Published online Jun 26, 2014. doi: 10.4330/wjc.v6.i6.353
Revised: March 1, 2014
Accepted: May 8, 2014
Published online: June 26, 2014
Processing time: 206 Days and 15.1 Hours
Essential hypertension is a highly prevalent pathological condition that is considered as one of the most relevant cardiovascular risk factors and is an important cause of morbidity and mortality around the world. Despite the fact that mechanisms underlying hypertension are not yet fully elucidated, a large amount of evidence shows that oxidative stress plays a central role in its pathophysiology. Oxidative stress can be defined as an imbalance between oxidant agents, such as superoxide anion, and antioxidant molecules, and leads to a decrease in nitric oxide bioavailability, which is the main factor responsible for maintaining the vascular tone. Several vasoconstrictor peptides, such as angiotensin II, endothelin-1 and urotensin II, act through their receptors to stimulate the production of reactive oxygen species, by activating enzymes like NADPH oxidase and xanthine oxidase. The knowledge of the mechanism described above has allowed generating new therapeutic strategies against hypertension based on the use of antioxidants agents, including vitamin C and E, N-Acetylcysteine, polyphenols and selenium, among others. These substances have different therapeutic targets, but all represent antioxidant reinforcement. Several clinical trials using antioxidants have been made. The aim of the present review is to provide new insights about the key role of oxidative stress in the pathophysiology of essential hypertension and new clinical attempts to demonstrate the usefulness of antioxidant therapy in the treatment of hypertension.
Core tip: This review focuses on one of the most prevalent diseases worldwide: hypertension, providing new insights about the key role of oxidative stress in the pathophysiology of essential hypertension and new clinical attempts to demonstrate the usefulness of antioxidant therapy in its treatment.
- Citation: González J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: New insights. World J Cardiol 2014; 6(6): 353-366
- URL: https://www.wjgnet.com/1949-8462/full/v6/i6/353.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i6.353
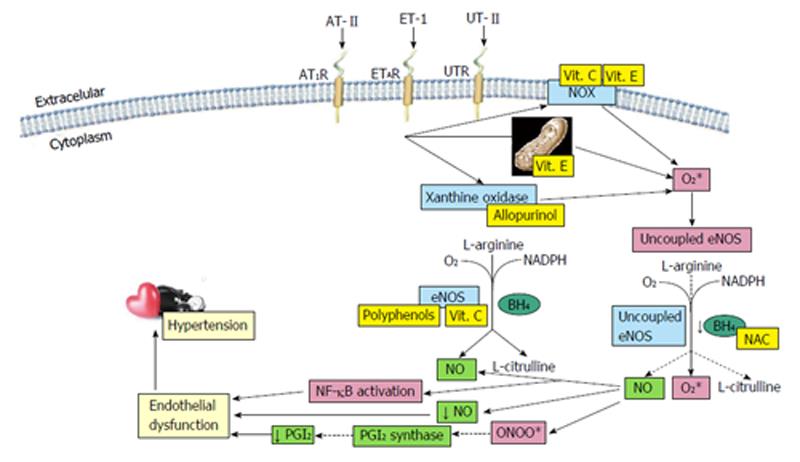
Hypertension is considered the most important risk factor for the occurrence of cardiovascular disease[1]. Oxidative stress has gained attention as one of the fundamental mechanisms responsible for the development of hypertension. Reactive oxygen species (ROS) have an important role in the homeostasis of the vascular wall, hence they could contribute to the mechanism of hypertension[2-4]. Thus, increased ROS production, and reduced nitric oxide (NO) and antioxidants bioavailability were demonstrated in experimental and human hypertension. Vascular superoxide is derived primarily from NADPH oxidase (NOX) when stimulated by hormones such as angiotensin II (AT-II), endothelin-1 (ET-1) and urotensin II (UT-II), among others. In addition, increased ROS production may be generated by mechanical forces, which increase with hypertension. ROS-induced vasoconstriction results from increased intracellular calcium concentration, thereby contributing to the pathogenesis of hypertension[2]. Vasomotor tone is dependent upon a delicate balance between vasoconstrictor and vasodilator forces resulting from the interaction of the components of the vascular wall and the blood, and both of them can be altered by oxidative stress. These findings have stimulated the interest on antihypertensive therapies targeted to decrease ROS generation and/or increase NO bioavailability. This review examines the available studies pointing to a role of oxidative stress in the mechanism of production of high blood pressure, as well as the use of antioxidants in the prevention or treatment of this disorder.
Endothelial dysfunction has been implicated in the pathophysiology of different forms of cardiovascular disease, including hypertension. It may be defined as impairment characterized by a shift of the actions of the endothelium toward reduced vasodilation, a proinflammatory state, and prothrombotic setting. These events lead to a state of vascular inflammation, which may be mediated, partly, by ROS formed by activated mononuclear cells.
Oxidative stress constitutes a unifying mechanism of injury of many types of disease processes, it occurs when there is an imbalance between the generation of ROS and the antioxidant defense systems in the body. The ROS family comprises many molecules that have divergent effects on cellular function. Importantly, many of these actions are associated with pathological changes observed in cardiovascular disease. The effects of ROS are mediated through redox-sensitive regulation of multiple signaling molecules and second messengers[5-7]. Several studies have demonstrated that essential hypertensive patients and various animal models of hypertension produce excessive amount of ROS[8-12], and have abnormal levels of antioxidant status[13], thereby contributing to the accumulating evidence that increased vascular oxidative stress could be involved in the pathogenesis of essential hypertension[2,3,14]. Recently, it was demonstrated a strong association between blood pressure and some oxidative stress-related parameters[15]. Other studies show that mouse models with genetic deficient in ROS-generating enzymes have lower blood pressure compared with wild-type counterparts[16,17]. In addition, in cultured vascular smooth muscle cells (VSMC) and isolated arteries from hypertensive rats and humans, ROS production is enhanced, redox-dependent signaling is amplified, and antioxidant bioactivity is reduced[18]. Classical antihypertensive agents such as β-adrenergic blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor antagonists, and calcium channel blockers may be mediated, in part, by decreasing vascular oxidative stress[19,20].
A variety of enzymatic and non-enzymatic sources of ROS exist in blood vessels. The best characterized source of ROS is NOX. In addition to NOX, several other enzymes may contribute to ROS generation, including xanthine oxidase, NO synthase and the mitochondrion.
NOX: NOX is the primary biochemical source of ROS in the vasculature, particularly of superoxide. The kidney and vasculature are rich sources of NOX-derived ROS, which under pathological conditions play an important role in renal dysfunction and vascular damage[12,21]. This system catalyses the reduction of molecular oxygen by NADPH as electron donor, thus generating superoxide. NOX is up-regulated in hypertension by humoral and mechanical signals. AT-II is the most studied stimulus, but ET-1 and UT-II may also participate in activation of NOX, thereby resulting in increased ROS. Likely the most well-known function of NOX derived superoxide is inactivation of NO to form peroxynitrite, leading to impaired endothelium dependent vasodilation and uncoupling of endothelial nitric oxide synthase (eNOS) to produce additional superoxide[16,22]. In the vasculature, NOX activation has been strongly associated with hypertension[23].
Uncoupled endothelial NO synthase: The primary function of eNOS is NO production which regulates vasodilation. Nevertheless, L-arginine and tetrahydrobiopterin (BH4)-two essential cofactors for its action-deficiency or oxidation are associated with uncoupling of the L-arginine-NO pathway resulting in decreased formation of NO, and increased eNOS-mediated generation of superoxide. NOX is the initial source of ROS. Superoxide combines with NO, which is synthesized by eNOS, to form peroxynitrite[24]. In turn, peroxynitrite oxidizes and destabilizes eNOS to produce more superoxide[22,25]. Superoxide also leads to BH4 oxidation (in fact, BH4 is highly sensitive to oxidation), which promotes eNOS uncoupling and ROS production.
Xanthine oxidase: Xanthine oxidase is also an important source for oxygen free radical present in the vascular endothelium[23,26]. It catalyzes the last two steps of purine metabolism. During this process oxygen is reduced to superoxide. There is evidence suggesting involvement of this enzyme in hypertension. Spontaneously hypertensive rats demonstrate elevated levels of endothelial xanthine oxidase and increased ROS production, which is associated with increased arteriolar tone[21]. In addition to effects on the vasculature, xanthine oxidase may play a role in end-organ damage in hypertension[27].
Mitochondrion: The mitochondrion is a major source and target of ROS. Part of the superoxide produced in the intermembrane space may be carried to the cytoplasm[28]. Ubiquinol or coenzyme Q is a source of superoxide when partially reduced (semiquinone form) and an antioxidant when fully reduced[29]. Complex I produces most of the superoxide generated by mammalian mitochondria in vitro. Complexes II and IV are not normally significant sites of ROS production. Mild uncoupling very effectively decreases the high superoxide production that occurs from complex I. A reduction in antioxidant enzymatic activity in patients with hypertension has been reported[30].
The endothelium senses mechanical and hormonal stimuli. In response, it releases agents that regulate vasomotor function. There is no doubt that endothelium plays a regulatory and protective role by generating vasorelaxing substances. Under some pathophysiological circumstances, endothelium derived vasoconstricting factors, such as ET-1, AT-II, UT-II, superoxide anions, vasoconstrictor prostaglandins and thromboxane A2, can be released and contribute to the paradoxical vasoconstrictor effects. VSMC are fit not only for short-term regulation of the blood vessel diameter and therefore of blood pressure, but also for long-term adaptation, via structural remodeling. ROS mediate many of these pathophysiological processes. The adventitia can contribute to hypertension by either reducing NO bioavailability or participating in vascular remodeling through ROS.
NO: NO is known to play an important role as a key paracrine regulator of vascular tone. Physiologically, NO inhibits leukocyte-endothelial cell adhesion, VSMC proliferation and migration, and platelet aggregation to maintain the health of the vascular endothelium. Therefore it has many beneficial effects. The decrease in bioavailability of NO in the vasculature reduces vasodilatory capacity and contributes to hypertension. The enzyme that catalyzes the formation of NO from oxygen and arginine is NOS, which in fact is a whole family of enzymes. eNOS is the predominant NOS isoform in the vessel wall. Receptor-mediated agonist stimulation leads to rapid enzyme activation. In addition, shear stress and allosteric modulators are also an important modulator of eNOS activity[31]. Except the vasorelaxing and antiproliferative properties per se, NO plays an important role in antagonizing the effects of AT-II, endothelins and ROS. Nitric oxide diffuses as a gas to the adjacent smooth muscle where it interacts with different receptor molecules such as the soluble guanylyl cyclase. It is accepted that the normal production of NO plays a crucial role in the maintenance of the physiologic conditions within the cardiovascular system. L-arginine, a substrate for eNOS, seems to be promising in preserving NO formation. However, L-arginine failed to prevent blood pressure increase and left ventricle remodeling due to chronic treatment with L-methyl ester of N-nitro-L-arginin (NAME), an inhibitor of eNOS[32]. The ACE inhibitor captopril completely prevented NO-deficient hypertension, yet without improving NOS activity. NO also has an ACE down-regulation effect. Thiols protect NO from oxidation by scavenging oxygen-free radicals and by forming nitrosothiols, both effects prolonging NO half-life and duration of NO action[33,34]. Reduced NO levels can be attributed to elevated levels of ROS. Superoxide combines with NO to form peroxynitrite that oxidizes BH4 and destabilizes eNOS to produce more superoxide[22,24,25] thus further enhancing the development of oxidative stress. The balance between NO and AT-II in the vasomotor centers seems to play important role in the regulation of the sympathetic tone.
Renin-angiotensin system: The renin-angiotensin system plays a key role in the development of cardiovascular disease. AT-II is a potent vasoactive peptide that can be formed in vascular beds rich in ACE. When AT-II production increases above normal levels, it induces vascular remodeling and endothelial dysfunction in association with increases in levels of blood pressure. As a potent activator of NOX, AT-II contributes to the production of ROS[35,36]. In rats and mice made hypertensive by AT-II infusion, expression of NOX subunits, oxidase activity, and generation of ROS are all increased[37]. AT-II not only increases NOX activity but also upregulates superoxide dismutase activity, possibly to compensate for the increased ROS. In situations where this compensatory effect is efficient, ROS levels may appear normal even in the face of prooxidant. However, when ROS production becomes overwhelming, compensatory mechanisms are inadequate and pathophysiological consequences ensue[38]. Captopril and enalapril prevented blood pressure rise in young spontaneously hypertensive rats inhibiting ACE. Captopril, probably due to the antioxidant role of its thiol group, had more effective hypotensive effect than enalapril[39,40]. In contrast, NO not solely antagonizes the effects of AT-II on vascular tone, cell growth, and renal sodium excretion, but also down-regulates the synthesis of ACE and AT1 receptors. On the other hand, ACE inhibition up-regulates eNOS expression. The ability of AT-II to induce endothelial dysfunction is also due to its ability to down-regulate soluble guanylyl cyclase, thereby leading to impaired NO/cGMP signaling. Recently, it has been proposed that Ca2+/calmodulin-dependent protein kinase II is an important molecule linking AT-II, ROS and cardiovascular pathological conditions[41].
Acetylcholine: In vascular vessels, acetylcholine induces endothelium-dependent dilation via production of endothelial factors, mainly NO. NO then diffuses to underlying VSMC, where it induces vascular smooth muscle cell relaxation. The diminution in NO bioavailability will lead to significantly reduced acetylcholine-mediated vasodilation[39,40]. The consequence of an overall increase in ROS is a reduce bioavailability of NO.
ET-1: Endothelins are potent vasoconstrictor isopeptides produced in different vascular tissues, including vascular endothelium. ET-1 is the main endothelin generated by the endothelium and the most important in the cardiovascular system. When ET-1 is administered in large concentrations, it behaves as a potent vasoconstrictor capable of exerting an array of physiological effects, including the potential to alter arterial pressure. ET-1 mediates its effects through two receptors, ETA and ETB. ETA mediates contractions via activation of NOX, xanthine oxidase, lipoxygenase, uncoupled NO synthase, and mitochondrial respiratory chain enzymes. The ETB induces relaxation on endothelial cells[42]. Many factors that normally stimulate ET-1 synthesis, (e.g., thrombin, AT-II) also cause the release of vasodilators such as prostacyclin (PGI2) and/or NO, which oppose the vasoconstricting action of ET-1. It was reported that essential hypertension is characterized by increased ET-1 vasoconstrictor tone, an effect that seems to be dependent on decreased endothelial ETB-mediated NO production attributable to the impaired NO bioavailability.
UT-II: UT-II is a potent vasoactive peptide[43], indeed the most potent vasoconstrictor identified. It acts trough activation of NOX. The role of UT-II in disease is not well elucidated. The constrictor response to UT-II appears to be variable and highly dependent on the vascular bed examined. Vasoconstriction is not its only effect, because UT receptors have been found in other organs[44,45]. UT-II has also been shown to act as a potent vasodilator in some isolated vessels[46].
Norepinephrine: VSMC is innervated primarily by the sympathetic nervous system through adrenergic receptors. Three types of adrenoceptors are present within VSMC: α1, α2 and β2. Norepinephrine stimulates VSMC proliferation. In addition, over-expression of inducible NOS increases blood pressure via central activation of the sympathetic nervous system, which is mediated by an increase in oxidative stress[5].
Prostaglandins: PGI2, another endothelium-dependent vasodilator, relaxes the VSMC. PGI2 is released in higher amount in response to ligand binding such as thrombin, arachidonic acid, histamine, or serotonin. The enzymes prostaglandin H2 synthase uses arachidonic acid as a substrate, forming prostaglandin H2. Prostaglandin H2 is converted to vasoactive molecules, such as PGI2. The isoform prostaglandin H2 synthase-2 may mediate vascular dysfunction in conditions characterized by oxidative stress. Thus, peroxynitrite inhibits the enzymatic activity of prostacyclin synthase, thereby causing impairment in the PGI2-mediated vasodilation.
Homocysteine: This molecule may play an important role in the pathogenesis of essential hypertension[3]. Elevated homocysteinemia diminishes the vasodilation by nitric oxide, increases oxidative stress, stimulates the proliferation of VSMC, and alters the elastic properties of the vascular wall. Thus, homocysteine contributes to elevate the blood pressure[47]. It is also known that elevated homocysteine levels could lead to oxidant injury of the endothelium[3]. The correction of elevated homocysteinemia by administration of vitamins B12 and B6 plus folic acid, could be a useful adjuvant therapy of hypertension[3,48]. However, further controlled randomized trials are necessary to establish the efficacy of these therapeutic agents.
A hypothesis for the role of vascular oxidative stress in hypertension is depicted in Figure 1.
This review has discussed the importance of ROS in the vasculature and its relation to hypertension, but it is important to emphasize the evidence that hypertensive stimuli, such as high salt and AT-II, promote the production of ROS not only in the vasculature, but also in kidney and the central nervous system (CNS) and that each of these sites contributes either to hypertension or to the untoward sequels of this disease[48].
Evidence proposes that ROS play a key role in the pathophysiological processes of several renal diseases; these diseases are considered to be cause and consequence of hypertension. Regarding glomerular alterations, ROS mediates lipoprotein glomerulopathy and other inflammatory glomerular lesions[49]. A recent study demonstrates that NOX activation and production of ROS through lipid raft clustering is an important molecular mechanism triggering oxidative injury of podocytes induced by homocysteine. This may represent an early event initiating glomerulosclerosis during hyperhomocysteinemia[50]. Concerning ROS mediated tubulointerstitial injury, one of the mechanisms is the exposure of tubular cells to low-density lipoproteins which may result in tubulointerstitial damage due to ROS production mediated by NOX[51]. AT-II also plays a pivotal role in the progression of tubulointersitial injury but also in obstructive nephropathy[52,53]. It activates NOX and, subsequently, generates superoxide that leads to hypertrophy of renal tubular cells[54].
There is evidence suggesting that a high-fat diet induces renal inflammation and aggravation of blood pressure in spontaneously hypertensive rats, via ROS[55]. It is also known that the metabolic syndrome is a risk factor for chronic kidney disease (CKD) at least in part independent of diabetes and hypertension per se, probably mediated by ROS. Moreover, the onset and maintenance of renal damage may worsen metabolic syndrome features like hypertension, leading to potential vicious cycles[56].
There are several oxidative stress-mediated mechanisms involved in endothelial dysfunction in CKD[57]. ROS are elevated in CKD and related to endothelium-dependent vascular reactivity and systolic blood pressure[58]. High ROS and increased level of the endogenous asymmetric dimethylarginine (ADMA) was reported to be a novel risk factor for endothelial dysfunction[59]. Moreover, high levels of ADMA were reported in CKD and were associated with higher intima-media thickness and cardiovascular events[60]. In renovascular hypertension, oxidative stress in the ischemic kidney plays a major role in the maintenance of hypertension in two kidney-one clip rats[61].
Just like the kidney and the vasculature itself, the sympathetic nervous system (SNS), regulated in the CNS, plays an important role in the pathogenesis of hypertension[62]. Recent studies strongly suggest that central sympathetic outflow is increased in hypertension[63]. There is also evidence that increased ROS generation in the brainstem contributes to the neural mechanisms of hypertension in hypertensive rats[64].
The rostral ventrolateral medulla (RVLM) is the major vasomotor center and is essential for the maintenance of basal vasomotor tone[65,66]. There are findings that strongly indicate that ROS in the RVLM is increased in stroke-prone spontaneously hypertensive rats and thereby contributes to the neural mechanisms of hypertension through activation of the SNS[65]. The paraventricular nucleus of the hypothalamus is most likely also involved in the ROS mediated neural mechanism of hypertension[61,67]. There is evidence that other regions of the brain are also involved in ROS mediated hypertension. These investigations suggest that increased intracellular superoxide production in the subfornical organ is critical in the development of AT-II-induced hypertension[68].
This section refers to the antihypertensive role of endogenous and exogenous antioxidants that have demonstrated their ability to alter the blood vessels function and to participate in the main redox reactions involved in the pathophysiology of hypertension.
Vitamin C: Vitamin C is a potent water-soluble antioxidant. On the vascular wall behaves as enzyme modulator exerting up-regulation on eNOS and down regulation of NOX[69]. Most studies have demonstrated an inverse relationship between plasma ascorbate levels and blood pressure in both normotensive and hypertensive populations[15]. It has been shown that treatment with antioxidants improves the vascular function and reduces the blood pressure in animal models[70,71] and in human hypertension[72,73]. Vitamin C may have favorable effects on vascular dilation, possibly through its antioxidant effects on NO[74-76].
Nevertheless, there are several small and short-term clinical trials in which the effect of vitamin C supplements on blood pressure have yielded inconsistent findings[77-82]. The lack of antihypertensive efficacy observed in studies using supplementation with vitamin C alone could be due to the decreased bioavailability of NO under conditions of oxidative stress. It was shown that these effects are mediated in part by the ability of vitamin C to protect BH4 from oxidation and thereby increase the enzymatic activity of eNOS[83]. In addition, this uncertain clinical beneficial effect of vitamin C in vivo as an antihypertensive agent could be due to the lack of consideration of their pharmacokinetic properties. It was experimentally determined that the antihypertensive effect of vitamin C is expected to occur at a concentration by 10μmo/L[75], a plasma level unreachable in humans through oral administration, but that would be required to compete efficiently with the reaction of NO with superoxide. The renal ascorbic acid threshold occurs at vitamin C dose between 60 and 100 mg daily. Plasma is completely saturated at doses of 400 mg daily and higher, producing a steady-state plasma concentration of approximately 80 μmo/L[84]. Thus, the antihypertensive effect may only be active in plasma following vitamin C infusion at high doses.
Vitamin E: This major lipid-soluble antioxidant has received considerable attention for their antioxidant potential. Epidemiological data support a role of high dietary vitamin E intake and a reduced incidence of cardiovascular disease[57]. Increasing evidence indicates that vitamin E can act as a biological modifier independently of its antioxidant activity. Experimental evidence available shows that vitamin E is capable of dose-dependently regulating mitochondrial generation of superoxide and hydrogen peroxide.
However, intervention trials have not been convincing, with a number of studies demonstrating no beneficial effect of vitamin E on cardiovascular disease outcomes[85-88]. Moreover, a study using supplementation with vitamin E, either as α-tocopherol or mixed tocopherols, showed a significant increase in blood pressure, pulse pressure and heart rate in individuals with type 2 diabetes[89]. It should be noted that it is unlikely to achieve sufficiently high concentrations in the vascular microenvironment to interfere effectively with all components of oxidative stress[90].
Association of vitamins C and E: Ascorbic acid may reduce the α-tocopheroxyl radical and may be required for beneficial vascular effects of α-tocopherol[91]. In fact, the effect of α-tocopherol seems to be dependent on tissue saturation with vitamin C, and both vitamins may act synergistically to provide optimal conditions for endothelial NO formation[92]. Thus, the association of vitamins C and E is expected to have an antihypertensive effect probably because this combined therapy provides a reinforcement of their individual properties through a complementary effect[93].
Despite the biological effects of both vitamin C and E, long-term clinical trials have failed to consistently support their antihypertensive effects in patients at high cardiovascular risk. Some short-term trials have shown that supplemental antioxidant vitamin intake lowers blood pressure[78,81,82,94] but the majority of clinical long-term trials did not find any antihypertensive effects of antioxidant vitamins. However, most of these studies lack rigorous exclusion criteria in the selection of subjects to avoid the influence of confounders[95]. It deserves special mention that regarding cohorts included in large trials, most subjects had irreversible cardiovascular disease.
Allopurinol: Xanthine oxidase is an important source of ROS in the vascular endothelium[24]. It catalyzes the last two steps of purine metabolism, producing uric acid. Xanthine oxidase activity is associated with an increasing arteriolar tone and therefore, hypertension[96,97]. Xanthine oxidase inhibitors such as allopurinol have shown marked improvements in endothelial function in various cohorts at risk of cardiovascular events. Treatment with allopurinol result in reduction of blood pressure in adolescents[98], spontaneously hypertensive rats[99] and patients with CKD[100]. Nevertheless, most of the evidence so far comes from smaller mechanistic studies, and the few large randomized controlled trials have not shown significant mortality benefit using these agents[101].
Selenium: Selenium is an essential trace element and an integral part of many proteins with catalytic and structural functions. It exerts an antioxidant function mainly in the form of selenocysteine residues, an integral constituent of ROS-detoxifying selenoenzymes, such as glutathione peroxidase (GSH-Px), thioredoxin reductases (TR) and selenoprotein P[102]. Maintenance of full GSH-Px and TR activity by adequate dietary selenium supply has been proposed to be useful for the prevention of several cardiovascular disorders[83]. In addition, selenium is capable of preventing the union of nuclear factor kappa B (NF-κB) to its nuclear response elements in DNA[103]. NF-κB has a key role in inflammation and production of ROS. The inhibition of NF-κB is presumed to be the result of the binding of the selenium to the essential thiols of this transcription factor[104].
Its antioxidant properties have been documented in several trials[103,105-110]. Selenium at low doses can provide significant protection of the human coronary artery endothelium against damage by oxidative stress[102]. In an animal model, dietary supplementation with selenium was associated with lower levels of cardiac oxidative damage and increased antioxidant expression, as well as a reduction in disease severity and mortality in spontaneously hypertensive rats[111]. A reduced selenium concentration in hypertensive pregnancies has been associated with a diminution of GSH-Px activity[112]. Thus it is reasonable to say that deficiency of selenium might be an underestimated risk factor for the development of high blood pressure[113].
N-acetylcysteine: The antioxidant N-acetylcysteine (NAC), a sulfhydryl group donor, improves renal dysfunction and decreases arterial pressure and renal injury in salt-sensitive hypertension[114]. The inhibition of oxidative stress in hypertensive states probably contributes to the therapeutic effects of NAC, an effect likely mediated by an NO-dependent mechanism[115]. This protective mechanism is exerted by prevention of BH4 oxidation by the increased superoxide[116]. In addition, this molecule can protect against oxidative damage inhibiting lipid peroxidation and scavenging ROS[117,118].
Polyphenols: Polyphenols are the most abundant antioxidant in diet. They can act as ROS scavengers, iron chelators, enzyme modulators[119,120], and possibly enhancing the production of NO[121,122]. In humans, after the consumption of polyphenols, circulating NO concentration increases[123]. Polyphenols also increase glutathione, and inhibit ROS-producing enzymes such as NADPH and xanthine oxidases. These pathways lead to improved endothelial function[124]. However, some studies have shown increased blood pressure by association of polyphenols with vitamin C[125].
Diet: There is sufficient evidence to suggest that dietary approaches may help to prevent and control high blood pressure. There are dietary approaches regarding the prevention and management of hypertension: i.e., moderate use of sodium, alcohol, an increased potassium intake, plant fibers, calcium, and foods like salmon, nuts, wine, among others[126]. In a Mediterranean population with an elevated fat consumption, a high fruit and vegetable intake is inversely associated with blood pressure levels[127]. Short-term studies indicate that specialized diets may prevent or ameliorate mild hypertension, most notable are the Dietary Approaches to Stop Hypertension (DASH) diet, which is high in fruits, vegetables, and low-fat dairy products[128]. It has been reported that a low sodium DASH diet is effective in reducing blood pressure in hypertensive patients, particularly in those taking antihypertensive medications[129]. In addition, DASH diet had significant beneficial effects on cardiovascular risk[130-132]. In overweight or obese persons with above-normal blood pressure, the addition of exercise and weight loss to the DASH diet resulted in even larger blood pressure reductions, greater improvements in vascular and autonomic function, and reduced left ventricular mass[133,134].
Recent advances in understanding the complexity of redox signaling in the vascular system points to a central role of oxidative stress in the pathogenesis of vascular dysfunction. This is how hypertension is associated with impaired endothelium-dependent vasodilation with inactivation of endothelium-derived nitric oxide by oxygen free radicals. In this regard, it has arisen a growing interest concerning the therapeutic possibilities to target ROS in the management of essential hypertension.
In support of this view, epidemiological studies suggest that individuals with higher antioxidant intake have reduced cardiovascular risk. In fact, population-based observational studies have shown an inverse association between diverse plasma antioxidant concentrations, obtained by dietary intake, with blood pressure[113,135], providing justification for trials evaluating antioxidant supplementation as adjunct anti-hypertensive therapy favoring blood pressure reduction.
Antihypertensive effects of vitamin C were hypothesized as early as 1946[136], and it has been proven that vitamin C enhances endothelial function through effects on nitric oxide production[75]. Most studies have demonstrated an inverse relation between vitamin C plasma levels and blood pressure, in normotensive and hypertensive populations[27,137]. However, evidence for blood pressure-lowering effects of vitamin C in clinical trials is still inconsistent. Nevertheless, laboratory[138,139] and human studies[140,141] have established biological plausibility for a clinical use of antioxidants concerning hypertension.
Taddei et al[142] made one of the first trials in 1998, where patients with essential hypertension received intra-arterial infusion of vitamin C, and showing that in essential hypertensive patients vitamin C significantly increased the vasodilation effect of the muscarinic agonist, acetylcholine, indicating that antioxidant vitamin C improves endothelium-dependent vasodilation in hypertensive patients. As ratifying evidence, On et al[143] in 2002 conducted a study that achieved similar results on endothelium dysfunction, using vitamin C as an adjunctive therapy to Amlodipine.
Despite the evidence points to the use of vitamin C as an adjunct in the treatment on essential hypertension, there is still lack of long-term studies that support its use. Up to date there are few trials that have used chronic supplementation. In a small randomized, double-blind controlled trial[144], patients were followed for 8 mo and were randomized to receive 500, 1000 and 2000 mg of vitamin C once daily. Results of this study showed a significant diminution of both, mean systolic blood pressure and diastolic blood pressure, with no differences between the increasing doses of vitamin C. Additionally, these effects were only seen during the first month of supplementation, suggesting only a short term benefit. Besides this, other trial aimed to study the effects of ascorbic acid on ambulatory blood pressure in elderly patients, showing that chronic supplementation of vitamin C (600 mg/daily) markedly reduced systolic blood pressure and pulse pressure in ambulatory patients[145]. Furthermore, this was accompanied by decreases of oxidative stress biomarkers such as levels of 8-isoprostane and malondialdehyde.
The strongest evidence of the possible role of vitamin C on hypertension treatment was handed by a recent a meta-analysis that included twenty-nine trials, concluding that in short-term trials, vitamin C supplementation reduces systolic and diastolic blood pressure. But it also highlights that long-term trials on the effects of vitamin C on blood pressure and clinical events are still needed to elucidate its true benefit[146].
Because supplementation made only with vitamin C has achieved inconsistent clinical outcomes, the scientific rational approach has led to the suggestion that the combined intake of antioxidants could achieve better clinical results. To prove this concept, a small randomized double-blind placebo-controlled trial was made including 38 subjects, 21 hypertensive and 17 normotensive[81]. Groups were assigned to receive in a crossover design placebo or a combination of antioxidants consisting of zinc, ascorbic acid, alpha-tocopherol and beta-carotene daily for 8 wk. Although it was a short-term following, this combined therapy of antioxidants showed that systolic blood pressure fell significantly on hypertensive subjects while been on the antioxidant phase compared with placebo. Additional evidence was given by another study aimed to evaluate the effect of short-term combined treatment with antioxidants vitamin C and E[95]: 30 essential hypertensive patients were assigned randomly either to vitamin C plus vitamin E or placebo for 8 wk. Results showed that parameters of flow-mediated dilation of the brachial artery and central pulse wave velocity were significantly improved after antioxidant supplementation, concluding that treatment with vitamins C and E has beneficial effects on endothelium-dependent vasodilation in untreated essential hypertensive patients.
Following the same consideration, recently a randomized double-blind placebo-controlled clinical trial was conducted to test the hypothesis that oral administration of vitamin C and E together causes decrease in blood pressure in patients with mild-to-moderate essential hypertension, 110 men with recent diagnosis of grade 1 essential hypertension were randomly assigned to receive either vitamin C (1 gr) plus Vitamin E (400 UI) daily or placebo for 8 wk. The results of this study, showed for the first time a specific association between oxidative-stress related parameters and blood pressure. Following administration of vitamins C plus E, patients with essential hypertension had significantly lower systolic, diastolic and mean arterial blood pressure[147].
According to the theoretical possibility of the role of antioxidants, further trials have been performed using different compounds with antioxidant activity. This is how Barrios et al[148] in 2002 conducted a patient crossover study with the aim to investigate the potential effect of NAC added to the Angiotensin-converting enzyme inhibitors (ACEI) antihypertensive action. A significant decrease in systolic and diastolic blood pressure was achieved with the combination of ACEI and NAC compared to ACEI-only period[148].
A more recent study tried the use of melatonin to evaluate its effectiveness as an adjunct for a combined treatment adding melatonin to standard anti-hypertensive drugs[149]. This study showed that combined therapy had better outcomes than standard therapy alone on essential hypertensive patients.
Although there is objective compelling evidence supporting the use of antioxidants in the management of hypertensive patients, there are also several studies that have not shown beneficial effects. As an example: Vitamin E[150], Coenzyme Q10[151], NAC[152] and vitamin C[153] have failed to obtain beneficial effects on clinical settings.
A summary of the antioxidant approaches as clinical interventions on essential hypertension is presented on Table 1.
| Details of Study | Study | n | Results | Ref. |
| Intrabrachial vitamin C (2.4 mg/100 mL forearm tissue per minute) | Randomized placebo-controlled trial | 28 | In hypertensive patients but not in control subjects, vitamin C increased the impaired vasodilation to acetylcholine | [141] |
| Intra-arterial infusion of vitamin C at 24 mg/min for 10 min | Randomized trial | 16 | Forearm blood flow response to acetylcholine was significantly enhanced with intra-arterial infusion of vitamin C in hypertensive group before antihypertensive treatment | [142] |
| Oral administration of 500, 1000 or 2000 mg of vitamin C once daily | Randomized double-blind, placebo-controlled trial | 31 | Significant diminution of mean systolic blood pressure and diastolic blood pressure, with no differences between the increasing doses of vitamin C | [143] |
| Chronic supplementation of 600 mg/daily of vitamin C | Randomized placebo-controlled trial | 24 | Reduced systolic blood pressure and pulse pressure in ambulatory elderly patients, but not in adult group | [144] |
| Included 29 trials of vitamin C supplementation | Meta-analysis | - | In short-term trials, vitamin C supplementation reduces systolic and diastolic blood pressure | [145] |
| Crossover design Placebo or antioxidant combination: 200 mg zinc 500 mg vitamin C 600 mg vitamin E 30 mg of β-carotene | Randomized double-blind placebo-controlled trial | 38 | Combined therapy of antioxidants showed that systolic blood pressure fell significantly on hypertensive subjects | [80] |
| Oral supplementation: 1 g vitamin C 400 UI vitamin E or Placebo for 8 wk | Randomized double-blind, placebo-controlled, crossover study | 30 | Treatment with vitamins C and E has beneficial effects on endothelium-dependent vasodilation in untreated essential hypertensive patients | [153] |
| Oral supplementation: 1 g vitamin C 400 UI vitamin E or Placebo for 8 wk | Randomized double-blind placebo-controlled trial | 110 | Specific association between oxidative-stress related parameters and blood pressure Patients with essential hypertension had significantly lower systolic, diastolic and mean arterial blood pressure | [146] |
| ACEI plus NAC (600 mg three times a day) or ACEI only | Randomized controlled trial, crossover study | 18 | Significant decrease in systolic and diastolic blood pressure with the combination of ACEI and NAC compared to ACEI-only | [147] |
| Standard therapy or Melatonin plus antihypertensive standard therapy | Randomized controlled trial | 170 | Combined therapy had better outcomes than standard therapy alone on essential hypertensive patients | [148] |
| Intra-arterial administration: NAC (48 g/min) or vitamin C (18 mg/min) | Cross-over randomized study | 30 | The intra-arterial administration of NAC had no effect on endothelium-dependent vasodilation Intra-arterial vitamin C improved endothelium-dependent vasodilation | [151] |
| Coenzyme Q10, 100 mg twice daily or placebo | Randomized, double-blind, placebo-controlled crossover study | 30 | There was not statistically significant reductions systolic or diastolic blood pressure | [150] |
| Vitamin C supplement daily Either 50 mg or 500 mg, for 5 yr | Randomized double-blind controlled trial | 244 | Neither systolic nor diastolic blood pressure was significantly related with the serum vitamin C concentration | [152] |
There is considerable evidence supporting the view that oxidative stress is involved in the pathophysiology of hypertension. ROS are mediators of the major physiological vasoconstrictors, increasing intracellular calcium concentration. In addition, superoxide reduces the bioavailability of NO and enhances superoxide production via uncoupled eNOS, further enhancing oxidative stress, a major factor of hypertension.
Antioxidant therapy can curtail the development of hypertension in animal models, but remains controversial in humans. Possible confounding factors in patients include co-existing pathologies and treatments, lack of selection of treatments according to ROS levels, among others. However, the dietary intake of antioxidants and polyphenols could have an effect in the primary prevention or reduction of hypertension. Despite existing molecular basis and in-vitro evidence supports the use of diverse antioxidants, clinical evidence continues to be controversial. It is necessary to collect efforts in performing basic/clinical trials that augment the current, which could eventually help to elucidate the role of antioxidants as novel therapy for essential hypertension. Also available data lead us to think that antioxidants may not play the same role in different stages of disease, suggesting that supplementation could be only beneficial during the phase of endothelial dysfunction, which precedes an established vascular damage. In this setting antioxidants would be more likely to have a role on early stages of hypertension with the potential to reverse or counteract deleterious effects of ROS. In contrast, it should not be expected an anti-hypertensive effect in patients having an advanced state of cardiovascular disease, in which chronic damaging effects of oxidative stress may be unreachable for antioxidant approach.
P- Reviewers: Kyaw T, Rentoukas E, Xie LH S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7301] [Cited by in RCA: 7415] [Article Influence: 353.1] [Reference Citation Analysis (1)] |
| 2. | Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 420] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J Cardiovasc Pharmacol. 2003;42:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Lassègue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852-860. [PubMed] |
| 5. | Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9:409-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Yoshioka J, Schreiter ER, Lee RT. Role of thioredoxin in cell growth through interactions with signaling molecules. Antioxid Redox Signal. 2006;8:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Lacy F, Kailasam MT, O’Connor DT, Schmid-Schönbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens. 2002;20:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Redón J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Sáez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Tanito M, Nakamura H, Kwon YW, Teratani A, Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R, Yodoi J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal. 2004;6:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 13. | Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol. 2003;30:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Rodrigo R, Prat H, Passalacqua W, Araya J, Guichard C, Bächler JP. Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007;30:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 526] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 17. | Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens. 2001;19:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Salvetti A. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, Ohtani T, Miwa T, Hori M, Masuyama T. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Feairheller DL, Brown MD, Park JY, Brinkley TE, Basu S, Hagberg JM, Ferrell RE, Fenty-Stewart NM. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med Sci Sports Exerc. 2009;41:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277-R297. [PubMed] |
| 24. | Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546-22554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 524] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 25. | Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 473] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H281-H288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Laakso JT, Teräväinen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557-5563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 522] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 29. | Eto Y, Kang D, Hasegawa E, Takeshige K, Minakami S. Succinate-dependent lipid peroxidation and its prevention by reduced ubiquinone in beef heart submitochondrial particles. Arch Biochem Biophys. 1992;295:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Zhou L, Xiang W, Potts J, Floyd M, Sharan C, Yang H, Ross J, Nyanda AM, Guo Z. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. Free Radic Biol Med. 2006;41:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem. 1997;272:25907-25912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Simko F, Luptak I, Matuskova J, Krajcirovicova K, Sumbalova Z, Kucharska J, Gvozdjakova A, Simko J, Babal P, Pechanova O. L-arginine fails to protect against myocardial remodelling in L-NAME-induced hypertension. Eur J Clin Invest. 2005;35:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Hogg N. S-Nitrosothiols: cellular formation and transport. Free Radic Biol Med. 2005;38:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Sládková M, Kojsová S, Jendeková L, Pechánová O. Chronic and acute effects of different antihypertensive drugs on femoral artery relaxation of L-NAME hypertensive rats. Physiol Res. 2007;56 Suppl 2:S85-S91. [PubMed] |
| 35. | Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 438] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 38. | Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 758] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 39. | Pechánová O. Contribution of captopril thiol group to the prevention of spontaneous hypertension. Physiol Res. 2007;56 Suppl 2:S41-S48. [PubMed] |
| 40. | Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Wen H, Gwathmey JK, Xie LH. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J Hypertens. 2012;2:34-44. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 42. | Gomez-Alamillo C, Juncos LA, Cases A, Haas JA, Romero JC. Interactions between vasoconstrictors and vasodilators in regulating hemodynamics of distinct vascular beds. Hypertension. 2003;42:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Görlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Matsushita M, Shichiri M, Imai T, Iwashina M, Tanaka H, Takasu N, Hirata Y. Co-expression of urotensin II and its receptor (GPR14) in human cardiovascular and renal tissues. J Hypertens. 2001;19:2185-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Jégou S, Cartier D, Dubessy C, Gonzalez BJ, Chatenet D, Tostivint H, Scalbert E, LePrince J, Vaudry H, Lihrmann I. Localization of the urotensin II receptor in the rat central nervous system. J Comp Neurol. 2006;495:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, Kirk A, Richardson M, MacLean MR. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H925-H928. [PubMed] |
| 47. | Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Homocysteine and essential hypertension. J Clin Pharmacol. 2003;43:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 49. | Rodrigo R, Rivera G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 2002;33:409-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Zhang C, Hu JJ, Xia M, Boini KM, Brimson C, Li PL. Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes. Biochim Biophys Acta. 2010;1803:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Piccoli C, Quarato G, D’Aprile A, Montemurno E, Scrima R, Ripoli M, Gomaraschi M, Cirillo P, Boffoli D, Calabresi L. Native LDL-induced oxidative stress in human proximal tubular cells: multiple players involved. J Cell Mol Med. 2011;15:375-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Klahr S. Urinary tract obstruction. Semin Nephrol. 2001;21:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Grande MT, Pérez-Barriocanal F, López-Novoa JM. Role of inflammation in túbulo-interstitial damage associated to obstructive nephropathy. J Inflamm (Lond). 2010;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 55. | Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, Kim HW, Kim BS, Lee JH, Kim GH. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Guarnieri G, Zanetti M, Vinci P, Cattin MR, Pirulli A, Barazzoni R. Metabolic syndrome and chronic kidney disease. J Ren Nutr. 2010;20:S19-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Costa-Hong V, Bortolotto LA, Jorgetti V, Consolim-Colombo F, Krieger EM, Lima JJ. Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol. 2009;92:381-386, 398-403, 413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 797] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 60. | Nanayakkara PW, Teerlink T, Stehouwer CD, Allajar D, Spijkerman A, Schalkwijk C, ter Wee PM, van Guldener C. Plasma asymmetric dimethylarginine (ADMA) concentration is independently associated with carotid intima-media thickness and plasma soluble vascular cell adhesion molecule-1 (sVCAM-1) concentration in patients with mild-to-moderate renal failure. Kidney Int. 2005;68:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol. 2011;38:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 63. | Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1369] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 64. | Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 65. | Hirooka Y, Sagara Y, Kishi T, Sunagawa K. Oxidative stress and central cardiovascular regulation. - Pathogenesis of hypertension and therapeutic aspects -. Circ J. 2010;74:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep. 2003;5:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens. 2009;22:484-492. [PubMed] |
| 68. | Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 359] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 69. | Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Nishikawa Y, Tatsumi K, Matsuura T, Yamamoto A, Nadamoto T, Urabe K. Effects of vitamin C on high blood pressure induced by salt in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2003;49:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Reckelhoff JF, Kanji V, Racusen LC, Schmidt AM, Yan SD, Marrow J, Roberts LJ, Salahudeen AK. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am J Physiol. 1998;274:R767-R774. [PubMed] |
| 72. | Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Atarashi K, Ishiyama A, Takagi M, Minami M, Kimura K, Goto A, Omata M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Am J Hypertens. 1997;10:116S-119S. [PubMed] |
| 74. | Vita JA, Frei B, Holbrook M, Gokce N, Leaf C, Keaney JF. L-2-Oxothiazolidine-4-carboxylic acid reverses endothelial dysfunction in patients with coronary artery disease. J Clin Invest. 1998;101:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Jackson TS, Xu A, Vita JA, Keaney JF. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Duffy SJ, Gokce N, Holbrook M, Hunter LM, Biegelsen ES, Huang A, Keaney JF, Vita JA. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H528-H534. [PubMed] |
| 77. | Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Fotherby MD, Williams JC, Forster LA, Craner P, Ferns GA. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J Hypertens. 2000;18:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001;37:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Ghosh SK, Ekpo EB, Shah IU, Girling AJ, Jenkins C, Sinclair AJ. A double-blind, placebo-controlled parallel trial of vitamin C treatment in elderly patients with hypertension. Gerontology. 1994;40:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Galley HF, Thornton J, Howdle PD, Walker BE, Webster NR. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci (Lond). 1997;92:361-365. [PubMed] |
| 82. | Mullan BA, Young IS, Fee H, McCance DR. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension. 2002;40:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 84. | Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1018] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 85. | Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 378] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 86. | Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2911] [Cited by in RCA: 2548] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 87. | Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 813] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 88. | Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 757] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 89. | Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens. 2007;25:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Münzel T, Keaney JF. Are ACE inhibitors a “magic bulle“ against oxidative stress? Circulation. 2001;104:1571-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Heller R, Werner-Felmayer G, Werner ER. Antioxidants and endothelial nitric oxide synthesis. Eur J Clin Pharmacol. 2006;62:21-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Heller R, Werner-Felmayer G, Werner ER. Alpha-Tocopherol and endothelial nitric oxide synthesis. Ann N Y Acad Sci. 2004;1031:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Bilodeau JF, Hubel CA. Current concepts in the use of antioxidants for the treatment of preeclampsia. J Obstet Gynaecol Can. 2003;25:742-750. [PubMed] |
| 94. | Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392-397. [PubMed] |
| 95. | Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, Schmid-Schönbein GW. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci USA. 1998;95:4754-4759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schönbein GW. Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation. 2006;13:551-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 742] [Cited by in RCA: 665] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 99. | Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 893] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 100. | Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 101. | George J, Struthers A. The role of urate and xanthine oxidase in vascular oxidative stress: future directions. Ther Clin Risk Manag. 2009;5:799-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Miller S, Walker SW, Arthur JR, Nicol F, Pickard K, Lewin MH, Howie AF, Beckett GJ. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci (Lond). 2001;100:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Faure P, Ramon O, Favier A, Halimi S. Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Invest. 2004;34:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Kim IY, Stadtman TC. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci USA. 1997;94:12904-12907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Campbell L, Howie F, Arthur JR, Nicol F, Beckett G. Selenium and sulforaphane modify the expression of selenoenzymes in the human endothelial cell line EAhy926 and protect cells from oxidative damage. Nutrition. 2007;23:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Takizawa M, Komori K, Tampo Y, Yonaha M. Paraquat-induced oxidative stress and dysfunction of cellular redox systems including antioxidative defense enzymes glutathione peroxidase and thioredoxin reductase. Toxicol In Vitro. 2007;21:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Faure P. Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin Chem Lab Med. 2003;41:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Brigelius-Flohé R, Banning A, Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal. 2003;5:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Ito Y, Fujita T. [Trace elements and blood pressure regulation]. Nihon Rinsho. 1996;54:106-110. [PubMed] |
| 110. | Zhou X, Ji WJ, Zhu Y, He B, Li H, Huang TG, Li YM. Enhancement of endogenous defenses against ROS by supra-nutritional level of selenium is more safe and effective than antioxidant supplementation in reducing hypertensive target organ damage. Med Hypotheses. 2007;68:952-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Lymbury RS, Marino MJ, Perkins AV. Effect of dietary selenium on the progression of heart failure in the ageing spontaneously hypertensive rat. Mol Nutr Food Res. 2010;54:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Mistry HD, Wilson V, Ramsay MM, Symonds ME, Broughton Pipkin F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 2008;52:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 113. | Nawrot TS, Staessen JA, Roels HA, Den Hond E, Thijs L, Fagard RH, Dominiczak AF, Struijker-Boudier HA. Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J. 2007;28:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 114. | Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Pechánová O, Zicha J, Kojsová S, Dobesová Z, Jendeková L, Kunes J. Effect of chronic N-acetylcysteine treatment on the development of spontaneous hypertension. Clin Sci (Lond). 2006;110:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ. Inhibition of endothelial nitric oxide synthase by ebselen. Prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J Pharmacol Exp Ther. 1993;267:1112-1118. [PubMed] |
| 117. | De la Fuente M, Victor VM. Ascorbic acid and N-acetylcysteine improve in vitro the function of lymphocytes from mice with endotoxin-induced oxidative stress. Free Radic Res. 2001;35:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N. Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res. 2005;1056:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Pietta P, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardelli E. Relationship between rate and extent of catechin absorption and plasma antioxidant status. Biochem Mol Biol Int. 1998;46:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Duarte J, Andriambeloson E, Diebolt M, Andriantsitohaina R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol Res. 2004;53:595-602. [PubMed] |
| 122. | Zenebe W, Pechánová O, Andriantsitohaina R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol Res. 2003;52:425-432. [PubMed] |
| 123. | Pechánová O, Rezzani R, Babál P, Bernátová I, Andriantsitohaina R. Beneficial effects of Provinols: cardiovascular system and kidney. Physiol Res. 2006;55 Suppl 1:S17-S30. [PubMed] |
| 124. | Rodrigo R, Gil D, Miranda-Merchak A, Kalantzidis G. Antihypertensive role of polyphenols. Adv Clin Chem. 2012;58:225-254. [PubMed] |
| 125. | Ward NC, Hodgson JM, Croft KD, Burke V, Beilin LJ, Puddey IB. The combination of vitamin C and grape-seed polyphenols increases blood pressure: a randomized, double-blind, placebo-controlled trial. J Hypertens. 2005;23:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3896] [Cited by in RCA: 3728] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 127. | Alonso A, de la Fuente C, Martín-Arnau AM, de Irala J, Martínez JA, Martínez-González MA. Fruit and vegetable consumption is inversely associated with blood pressure in a Mediterranean population with a high vegetable-fat intake: the Seguimiento Universidad de Navarra (SUN) Study. Br J Nutr. 2004;92:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 128. | Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 129. | Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium Dietary Approaches to Stop Hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, Esmaillzadeh A, Willett WC. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care. 2011;34:55-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 131. | Chen ST, Maruthur NM, Appel LJ. The effect of dietary patterns on estimated coronary heart disease risk: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Circ Cardiovasc Qual Outcomes. 2010;3:484-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 132. | Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. 2009;104:1416-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 133. | Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 134. | Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 380] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 135. | Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, Feldman DS. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. Am J Clin Nutr. 1993;57:213-217. [PubMed] |
| 136. | Hoitink AWJH. Research on the influence of vitamin C administration on the human organism, in particular in connection with the working capacity. Verh Nederlands Inst Praevent. 1946;4:62-63. |
| 137. | Houston MC. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiovasc Dis. 2005;47:396-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 138. | Yoshioka M, Aoyama K, Matsushita T. Effects of ascorbic acid on blood pressure and ascorbic acid metabolism in spontaneously hypertensive rats (SH rats). Int J Vitam Nutr Res. 1985;55:301-307. [PubMed] |
| 139. | Ettarh RR, Odigie IP, Adigun SA. Vitamin C lowers blood pressure and alters vascular responsiveness in salt-induced hypertension. Can J Physiol Pharmacol. 2002;80:1199-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Koh ET. Effect of vitamin C on blood parameters of hypertensive subjects. J Okla State Med Assoc. 1984;77:177-182. [PubMed] |
| 141. | Feldman EB, Gold S, Greene J, Moran J, Xu G, Shultz GG, Hames CG, Feldman DS. Ascorbic acid supplements and blood pressure. A four-week pilot study. Ann N Y Acad Sci. 1992;669:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 142. | Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 143. | On YK, Kim CH, Sohn DW, Oh BH, Lee MM, Park YB, Choi YS. Improvement of endothelial function by amlodipine and vitamin C in essential hypertension. Korean J Intern Med. 2002;17:131-137. [PubMed] |
| 144. | Hajjar IM, George V, Sasse EA, Kochar MS. A randomized, double-blind, controlled trial of vitamin C in the management of hypertension and lipids. Am J Ther. 2002;9:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Sato K, Dohi Y, Kojima M, Miyagawa K, Takase H, Katada E, Suzuki S. Effects of ascorbic acid on ambulatory blood pressure in elderly patients with refractory hypertension. Arzneimittelforschung. 2006;56:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 146. | Juraschek SP, Guallar E, Appel LJ, Miller ER. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 147. | Rodrigo R, Prat H, Passalacqua W, Araya J, Bächler JP. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin Sci (Lond). 2008;114:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 148. | Barrios V, Calderón A, Navarro-Cid J, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Zaslavskaia RM, Shcherban’ EA, Lilitsa GV, Logvinenko SI. [Melatonin in the combined treatment of cardiovascular diseases]. Klin Med (Mosk). 2010;88:26-30. [PubMed] |
| 150. | Palumbo G, Avanzini F, Alli C, Roncaglioni MC, Ronchi E, Cristofari M, Capra A, Rossi S, Nosotti L, Costantini C. Effects of vitamin E on clinic and ambulatory blood pressure in treated hypertensive patients. Collaborative Group of the Primary Prevention Project (PPP)--Hypertension study. Am J Hypertens. 2000;13:564-567. [PubMed] |
| 151. | Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Nicholls MG, Scott RS, George PM. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am J Hypertens. 2012;25:261-270. [PubMed] |
| 152. | Schneider MP, Delles C, Schmidt BM, Oehmer S, Schwarz TK, Schmieder RE, John S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am J Hypertens. 2005;18:1111-1117. [PubMed] |
| 153. | Kim MK, Sasaki S, Sasazuki S, Okubo S, Hayashi M, Tsugane S. Lack of long-term effect of vitamin C supplementation on blood pressure. Hypertension. 2002;40:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |