Published online Dec 26, 2014. doi: 10.4330/wjc.v6.i12.1262
Revised: September 13, 2014
Accepted: October 1, 2014
Published online: December 26, 2014
Processing time: 156 Days and 16.4 Hours
Cardiovascular disease (CVD) is a prevalent condition in general population and the first cause of death overall. Klotho, a pleiotropic protein related to longevity that acts as a co-receptor of the fibroblast growth factor 23, has been proposed as a key regulator of the development of CVD. In the few clinical studies made, it has been observed a relationship between low levels of soluble Klotho and the occurrence and severity of CVD, as well as a reduction of cardiovascular risk when they are high. Also, different polymorphisms of human Klotho gene have been related to the incidence of cardiovascular events. Moreover, several experimental studies indicate that this protein acts in the maintenance of vascular homeostasis. Klotho improves endothelial dysfunction through promotion of NO production and mediates anti-inflammatory and anti-aging effects such as suppression of adhesion molecules expression, attenuation of nuclear factor-kappa B or inhibition of Wnt signaling. Furthermore, this protein is related to the attenuation of vascular calcification as well as prevention of cardiac hypertrophy. The expression of this protein in the vascular wall implies a new scenario for the treatment of vascular disorders. The purpose of this review is to provide an overview of the relationship between the Klotho protein and CVD, in addition to its role in the maintenance of functional vascular integrity.
Core tip: Cardiovascular disease (CVD) is the first cause of death worldwide. The anti-aging factor Klotho has been linked to the development of CVD since clinical studies relate circulating levels of Klotho with the appearance of vascular disease and different Klotho gene variants are associated with increased cardiovascular risk. Furthermore, Klotho is involved in promotion of vascular health through different mechanisms. The recent description of its expression in vascular tissue opens up new options for the treatment of cardiovascular diseases.
- Citation: Martín-Núñez E, Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Implications of Klotho in vascular health and disease. World J Cardiol 2014; 6(12): 1262-1269
- URL: https://www.wjgnet.com/1949-8462/full/v6/i12/1262.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i12.1262
The cardiovascular disease (CVD) is highly prevalent in the general population and the leading cause of death worldwide[1], maintaining these projections in the future[2]. CVD broadly comprises coronary artery disease (CAD), myocardial infarction, vascular stiffening and left ventricular hypertrophy[3].
Klotho, a gene originally identified in 1997 codifying for a novel anti-aging protein, has been implicated in a multitude of biological processes, most of them related to human longevity[4]. Mice lacking the Klotho gene develop a phenotype similar to premature human aging, which includes endothelial dysfunction, vascular calcification, progressive atherosclerosis and shortened lifespan[5]. A reduction in Klotho levels is observed in chronic kidney disease (CKD) patients, similar to other premature vascular aging diseases, such as hypertension or diabetes mellitus. Even normal aging is associated with a reduction in serum and urine concentration of Klotho[6-8].
The first function described for Klotho is its role in the metabolism of phosphorus as the obligatory co-receptor of fibroblast growth factor 23 (FGF23), a bone-derived hormone responsible of the phosphate balance in the body through promotion of renal phosphate excretion. Klotho directly binds to FGF receptors (FGFRs) constituting a high affinity complex for FGF23 which mediates the intracellular effects of this phosphatonin[9]. More recently, the involvement of Klotho in vascular protection through different mechanisms has been demonstrated. These mechanisms include inhibition of oxidative stress, modulation of inflammation or attenuation of vascular calcification[10-12]. Therefore, Klotho has been suggested as a master regulator of CVD[13]. The aim of this review is to provide an overview of what is known so far about Klotho and its relationship with CVD, besides its role in the maintenance of vascular homeostasis.
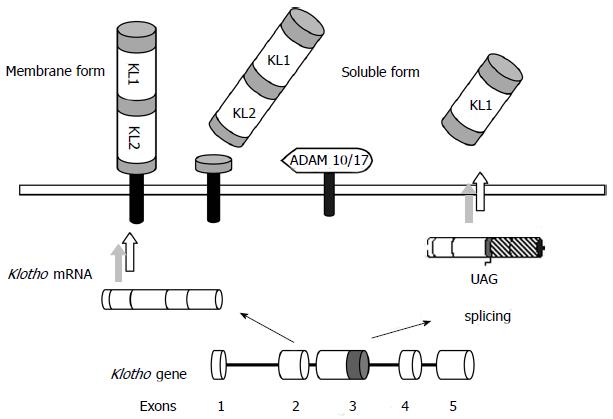
The human Klotho gene comprises 5 exons and is located in a region of approximately 50 kb on chromosome 13q12. This gene encodes for two possible transcripts: a full-length, translated into a single-pass transmembrane protein of 1012 amino acids (130 kDa), or an alternative spliced transcript, which encodes the N-terminal half of 549 amino acids (65-70 kDa) and is secreted to the extracellular space. Another form of soluble Klotho can also be generated through proteolytic cleavage of the transmembrane form by membrane-anchored A Desintegrin and metalloproteinase (ADAM) -17 and ADAM-10, so that the full-length extracellular domain is released into the circulation[14-17] (Figure 1). Soluble Klotho predominates in humans over the membrane form and is detectable in urine, serum and cerebrospinal fluid[18]. This circulating form acts as a humoral factor with multitude of functions such as anti-oxidation, modulation of renal ion channels, anti-Wnt signaling or anti-apoptosis and senescence effects[19].
The Klotho protein comprises an extracellular domain composed of two repeat sequences (KL1 and KL2), two short membrane-spanning regions (21 amino acids) and an intracellular carboxyl (11 amino acids) domain. The KL1 and KL2 sequences share 20%-40% sequence identity with the Family 1 glycosidases[4,16].
In humans, Klotho is mainly expressed in the kidneys, but its tissue distribution also includes brain, reproductive organs, pituitary gland, parathyroid glands, urinary bladder, skeletal muscle, placenta, thyroid gland, colon[4], and more recently described, human vascular tissue[20,21]. The membrane form mainly acts as the obligatory co-receptor for FGF23, thereby tissues expressing Klotho are potential targets for FGF23 to exert its actions[9,22,23].
Although the circulating levels of soluble Klotho have been initially proposed as biomarker of renal function, since some works show a decrease in serum levels during development of CKD[6], its association with cardiovascular risk has been less extensively explored.
In a first work, Semba et al[24] found that in community-dwelling adults higher plasma Klotho concentrations are independently associated with a lower likelihood of having CVD, defined as CAD, heart failure stroke, or peripheral arterial disease. Likewise, in a recent study developed by our group, we observed that patients with significant CAD have lower soluble concentrations of soluble Klotho, as well as a reduced expression level of Klotho mRNA in the vascular wall. Besides, the reduced serum Klotho levels and decreased vascular gene expression were associated with the presence and severity of CAD independently of established cardiovascular risk factors such as age, diabetes, hypertension, smoking, dyslipidemia, and inflammation[25].
Moreover, Kitagawa et al[26] observed that serum Klotho level is an independent determinant of marked arterial stiffness but not of other types of vascular dysfunction such as atherosclerosis, endothelial dysfunction or vascular calcification, in CKD patients[26]. In contrast, in a very recent work, Seiler et al[27] found no significant relationship between soluble Klotho and cardiovascular outcomes in a CKD stages 2-4 cohort.
Taken together, these studies suggest that a reduction in the levels of soluble Klotho may promote or encourage the development and progression of CVD, while high levels of this factor prevents the risk of CVD. In any case, further studies are needed to clarify the relationship between circulating Klotho levels and cardiovascular risk.
Genetic variation studies have demonstrated that Klotho gene polymorphisms might be associated with longevity[28] and CAD[29-32]. In particular, the KL-VS allele, characterized by six SNPs in a region of 800 bp in exon 2 and flanking sequence, is prevalent in the population and is associated with a reduced longevity[28]. In a study where two different groups of healthy siblings were tested, Arking et al[29] found that this functional variant of Klotho gene is an independent risk factor for CAD. The risk associated with this allele is modulated by modifiable risk factors, such as hypertension, increased high-density lipoprotein cholesterol levels or smoking[29]. Likewise, in an Ashkenazi Jew group it was found that homozygous KL-VS individuals were at higher risk of stroke than wild-type subjects[33].
In the case of G-395A polymorphism, the A allele has been found to be an independent predictor of atherosclerotic CAD but not of vasospastic angina in Japanese population[30]. This polymorphism affects the promoter of the Klotho gene, so that the G→A substitution impairs protein binding to the region and consequently affects gene expression[34] and soluble Klotho levels. Similarly, Jo et al[32] observed an association of the G-395A allele with CAD but not with coronary artery calcification in Korean patients. Besides, subjects with the T allele for the C1818T polymorphism (located in exon 4) have lower prevalence of CAD than those with CC genotype[31].
One of the first vasculoprotective activities described for Klotho is its role in maintenance of endothelial homeostasis. Kl+/- mice show attenuated aortic and arteriolar vasodilatation, which can be increased after two weeks of parabiosis with wild type mice[35]. Moreover, these Kl heterozygous mice show a significantly reduction of urinary excretion of NO2- and NO3- (NO metabolites), suggesting a decrease in NO production[35]. In Otsuka Long-Evans Tokushima Fatty rats, an animal model which displays multiple atherogenic risk factors, adenovirus-mediated klotho gene delivery results in improvement of aortic relaxation and increased NO production[36]. These findings point to a direct involvement of Klotho in improving endothelial dysfunction through pathways involving NO. Consistent with this, Shimada et al[37] observed impaired angiogenesis, a NO-dependent process, and reduced endothelium-derived NO release in kl/kl mice.
This reduction of NO mediated by Klotho deficiency can be due to its accelerated degradation because of increased oxidative stress associated with aging. Klotho is able to increase resistance to oxidative stress inducing expression of manganese superoxide dismutase (Mn-SOD) through activation of FoxO forkhead transcription factor[38]. In regard of this, Klotho increases Mn-SOD activity and NO production via c-AMP-PKA-dependent pathway in human umbilical vascular endothelial cells (HUVECs)[10], and it also reduces H2O2-induced apoptosis and cellular senescence[39]. Likewise, Klotho transfection of cultured vascular smooth muscle cells (VSMCs) also reduces superoxide production and decrease angiotensin II-induced oxidative stress[40].
Another possibility is that Klotho regulates expression levels of the endothelial NO synthase (eNOS). Six et al[41] recently observed that attenuation mediated by Klotho of FGF23 or phosphate-induced vasoconstriction is abolished by adding nitro-L-arginine, a competitive inhibitor of NOS. Moreover, they observed that exposure of HUVECs to Klotho increased NO production and induced eNOS phosphorylation and iNOS expression. Interestingly, Klotho was able to increase H2O2 production in cultured human VSMCs (HVSMCs), which suggests a more complex effect of this protein on the regulation of vascular tone through mediation of a ROS/NO balance[41].
Inflammation is a central process in CVD[42,43] and Klotho has been suggested to play a protective role in the vessels since it mediates anti-inflammatory actions. In cultured HUVECs, incubation with Klotho results in suppression of expression of cell adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)[11]. These Klotho effects in ECs also include attenuation of the activation of NF-κB and blockade of tumor necrosis factor-α induced monocyte adhesion[11]. Likewise, the intracellular form of Klotho is capable to inhibit RIG-I-induced expression of interleukin (IL)-6 and IL-8 both in vitro and in vivo[44].
Moreover, it is known that soluble form of Klotho is able to bind to various members of Wnt family, and thereby suppress Wnt biological activity[45,46]. Although this signal is essential for stem cells proliferation, continued activation of Wnt can contribute to cellular depletion and accelerated cellular senescence[47]. Therefore, Klotho could exert an anti-aging effect by attenuation of Wnt signaling, preventing cellular senescence[48].
Vascular calcification (VC) is one of the major complications of CKD and is associated with mineral and bone disorders. Since CKD patients, who have low levels of Klotho protein, and Klotho-deficient animals develop medial vascular calcification[4], the absence of this protein has been associated with the appearance of VC. Initially, the involvement of Klotho in the protection against VC was believed to be related to its role in the regulation of phosphate metabolism as co-receptor for FGF23. However, in recent years Klotho has shown to have direct effects on vasculature to prevent this pathology.
High levels of extracellular Pi induce mineralization of VSMCs through inorganic Pi influx mediated by cotransporters NaPi type 3 (Pit-1 and Pit-2)[49]. This process is accompanied by overexpression of osteogenic markers, such as RunX2, which leads to dedifferentiation of VSMCs[50,51]. In 2011, Hu et al[12] found that Klotho deficiency in mouse involved increased arterial calcification, and aortic downregulation of SM22 (a smooth muscle cell marker) expression and upregulation of the transcripts for Pit-1, Pit-2 and RunX2. A similar expression profile was observed in the mouse model of CKD, which was prevented by Klotho overexpression. Moreover, addition of recombinant soluble Klotho to rat VSMCs cultured in high-Pi decreased aortic calcium content and Na+-dependent Pi uptake, confirming Klotho direct modulation of NaPi-3 activity[12]. Administration of exogenous Klotho protein to kl/kl mice also attenuates aortic calcification[52]. Therefore, it seems that Klotho prevents vascular calcification through mediation of NaPi-3 cotransporters activity and modulating VSMCs differentiation.
Consistent with this, Lim et al[21] confirmed the importance of Klotho in arterial calcification in a study where they found that silencing of Klotho in human aortic smooth muscle cells (HA-SMCs) leads to increased calcification[21]. Interestingly, treatment with vitamin D receptor activators (VDRAs), such as calcitriol or paricalcitol, restores Klotho expression in pro-calcific cultured HA-SMCs and increases serum and urine Klotho in uremic mice[21,53]. This VDRA therapy is associated with improved aortic medial calcification and increased osteopontin expression, an anticalcification factor[53].
Cardiac hypertrophy is a high prevalent pathological condition among end stage renal disease patients, which leads to cardiac dysfunction and death[54-56]. Stress signals induce abnormal growth and remodeling that progress to heart failure. Klotho is involved in cardioprotection since its deficiency produce an exaggerated cardiac hypertrophy caused by isoprotenerol (ISO) injection in mice[57]. Likewise, its administration ameliorates ISO-induced structural changes in mouse hearts, e.g., disordered arrangement of myocardial fibers, fibroblastic hyperplasia, mononuclear cell infiltration or interstitial and perivascular fibrosis[58].
This cardiac protection by Klotho occurs through downregulation of TRPC6 channels, whose overexpression causes aberrant cardiac development and premature death[57]. Moreover, cardiomyocyte apoptosis is an important process in cardiac remodeling[59] and Klotho is able to suppress it by downregulation of endoplasmic reticulum stress and ROS production[58].
In recent years, the detection of Klotho in human vascular tissue[20,21,60] has extended the range of putative target tissues of FGF23 actions. Coexpression of two cognate FGF23 receptors, FGFR-1 and -3 in the vascular wall, along with Klotho[21], supports this idea. Furthermore, expression of Klotho protein appears to be limited to medial layer of the vessel, since it is detected by immunohistochemistry in tunica media of healthy subjects arteries[21] or in rat aorta[61], and by western blotting in human VSMCs[21]. Likewise, Klotho mRNA is detected in cultured HVSMCs rather than human vascular endothelial cells[60].
However, there are conflicting data which have led to a debate about the presence of Klotho in the vascular tissue. Scialla et al[62] detected no expression of Klotho in human or mouse VSMCs, neither in mouse aortas. Moreover, Lindberg et al[63] detected only low levels of Klotho transcript in different vascular tissues (aorta, mesenteric, femoral and lung arteries) and without significant differences between wild type and Sm22-KL-/- mice (a new experimental model with targeted deletion of Klotho in VSMCs). In this study, protein expression was undetectable in vascular tissue by immunohistochemistry or western blotting, and the absence of expression of Egr-1 in aortas of mice after injection of FGF23 indicates the lack of a functional Klotho-FGF23 signaling complex in vascular tissue[63]. Conversely, Fang et al[64] demonstrated vascular expression of Klotho in low-density lipoprotein-deficient (ldlr-/-) mice. In another study, Jimbo et al[61] demonstrated expression of Klotho protein in rat aortas but not in isolated VSMCs. Furthermore, they showed that extracellular signal-related kinase 1/2, an enzyme activated by FGF23 in Klotho-expressing cells[65], was phosphorylated by FGF23 in a dose-dependent manner in Klotho-overexpressing VSMCs but not in isolated VSMCs, suggesting that presence of Klotho only occurs in contractile VSMCs[61].
Some studies show a decreased Klotho vascular expression in CKD, similar to early reduction of this protein in the kidney during the disease[12]. Lim et al[21] observed a marked reduction of Klotho protein expression in arteries from patients with CKD. Furthermore, they showed that exposure of HA-SMC to uremic serum from patients with CKD, or to different conditions recalling CKD like hyperphosphatemia, hypercalcemia or proinflammatory stress, significantly reduced Klotho protein[21]. Moreover, Fang et al[64] also observed a reduction of Klotho activity in the aorta of a mice model of early CKD, although serum Klotho levels were increased. This decrease of vascular Klotho during disease could involve a FGF23 resistance state in the vascular bed. In contrast, Jimbo et al[61] showed that Klotho remained unchanged in aortas of nephrectomized rats.
As already suggested, all these discrepancies can be due to differences in experimental settings, like issues regarding specificity and sensitivity of anti-Klotho antibodies, different vasculature segments analyzed or differences in cell culture conditions, as well as, variance in CKD stage[66]. Although further studies are needed to characterize the vascular expression of Klotho in animal models, healthy subjects and CKD patients, as well as its stability under in vitro and ex vivo conditions, the set of results obtained so far seem to suggest that this tissue is sensitive to FGF23 and that CKD is a state of vascular Klotho deficiency. It is also interesting to note the relationship between the expression in human thoracic aorta tissue of vascular Klotho and ADAM-17[20], one of the metalloproteinases responsible for the shedding of Klotho from the cell surface, which suggests the possibility that vascular wall is a source of soluble Klotho, and therefore an important element in vascular protection.
Klotho is a novel factor involved in longevity and aging, which also has a central role in regulating phosphorus metabolism acting as co-receptor for FGF23[4,9]. But beyond these roles, several clinical studies have linked this protein to the development and progression of CVD. The reduction of circulating levels of Klotho is associated with the presence and severity of CAD and is also an independent marker of some forms of vascular dysfunction such as arterial stiffness[25,26]. Likewise, various genetic studies have shown the association between gene variants of human Klotho gene with CAD or stroke[29-32].
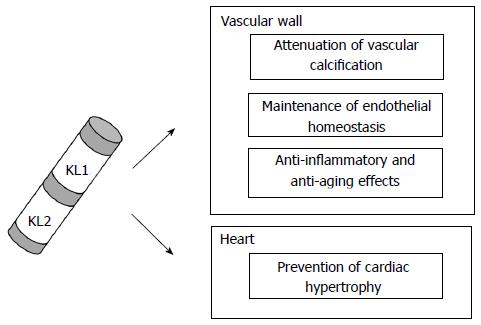
Klotho is involved in the protection of vasculature through various mechanisms, including prevention of endothelial dysfunction, anti-inflammatory effects, reduction of vascular calcification or attenuation of cardiac hypertrophy[11,12,35,58] (Figure 2). The disruption in the homeostasis of this factor seems to be a key element in the development of CVD. Furthermore, Klotho expression in the vessel wall, along with the enzymes responsible for generating its soluble form[20,21], makes the vascular context a new scenario to be considered for the treatment of vascular diseases.
The central role of Klotho in the development of CVD makes its possible use promising as a diagnostic biomarker or as a therapeutic factor for treatment of vascular diseases. However, further studies are needed to clarify the relationship between this factor and promotion of vascular health.
Research studies by the authors have been funded by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (PI13/01726), Sociedad Española de Nefrología and ACINEF. We also acknowledge cofunding by the Fondo Europeo de Desarrollo Regional, Unión Europea (“Una forma de hacer Europa”). Research activity of JFNG is supported by Programa de Intensificación de la Actividad Investigadora, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad (Convenio ISCIII-Comunidad Autónoma Canarias).
P- Reviewer: Kirmizis D S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Global status report on noncommunicable diseases 2010. Geneva: World Health Organization, 2011. Available from: http://www.who.int/nmh/publications/ncd_report2010/en/. |
| 2. | Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6517] [Cited by in RCA: 6896] [Article Influence: 362.9] [Reference Citation Analysis (0)] |
| 3. | Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 4. | Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45-51. [PubMed] |
| 5. | Kuro-o M. Klotho. Pflugers Arch. 2010;459:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI. Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis. 2013;61:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120-6123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1123] [Cited by in RCA: 1036] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 10. | Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 727] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 13. | Moe SM. Klotho: a master regulator of cardiovascular disease? Circulation. 2012;125:2181-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 516] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 15. | Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777-9784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Mian IS. Sequence, structural, functional, and phylogenetic analyses of three glycosidase families. Blood Cells Mol Dis. 1998;24:83-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 483] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 19. | Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, Muros-de-Fuentes M, Pérez H, Meneses-Pérez B, Cazaña-Pérez V, Navarro-González JF. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1372] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 24. | Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R, Mora-Fernández C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, Floege J, Fliser D, Heine GH. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol. 2014;9:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Arking DE, Krebsova A, Macek M, Macek M, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Imamura A, Okumura K, Ogawa Y, Murakami R, Torigoe M, Numaguchi Y, Murohara T. Klotho gene polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospastic angina in Japanese. Clin Chim Acta. 2006;371:66-70. [PubMed] |
| 31. | Rhee EJ, Oh KW, Lee WY, Kim SY, Jung CH, Kim BJ, Sung KC, Kim BS, Kang JH, Lee MH. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism. 2006;55:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Jo SH, Kim SG, Choi YJ, Joo NR, Cho GY, Choi SR, Kim EJ, Kim HS, Kim HJ, Rhim CY. KLOTHO gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in a Korean population. Int Heart J. 2009;50:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029-38034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 564] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 39. | Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, Drueke TB, Massy ZA. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014;9:e93423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4411] [Cited by in RCA: 4648] [Article Influence: 211.3] [Reference Citation Analysis (0)] |
| 44. | Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 45. | Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 589] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 46. | Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol. 2013;24:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 47. | Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 48. | Kuro-o M. A potential link between phosphate and aging--lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Nishiwaki-Yasuda K, Suzuki A, Kakita A, Sekiguchi S, Asano S, Nishii K, Nagao S, Oiso Y, Itoh M. Vasopressin stimulates Na-dependent phosphate transport and calcification in rat aortic smooth muscle cells. Endocr J. 2007;54:103-112. [PubMed] |
| 50. | Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44 Suppl 1:109-116. [PubMed] |
| 51. | Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 622] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 52. | Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol. 2013;698:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8524] [Article Influence: 405.9] [Reference Citation Analysis (0)] |
| 55. | Taddei S, Nami R, Bruno RM, Quatrini I, Nuti R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail Rev. 2011;16:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4 Suppl 1:S79-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 57. | Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 58. | Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8:e82968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 552] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 60. | Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, Takahashi T, Matoba S, Yamada H, Okigaki M. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. 2009;297:H1673-H1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014;85:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 62. | Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159-1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 63. | Lindberg K, Olauson H, Amin R, Ponnusamy A, Goetz R, Taylor RF, Mohammadi M, Canfield A, Kublickiene K, Larsson TE. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One. 2013;8:e60658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85:142-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 65. | Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y, Michigami T. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J Cell Biochem. 2010;111:1210-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Jimbo R, Shimosawa T. Cardiovascular Risk Factors and Chronic Kidney Disease-FGF23: A Key Molecule in the Cardiovascular Disease. Int J Hypertens. 2014;2014:381082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |