Accumulated evidence in clinical studies on diabetic cardiomyopathy suggests increased myocardial infarction and mortality in diabetic patients; however, experimental data regarding the increased resistance of diabetic animals to ischemic injury are quite controversial[9]. Conversely, chronic hyperglycemia is associated with increased incidence of long-term cardiovascular complications, although its effect on acute hyperglycemic response and mortality after acute myocardial infarction remains unclear[10].
One review study suggests that the diabetic heart may be more, equally, or even less susceptible to ischemia-reperfusion injury (novel cardioprotective strategy for the diabetic heart)[11]. Our review study, however, aims at demonstrating the role of conditioned hyperglycemia as a protective mechanism of the heart after ischemic injury and in the preservation of cardiac function.
Several studies have suggested that cardiomyocyte loss in ischemic cardiomyopathy may occur either by necrosis or by apoptosis, without significant inflammatory response[12,13]. This loss has been found to contribute to the decline of the left ventricular function in humans[14,15].
Indeed, experimental studies have shown that the chronic treatment of isolated cardiomyocytes with a high glucose content medium increased the rate of cell death[16]. In contrast, exposure to short periods of a high glucose medium or diabetes has been found to protect the heart against a variety of pathological insults, including ischemia, hypoxia, and calcium overload[17-19]. Several mechanisms have been proposed to explain the cardioprotective role of high glucose exposure, such as up-regulation of antiapoptotic factor Bcl-2, inactivation of proapoptotic factor Bad, and activation of prosurvival factors[17,20].
To investigate the mechanisms behind improved cardiac function (accompanied by a reduction in lesion area) in diabetic rats (30 d of hyperglycemia) undergoing myocardial infarction (15 d), we evaluated the gene expression regulating cardiac cellular survival factors: Bax, Fas, Bcl-2 e p53. In fact, gene expression was increased in diabetic animals after myocardial infarction, suggesting that the pro and anti apoptotic pathways can be activated simultaneously in this condition; this hypothesis was further strengthened by increased caspase-3 activity. These findings suggest an increased cell turnover acting to preserve cardiac function and reduce tissue injury[21].
Cell survival factors can be activated by increased Bcl-2, as the up-regulation of Bcl-2 in some cells prevents excessive accumulation of calcium by mitochondria[22], thus favoring cell survival. In this tissue, although calcium overload may be induced by ischemia, the association with hyperglycemia appears to reduce the activity of the Na+/Ca2+ exchanger[23].
Lending support to these findings, a study showed a reduction in protein expression of the Na+/Ca2+ exchanger in diabetic infarcted heats, which might contribute to mitochondrial disruption and contracture, inducing structural damage[24]. In fact, the improvement in cardiac function in diabetic infarcted rats may be associated with the protective effect of Bcl-2, which abolishes the damage caused by the accumulation of calcium in the heart of diabetic rats.
Cytosolic Ca2+ overload during ischemia may be due to Ca2+ entry by reverse-mode of Na+/Ca2+ exchanger (NCX) secondary to the rise in Na+ concentration. During ischemia, the anaerobic metabolism increases proton generation, which is extruded from the cell by Na+/H+ exchanger (NHE), resulting in increased cytosolic Na+ concentration[25]. This activates the reverse-mode of NCX exchanger, which in turn promotes an increase in Ca2+ concentration in the cardiomyocyte[26]. Research has suggested that Na+/H+ exchange activity is decreased in diabetic hearts[27]. Therefore, Ca2+ accumulation in the diabetic is lower than in the non-diabetic ischemic heart.
Several factors are related to cell survival: hypoxia inducible factor-1α (HIF-1α) is a transcription factor expressed in response to a decreased partial pressure of oxygen, and it is able to activate genes involved in angiogenesis, such as vascular endothelial growth factor (VEGF)[28]. As a result of diabetic hyperglycemia, these survival factors were increased in diabetic animals before and after myocardial infarction[21].
Interestingly, the expression of VEGF was also elevated before myocardial infarction in diabetic animals, and results were similar to the observed in interleukin 8 (IL-8) gene, i.e., chemokine regulating neutrophil influx and activation with angiogenic propriety[29-31]. IL-8 plays an important role in the recruitment of granulocytes in the infarcted myocardium, increasing cell adhesion (integrin) and activating the signaling pathways of cell survival mitogen-activated protein kinase and protein kinase C (PKC), which contribute to angiogenesis[32]. Ooie et al[33] have found that administration of streptozotocin for 12 wk in rats leads to increased tolerance to ischemic injury in an isolated heart model. These researchers also observed the translocation of protein kinase C-ε (PKC-ε) from the cytosol to the sarcolemal membrane, where the protein is activated. PKC-ε is a KATP channel opener in both the sarcolemal and mitochondrial membrane[34]. Opening mitochondrial KATP channel during ischemia stabilizes mitochondrial potential, reduces mitochondrial Ca2+ overload, prevents ATP depletion, and the generation of reactive oxygen species[34,35].
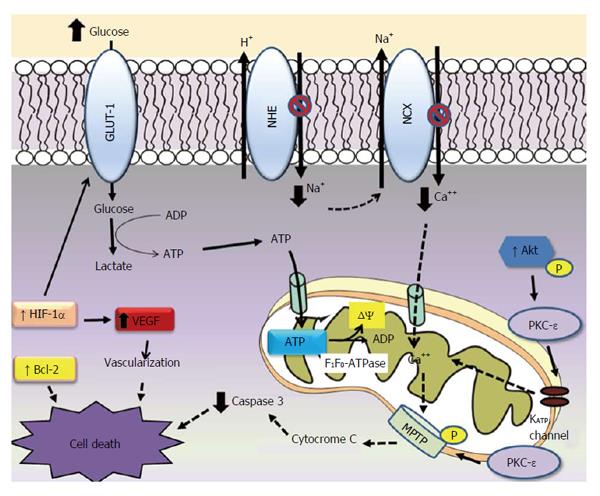
Mitochondrial permeability transition pore (MPTP) is a downstream of PKC-ε[36], which indicates that PKC interacts with MPTP, leading to phosphorylation of MPTP, and inhibits Ca2+ induced MPTP opening. Opening MPTP allows water and solutes to enter the mitochondria, increasing matrix volume and rupturing of the outer mitochondrial membrane. This results in the release of intermembrane cytochrome c, which can trigger apoptosis (Figure 1).
In this scenario, since hyperglycemia results in an increase of survival factors and induces angiogenesis, this may be interpreted as responses to repeated insults which eventually determine an ischemic conditioning in diabetic rats. These responses are strongly associated with improved left ventricle (LV) function observed after ischemic injury, suggesting the presence of a physiological mechanism of protection against heart damage.
Cardiac repair after myocardial infarction is dependent on the activation of tumor necrosis factor alpha (TNF-α), IL-1β and IL-6 cytokines, which results in leukocyte recruitment to the infarcted area[37]. In consequence, the immune imbalance between pro-inflammatory and anti-inflammatory properties can be modified in favor of more or less inflammatory factors, depending on the time course of the progression of heart failure. In this regard, changes in the concentration of TNF-α may have different effects on all the cell types involved in cardiac injury and repair, and in the suppression of cardiac contractility[38] to improve cardiomyocyte apoptosis [39].
In fact, Malfitano et al[21] have found a reduction of TNF-α in diabetic rats after myocardial infarction. The signaling of IL-1β is crucial for the activation of inflammatory and fibrogenic pathways in the healing of myocardial infarction, and it may play a role in the pathogenesis of post-infarction remodeling[40]. Moreover, the induction of members of the IL-6 family leads to a rapid recruitment of mononuclear cells and cardiomyocyte ischemic myocardium[41], thus indicating that the concentration of IL-6 was increased only in infarcted rats, but remained unchanged in diabetic animals after ischemic injury.
These three pro-inflammatory cytokines are not only associated with the inflammatory response, but are also involved in heart failure, cardiomyopathy and LV remodeling, suggesting that the reduction of inflammatory factors may be one of the mechanisms responsible for improved heart function observed in this group. These findings corroborate a previous study of our group, in which it was demonstrated that hyperglycemia in mice and in cell culture is capable of suppressing the expression of pro-inflammatory mediators by apoptosis of neutrophils and lymphocytes[42,43]. In fact, a high proportion of apoptotic lymphocytes in diabetic states strengthen the hypothesis that immune function is impaired in patients with poorly controlled diabetes[42].
Another result which is in line with our findings is the increased expression of glucose transporter type-1 (GLUT-1) in diabetic rats after myocardial infarction. Indeed, previous studies have shown that the supply of glucose, with the regulation of GLUT-1, plays a critical role in cardioprotective response to myocardial ischemia[21], with increased glucose supply during the acute ischemia[44,45], and progression to heart failure[46].
This is likely a result of increased availability and use of glucose, the preferred energy substrate of the heart in times of stress. Thus, the current clinical practice of tightly controlling blood glucose in patients having cardiac events may be detrimental to the heart in the acute setting[47].
Much of the ATP generated by anaerobic glycolysis is consumed for the maintenance of ion gradient thought membranes. Part of the ATP generated is hydrolyzed by reverse mode of the mitochondrial F1F0-ATPase, which uses the energy to generate mitochondrial membrane potential (ΔΨ)[48] (Figure 1).
Finally, the increase in survival pathways such as Bcl2, PKC-ε, Akt and in capillary density may effectively contribute to the reduction of ischemic injury and cardiac fibrosis (modulation of cardiac fibroblasts) in diabetic animals. This might be the key to a better heart function, as the increased GLUT-1 expression plays an important role in increasing glucose uptake in ischemic conditions. The clinical importance of the deficiency of glucose in the treatment of heart failure is not necessarily highlighted when blood glucose control is the pursued goal of treatment. In the DIGAMI II study reported on 1253 diabetic patients with acute myocardial infarction allocated to three treatment arms including acute insulin-glucose infusion followed by insulin-based long-term glucose control (group 1), insulin-glucose infusion followed by standard glucose control (group 2), and routine metabolic management according to local practice (group 3) that neither all-cause mortality nor morbidity (stroke and non-fatal reinfarctions) differed between the three groups[12].
The compensatory mechanism associated with the positive balance of regulatory genes related to program cell survival, reduction of inflammatory cytokines, and increased glucose use as energy substrate. Taken together, they promote greater plasticity and improved cellular resistance to ischemic injury in short term, suggesting an ischemic conditioning in hyperglycemia. These findings should be translated into more effective patient care strategies following ischemic events. Therefore, future studies should be conducted to further elucidate the mechanisms underlying conditioned hyperglycemia in cardioprotection after ischemia.
Possible cardioprotector mechanisms of conditionied hyperglycaemia or diabetes against ischemia and reperfusion injuries. Hyperglicaemia seems to be cardioprotective due to the increased glucose provision to heart during stress. In the ischaemia condition much of the ATP generated by glycolysis is breakdown by reverse mode of the mitochondrial F1F0-ATPase, wich uses the energy to maintain mitochondrial potential (ΔΨ). Diabetic heart accumulates less Ca2+ due the inhibition NCX and NHE exchange activities. PKC-ε activity increases in diabetes, activating mitochondrial KATP channel and closing MPTP in the mitochondrial outer membrane. These effects reduce calcium overload, increasing ATP production and decreasing cytochrome C from mitochondria during ischemia. Hyperglycaemia increases anti apoptotic Bcl-2 protein and reduces caspase-3 activity. The contents of HIF-1α mRNA and protein increase in diabetic heart. HIF-1α target genes which in turn improve cellular oxygenation (VEGF) and glucose metabolism (GLUT-1).
P- Reviewers: Sakabe K, Xanthos T S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 725] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 2. | Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791-2795. [PubMed] |
| 3. | Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab. 2007;292:E654-E667. [PubMed] |
| 4. | An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489-H1506. [PubMed] |
| 5. | Carvalho G, Pelletier P, Albacker T, Lachapelle K, Joanisse DR, Hatzakorzian R, Lattermann R, Sato H, Marette A, Schricker T. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. J Clin Endocrinol Metab. 2011;96:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95:4338-4344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Rana OA, Byrne CD, Greaves K. Intensive glucose control and hypoglycaemia: a new cardiovascular risk factor? Heart. 2014;100:21-27. [PubMed] |
| 8. | Deckers JW, van Domburg RT, Akkerhuis M, Nauta ST. Relation of admission glucose levels, short- and long-term (20-year) mortality after acute myocardial infarction. Am J Cardiol. 2013;112:1306-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ravingerová T, Neckár J, Kolár F. Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem. 2003;249:167-174. [PubMed] |
| 10. | Cao JJ, Hudson M, Jankowski M, Whitehouse F, Weaver WD. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol. 2005;96:183-186. [PubMed] |
| 11. | Whittington HJ, Babu GG, Mocanu MM, Yellon DM, Hausenloy DJ. The diabetic heart: too sweet for its own good? Cardiol Res Pract. 2012;2012:845698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650-661. [PubMed] |
| 13. | Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA. 1999;96:8144-8149. [PubMed] |
| 14. | Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296-306. [PubMed] |
| 15. | Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation. 1996;94:2285-2296. [PubMed] |
| 16. | Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363-2375. [PubMed] |
| 17. | Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000;278:H1948-H1954. [PubMed] |
| 18. | Xu G, Takashi E, Kudo M, Ishiwata T, Naito Z. Contradictory effects of short- and long-term hyperglycemias on ischemic injury of myocardium via intracellular signaling pathway. Exp Mol Pathol. 2004;76:57-65. [PubMed] |
| 19. | Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178-C187. [PubMed] |
| 20. | Ma G, Al-Shabrawey M, Johnson JA, Datar R, Tawfik HE, Guo D, Caldwell RB, Caldwell RW. Protection against myocardial ischemia/reperfusion injury by short-term diabetes: enhancement of VEGF formation, capillary density, and activation of cell survival signaling. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:415-427. [PubMed] |
| 21. | Malfitano C, Alba Loureiro TC, Rodrigues B, Sirvente R, Salemi VM, Rabechi NB, Lacchini S, Curi R, Irigoyen MC. Hyperglycaemia protects the heart after myocardial infarction: aspects of programmed cell survival and cell death. Eur J Heart Fail. 2010;12:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511-6519. [PubMed] |
| 23. | Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: the sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res. 1997;34:113-120. [PubMed] |
| 24. | Rodrigues B, Rosa KT, Medeiros A, Schaan BD, Brum PC, De Angelis K, Irigoyen MC. Hyperglycemia can delay left ventricular dysfunction but not autonomic damage after myocardial infarction in rodents. Cardiovasc Diabetol. 2011;10:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991;68:1250-1258. [PubMed] |
| 26. | Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1161] [Cited by in RCA: 1121] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 27. | Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med. 1998;131:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1507] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 30. | Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391-398. [PubMed] |
| 31. | Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr Opin Hematol. 2000;7:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Takami M, Terry V, Petruzzelli L. Signaling pathways involved in IL-8-dependent activation of adhesion through Mac-1. J Immunol. 2002;168:4559-4566. [PubMed] |
| 33. | Ooie T, Takahashi N, Nawata T, Arikawa M, Yamanaka K, Kajimoto M, Shinohara T, Shigematsu S, Hara M, Yoshimatsu H. Ischemia-induced translocation of protein kinase C-epsilon mediates cardioprotection in the streptozotocin-induced diabetic rat. Circ J. 2003;67:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Wang Y, Ashraf M. Role of protein kinase C in mitochondrial KATP channel-mediated protection against Ca2+ overload injury in rat myocardium. Circ Res. 1999;84:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. Am J Physiol Heart Circ Physiol. 2001;281:H1295-H1303. [PubMed] |
| 36. | Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 506] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 38. | Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 522] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 39. | Engel D, Peshock R, Armstong RC, Sivasubramanian N, Mann DL. Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression. Am J Physiol Heart Circ Physiol. 2004;287:H1303-H1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Pithon-Curi TC, De Melo MP, Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: a comparative study. Cell Biochem Funct. 2004;22:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, Sannomiya P. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 44. | King LM, Opie LH. Glucose and glycogen utilisation in myocardial ischemia--changes in metabolism and consequences for the myocyte. Mol Cell Biochem. 1998;180:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, Eberli FR. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation. 2000;101:2090-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res. 2001;52:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Chu LM, Osipov RM, Robich MP, Feng J, Oyamada S, Bianchi C, Sellke FW. Is hyperglycemia bad for the heart during acute ischemia? J Thorac Cardiovasc Surg. 2010;140:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |