Published online Nov 26, 2013. doi: 10.4330/wjc.v5.i11.426
Revised: September 29, 2013
Accepted: October 16, 2013
Published online: November 26, 2013
Processing time: 126 Days and 3.2 Hours
AIM: To investigate the effects of different doses of aspirin on coronary endothelial function.
METHODS: The study included 139 Japanese subjects (mean age, 60 years; 53 women) with angiographically normal coronary arteries. Patients were distributed into Group I (n = 63), who was administered aspirin and Group II (n = 76), the control, who were not administered aspirin. Group I was further divided into Group Ia (n = 50, low-dose aspirin, 100 mg) and Group Ib (n = 13, high-dose aspirin, 500 mg). After a routine coronary angiography, acetylcholine (ACh; 3 and 30 μg/min successively) and nitroglycerin (NTG) were infused into the left coronary ostium over 2 min. The change in the diameter of the coronary artery in response to each drug was expressed as the percentage change from baseline values.
RESULTS: The patient characteristics did not differ between the two groups. The change in coronary diameter in response to ACh was greater in Group I than in Group II (P = 0.0043), although the NTG-induced coronary vasodilation was similar between groups. ACh-induced dilation was greater in Group Ia than in Group Ib (P = 0.0231). Multivariate regression analysis showed that a low-dose of aspirin (P = 0.0004) was one of the factors associated with ACh-induced dilation at 30 μg/min.
CONCLUSION: In subjects with angiographically normal coronary arteries, aspirin only had a positive influence on coronary endothelial function at the low dose of 100 mg. This improvement of coronary endothelial function may be involved in the preventive effect of aspirin against future coronary events.
Core tip: We investigated the effect of aspirin on coronary endothelial function. Patients were distributed into Group I, who were administered aspirin and Group II, which was the control group. Group I was divided into Group Ia (low-dose aspirin) and Group Ib (high-dose aspirin). Acetylcholine (ACh)-induced coronary dilation was greater in Group I than in Group II and was greater in Group Ia than in Group Ib. Multivariate regression analysis showed that a low-dose of aspirin was associated with ACh-induced coronary dilation. A Low dose of aspirin has a positive influence on coronary endothelial function.
- Citation: Teragawa H, Mitsuba N, Ishibashi K, Kurisu S, Kihara Y. Positive influence of aspirin on coronary endothelial function: Importance of the dose. World J Cardiol 2013; 5(11): 426-433
- URL: https://www.wjgnet.com/1949-8462/full/v5/i11/426.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i11.426
Aspirin, an inhibitor of cyclooxygenase-1, helps prevent cardiovascular disease. However, its efficacy with respect to primary prevention of cardiovascular events remains controversial[1-6]. Although several studies have shown that primary prevention with aspirin has a positive effect on cardiovascular disease[1-3], others have not shown any such relationship[4-6]. Aspirin increases the risk of bleeding, particularly gastrointestinal bleeding[7]. In addition, the discrepancies in the effect of primary prevention with aspirin may depend on the cardiovascular risk of individual patients. These factors may contribute to the study findings reported for this population; therefore, aspirin may be effective in the primary prevention of cardiovascular disease if the degree of risk for cardiovascular burdens and gastrointestinal bleeding are appropriately assessed. The efficacy of aspirin in the secondary prevention of cardiovascular disease is, however, well established[8,9], and aspirin reduces reoccurrence in patients with established cardiovascular disease.
The dose of aspirin used to prevent cardiovascular disease ranges from 75 to 325 mg/d[8,10]. Aspirin inhibits the synthesis of thromboxane A2 in platelets and prostaglandin I2 in endothelial cells. Low-dose aspirin only inhibits thromboxane A2 in platelets, whereas high-dose aspirin inhibits both thromboxane A2 and prostaglandin I2[11]. Low-dose aspirin (approximately 81-162 mg) has been widely used as a preventive therapy against cardiovascular disease[10]. This preventive effect of aspirin may be primarily due to its prevention of thrombus formation, which is mediated by inhibition of platelet aggregation[11]. However, several studies have shown a favorable effect of aspirin on endothelial function[12-16], and there is some interest in the relationship between aspirin and endothelial function. To investigate the existence of such a relationship in the coronary arteries, and if one exists, to confirm whether this relationship depends on the dose of aspirin used, we investigated the effects of different doses of aspirin on coronary endothelial function in patients with angiographically normal coronary arteries.
One hundred and thirty-nine Japanese patients who underwent coronary angiography to evaluate chest pain were included in this study. All had angiographically normal epicardial coronary arteries, normal left ventricular function (contrast ventriculographic ejection fraction; LVEF, ≥ 60%), and normal coronary flow reserve (CFR; > 2.0). We excluded patients with vasospastic angina, previous myocardial infarction, left ventricular hypertrophy, moderate-severe valvular disease detected using echocardiography, heart failure, or other serious diseases.
The patients were divided into two groups based on their aspirin intake: Group I consisted of 63 patients who took aspirin and Group II consisted of 76 patients who did not. The 63 patients in Group I were subdivided into Group Ia, consisting of 50 patients who took aspirin 100 mg/d, and Group Ib, consisting of 13 patients who took aspirin 500 mg/d in Group Ia, 32 patients had taken aspirin for a possible coronary artery disease before admission. The remaining 18 patients in Group Ia and 13 patients in Group Ib began taking aspirin on admission. All patients in Group I took aspirin for at least 2 d. Written informed consent was obtained from all patients before their entry into the study. The protocol was approved by the Ethics Committee of our institution.
All anti-anginal agents were discontinued at least 48 h before catheterization, except for sublingual nitroglycerin, which was withheld for 1 h before catheterization but was otherwise unrestricted. Diagnostic left heart catheterization and coronary angiography were performed using a standard percutaneous brachial approach. A 6F-guide catheter was introduced into the left main coronary artery. A 0.0014-inch Doppler flow guidewire (Volcano FloWire; Volcano Therapeutics Inc., Rancho Cordova, CA) was subsequently advanced through the guide catheter into the proximal segment of the left anterior descending coronary artery. The wire tip was positioned in a straight segment of the vessel to obtain a reliable flow-velocity signal.
After baseline control conditions were established, incremental doses of acetylcholine (ACh) were infused into the left coronary artery (3 and 30 μg/min) for 2 min with 5-min intervals between consecutive doses. After re-establishment of control conditions, nitroglycerin was intracoronarily infused at a rate of 200 μg/min for 1 min. Finally, adenosine triphosphate (20 μg) was infused. ACh and nitroglycerin (NTG) were directly infused into the left coronary ostium using an infusion pump (TE-311; Terumo, Tokyo, Japan) at a rate of 1 mL/min.
Coronary angiography was performed under controlled conditions and at the end of each drug infusion. Coronary blood flow (CBF) velocity was continuously monitored using a 12-MHz pulsed Doppler velocimeter (FloMap; Volcano Therapeutics Inc.). Arterial pressure, heart rate, and electrocardiogram were continuously monitored and recorded using a multichannel recorder (Polygraph 1600; Nihon Electric Corporation, Tokyo, Japan).
The method used for measuring the coronary diameter was previously described in detail[17-20]. The coronary segment 2 mm distal to the Doppler wire tip was selected for quantitative analysis. In each patient, the luminal diameters of selected segments of the left anterior descending coronary artery were measured by a single investigator blinded to angiographic and clinical data to determine the effects of the different drugs on epicardial coronary diameter. The luminal diameters were measured on an end-diastolic frame using a computer-assisted coronary angiographic analysis system (CAAS II/QUANTCOR; Siemens, Berlin and Munich, Germany). Means of triplicate measurements of luminal diameter were used for analysis. Changes in coronary diameter in response to ACh and NTG infusions are expressed as the percentage change from the baseline measurement on the angiogram obtained before infusion. Intra- and inter-observer variability have previously been reported to be excellent[17].
CBF was calculated as the product of CBF velocity and vessel diameter using the following formula: π× average peak velocity × 0.125 × diameter2. For CBF calculations, the internal diameter of the vessel at the location of the flow measurements (2 mm distal to the wire tip) was measured using the method described above. CFR was calculated as the ratio of CBF velocity after adenosine triphosphate infusion relative to baseline velocity.
As described previously[17,18,21-23], in the present study, we adopted the percent changes in epicardial coronary diameter in response to ACh and NTG infusions as the endothelium-dependent and -independent functions, respectively, of the coronary artery at the level of conduit vessels. When the ACh-induced changes in coronary diameter is reduced despite of preserved NTG-induced dilation, it is accepted that coronary endothelial dysfunction at the level of conduit vessel is present. In addition, we adopted the percent change in CBF in response to ACh infusion and CFR as the endothelium-dependent and -independent functions, respectively, of the coronary artery at the level of resistance vessels. When the ACh-induced increase in CBF is reduced despite of preserved CFR, it is accepted that coronary endothelial dysfunction at the level of resistance vessel is present.
Blood samples were drawn from each patient on the same day as coronary angiography after fasting. Total cholesterol, triglyceride, high-density lipoprotein -cholesterol, low-density lipoprotein -cholesterol, glucose, hemoglobin A1C, high-sensitive C-reactive protein (CRP), and fibrinogen levels were subsequently measured.
All data are expressed as mean ± SEM. Baseline characteristics of the two groups were compared using Student’s unpaired t test or χ2analysis, as appropriate. Serial changes in hemodynamic variables and changes in coronary vasoreactivity in response to drug infusion were compared using a one-way analysis of variance. If the analysis of variance showed a significant difference between means, the level of significance was determined by contrast analysis. Serial percentage changes in the coronary vascular response to ACh infusion were compared between groups using a two-way analysis of variance. Univariate and multivariate regression analyses were also performed to identify factors associated with percent changes in coronary artery diameter induced by ACh. A P value < 0.05 was defined as indicative of statistical significance.
The patient characteristics are detailed in Table 1. Age, sex, body mass index, frequency of coronary risk factors, medications, and LVEF were similar between the two groups. The patient characteristics between Groups Ia and Ib were also similar.
| Group I | Group II | P value | |
| (n = 63) | (n = 76) | ||
| Age | 60 ± 1 | 59 ± 1 | NS |
| Men/women | 40/23 | 46/30 | NS |
| Body mass index (kg/m2) | 24.6 ± 0.3 | 24.2 ± 0.3 | NS |
| Coronary risk factors | |||
| Smoking (%) | 22 (35) | 19 (25) | NS |
| Hypertension (%) | 29 (46) | 29 (38) | NS |
| Hypercholesterolemia (%) | 23 (37) | 30 (39) | NS |
| Diabetes mellitus (%) | 9 (14) | 6 (8) | NS |
| Medications | |||
| Statins (%) | 11 (17) | 13 (17) | NS |
| ACI and/or ARB (%) | 8 (13) | 11 (14) | NS |
| LV ejection fraction (%) | 70 ± 1 | 71 ± 1 | NS |
Data on the biochemical parameters are detailed in Table 2. The biochemical parameters did not differ between Group I and Group II; the parameters were also similar between Group Ia and Group Ib.
| Group I | Group II | p value | |
| Total cholesterol (mg/dL) | 210 ± 5 | 206 ± 5 | NS |
| Triglyceride (mg/dL) | 155 ± 10 | 144 ± 9 | NS |
| HDL-cholesterol (mg/dL) | 54 ± 2 | 52 ± 2 | NS |
| LDL-cholesterol (mg/dL) | 125 ± 5 | 125 ± 4 | NS |
| Fasting blood sugar (mg/dL) | 100 ± 2 | 98 ± 2 | NS |
| Hemoglobin A1C (%) | 5.5 ± 0.1 | 5.4 ± 0.1 | NS |
| C-reactive protein (mg/L) | 1.4 ± 0.4 | 2.1 ± 0.4 | NS |
| Fibrinogen (mg/dL) | 340 ± 21 | 350 ± 22 | NS |
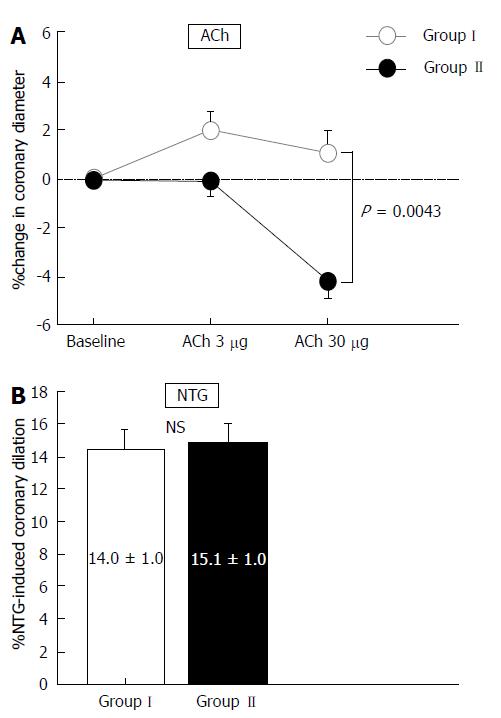
The hemodynamic and coronary vasoreactivity findings are shown in Table 3. Hemodynamics were similar between the two groups, as were the baseline coronary artery diameter and CBF. Changes in coronary artery diameter in response to ACh infusion were reduced in Group II compared with those in Group I (P = 0.0043), whereas NTG-induced coronary dilation did not differ between the two groups (Figure 1 and Table 3). The increase in CBF in response to ACh infusion and CFR did not differ between the two groups (Table 3).
| Group I | Group II | P value | |
| Baseline mean BP(mmHg) | 107 ± 2 | 104 ± 1 | NS |
| Baseline heart rate (/min) | 66 ± 1 | 67 ± 1 | NS |
| Coronary diameter | |||
| Baseline (mm) | 3.22 ± 0.07 | 3.07 ± 0.06 | NS |
| ACh at 3 μg/min (mm) | 3.28 ± 0.07 | 3.07 ± 0.07 | NS |
| (% change) | 1.8 ± 0.9 | 0.1 ± 0.8 | NS |
| ACh at 30 μg/min (mm) | 3.26 ± 0.08 | 2.95 ± 0.07 | 0.0030 |
| (% change) | 1.2 ± 1.1 | -3.9 ± 1.0 | 0.0008 |
| Nitroglycerin (mm) | 3.67 ± 0.07 | 3.51 ± 0.07 | NS |
| (% change) | 14.0 ± 1.1 | 15.1 ± 1.0 | NS |
| Coronary blood flow | |||
| Baseline (mL/min) | 88.1 ± 3.7 | 81.9 ± 3.3 | NS |
| ACh at 3 μg/min (mL/min) | 131.3 ± 7.8 | 123.3 ± 7.1 | NS |
| (% change) | 50.0 ± 6.3 | 52.1 ± 5.7 | NS |
| ACh at 30 μg/min (mm) | 219.9 ± 21.4 | 194.8 ± 19.5 | NS |
| (% change) | 172.8 ± 33.2 | 142.6 ± 30.3 | NS |
| Coronary flow reserve | 3.4 ± 0.2 | 3.4 ± 0.1 | NS |
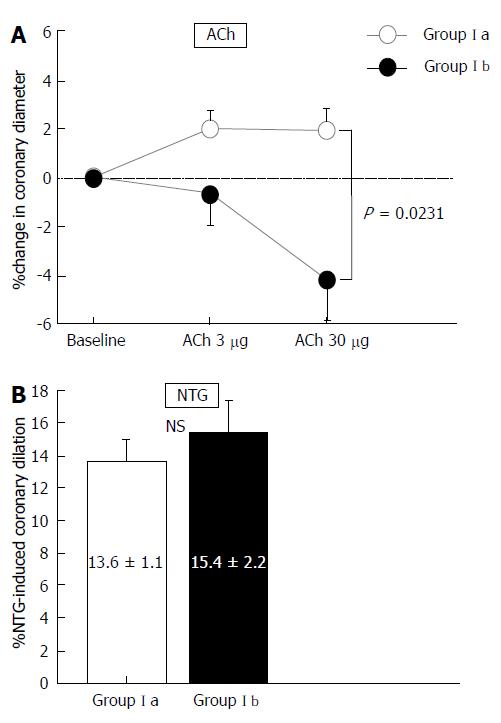
The hemodynamic and coronary vasoactivity findings for the subgroups of Group I are shown in Table 4. Hemodynamics, coronary artery diameter, and CBF at baseline did not differ between Group Ia and Group Ib (Table 4). However, changes in coronary artery diameter in response to ACh infusion were reduced in Group Ib compared with those in Group Ia (P = 0.0231). NTG-induced coronary dilation did not differ between the two groups (Table 4, Figure 2). The increase in CBF in response to ACh infusions or CFR did not differ between the two groups (Table 4). Statistically significant differences were observed in the percentage change in coronary diameter induced by ACh infusion at a dose of 30 μg/min, and the subsequent analyses were performed using this value.
| Group Ia | Group Ib | P value | |
| (n =50) | (n = 13) | ||
| Baseline mean BP (mmHg) | 108 ± 2 | 105 ± 3 | NS |
| Baseline heart rate (/min) | 67 ± 2 | 65 ± 3 | NS |
| Coronary diameter | |||
| Baseline (mm) | 3.23 ± 0.08 | 3.14 ± 0.16 | NS |
| ACh at 3 μg/min (mm) | 3.32 ± 0.08 | 3.05 ± 0.17 | NS |
| (% change) | 2.4 ± 0.9 | -0.6 ± 1.8 | NS |
| ACh at 30 μg/min (mm) | 3.32 ± 0.08 | 3.05 ± 0.17 | NS |
| (% change) | 2.6 ± 1.1 | -3.8 ± 2.9 | 0.0123 |
| Nitroglycerin (mm) | 3.67 ± 0.08 | 3.65 ± 0.16 | NS |
| (% change) | 13.6 ± 1.1 | 15.4 ± 2.2 | NS |
| Coronary blood flow | |||
| Baseline (mL/min) | 91.0 ± 4.6 | 76.8 ± 9.1 | NS |
| ACh at 3 μg/min (mL/min) | 137.7 ± 9.5 | 106.8 ± 18.7 | NS |
| (% change) | 53.4 ± 6.8 | 37.0 ± 13.4 | NS |
| ACh at 30 μg/min (mm) | 195.2 ± 15.9 | 188.6 ± 31.1 | NS |
| (% change) | 125.3 ± 16.2 | 141.9 ± 31.7 | NS |
| Coronary flow reserve | 3.4 ± 0.2 | 3.7 ± 0.3 | NS |
As noted above, statistically significant differences between the two groups were observed in the percentage change in coronary diameter induced by ACh infusion at a dose of 30 μg/min. Univariate analysis revealed that the presence or absence of aspirin (P = 0.0002), NTG-induced coronary dilation (P = 0.0142), and the increase in CBF in response to ACh infusion at a dose of 3 μg/min were associated with the change in coronary artery diameter response induced by ACh infusion at 30 μg/min; the mean blood pressure at baseline also showed a trend toward a positive association with the change in coronary artery diameter associated with ACh infusion at 30 μg/min (P = 0.0888). Multivariate regression analysis using these parameters demonstrated that low-dose aspirin (P = 0.0004), NTG-induced coronary dilation (P = 0.0077), and the increase in CBF induced by ACh infusion at a dose of 3 μg/min (P = 0.0344) were positively associated with the change in coronary artery diameter induced by ACh infusion at 30 μg/min, and that not taking aspirin (P = 0.0387) was negatively associated with this change (r2 = 0.212; Table 5).
| Variables | %change in coronary diameter | |
| induced by ACh 30 mg/min | ||
| t value | P value | |
| Taking aspirin | ||
| (+) at the low dose | 3.61 | 0.0004 |
| (-) | -2.09 | 0.0387 |
| Nitroglycerin-induced dilation | 2.71 | 0.0077 |
| %increase in CBF at ACh 3 mg/min | 2.14 | 0.0344 |
| Mean blood pressure at baseline | 1.79 | 0.0765 |
In the present study, we investigated the effects of different doses of aspirin on coronary endothelial function in patients with angiographically normal coronary arteries. We showed that the change in coronary artery diameter in response to ACh infusion was higher in patients who took aspirin than in those who did not take aspirin. However, NTG-induced coronary dilation, the increase in CBF in response to ACh infusion, and CFR were not significantly different between the two groups. In addition, among patients who took aspirin, the change in coronary artery diameter in response to ACh infusion was higher in those who took a low dose of aspirin (100 mg/d) than in those who took a higher dose of aspirin (500 mg/d). Multivariate regression analysis demonstrated that taking a low dose of aspirin was positively associated with ACh-induced coronary artery dilation and not taking aspirin was negatively associated with such dilation. These findings suggest that taking a low dose of aspirin has a positive influence on coronary endothelial function in patients with angiographically normal coronary arteries.
The preventive effect of aspirin against cardiovascular disease is mainly because of its inhibition of platelet aggregation, which is mediated by the inhibition of thromboxane A2 in platelets and prevention of thrombus formation[11]. However, there has been some interest in the relationship between aspirin and endothelial function[12-16]. Husain et al[12] reported that intra-arterial co-infusion of aspirin (1000 mg) restored ACh-induced microvascular endothelial dysfunction of the femoral vasculature in patients with coronary atherosclerosis and atherosclerotic burdens. In addition, Noon et al[13] showed that intra-arterial co-infusion of aspirin (600 mg) restored ACh-induced microvascular endothelial dysfunction of the forearm in hypercholesterolemic patients but not in control subjects. Monobe et al[14], Magen et al[15], and Furuno et al[16] have reported a relationship between aspirin and endothelial function using flow-mediated dilation (FMD) of the brachial artery. Monobe et al[14] reported that FMD was higher in hypercholesterolemic patients who took a low dose of aspirin (100 mg). Magen et al[15] reported that FMD was higher in hypertensive patients who took a low dose of aspirin (100 mg). Furuno et al[16] investigated the effects of various doses of aspirin on FMD in healthy male subjects and showed that aspirin had a positive influence even in healthy volunteers. Taking these studies into consideration, the effect of aspirin on endothelial function may, in part, depend on the severity of the atherosclerotic burden or on the dose of aspirin.
In the present study, we showed that aspirin has a positive influence on coronary endothelial function in patients with chest pain who have angiographically normal coronary arteries. When assessing coronary endothelial function, it is advantageous to simultaneously assess endothelial function at the level of both the conduit and resistance vessels[21,22]. In this study, aspirin only had a positive effect on coronary endothelial function at the level of the conduit vessels. In general, endothelium-derived nitric oxide (NO) and prostaglandin I2 act as an endothelium-derived vasodilators, primarily in large vessels[24-26]; this may account for the effect of aspirin on coronary endothelial function being limited to the level of the conduit vessels.
With regard to the dose of aspirin administered, 75-325 mg/d and particularly, 75-162 mg/dare widely used in the clinical setting[8,10]. Although a high dose of aspirin is effective in preventing cardiovascular disease[8], bleeding increases as the dose of aspirin increases[27], and this may explain the widespread use of low-dose of aspirin. Theoretically, a high dose of aspirin inhibits both thromboxane A2 in the platelets and prostaglandin I2 in endothelial cells; therefore, it is not unexpected that a high dose of aspirin has a negative influence on endothelial function. However, only one study has shown the relationship between the dose of aspirin administered and endothelial function[16]. Furuno et al[16] reported that the maximum effect of aspirin on endothelial function was observed at 162 mg/d, whereas the minimum effect was observed at 660 mg/d. In the present study, because a small number of patients took a high dose of aspirin, the multivariate regression analysis did not show that taking a high dose of aspirin led to a deterioration in endothelial function. However, in the subgroup analysis, the coronary endothelial function of patients who took a high dose of aspirin was significantly lower than that of patients who took a low dose. These results suggest that a low dose of aspirin is superior to a high dose of aspirin for improving endothelial function.
Several studies have examined possible mechanisms associated with the positive effect of aspirin on endothelial function[12,28-31]. Theoretically, a low dose of aspirin inhibits only thromboxane A2, which is an endothelium-derived, cyclooxygenase-dependent constricting factor, leading to vasodilation[12]. Furthermore, aspirin has a positive effect on endothelium-derived NO. Aspirin directly enhances NO synthesis in endothelial cells[28,29]; it delays the onset of endothelial senescence[30] and reduces oxidative stress[31]. These factors may contribute to aspirin-induced improvement of endothelial function. It is possible that vascular inflammation causes endothelial dysfunction[18], but aspirin has not been observed to have any influence on the assessment of high-sensitive CRP. Therefore, the anti-inflammatory effect of aspirin may not be involved in the mechanism through which aspirin exerts its positive effect on endothelial function.
The present study demonstrated that a low dose of aspirin had a positive effect on coronary endothelial function in patients with angiographically normal coronary arteries. However, this does not always imply that a low dose of aspirin should be administered for the primary prevention of cardiovascular disease. As mentioned above, there is no doubt that aspirin frequently causes gastrointestinal bleeding[27]; therefore, despite the positive effect of aspirin on coronary endothelial function, a low dose of aspirin should be used, particularly for primary prevention in consideration of the balance of atherosclerotic burden and bleeding risks.
There are several limitations to the present study. First, all patients in our study had chest symptoms and had undergone coronary angiography; thus, they may represent a specific group. Therefore, the results of the present study may not be representative of endothelial function in all patients. Second, the duration of aspirin intake was not consistent between the patients who took aspirin. This difference may have influenced the results, such as the high-sensitive CRP level. Third, the number of patients who took a high dose of aspirin was small, and this may also have influenced the results. However, it was not ethically possible to increase the number of patients in this subgroup. Finally, we did not measure biochemical parameters and platelet function associated with aspirin. Therefore, we cannot report on the precise mechanisms by which aspirin had a positive influence on coronary endothelial function in the present study.
In conclusion, our findings suggest that only low-dose aspirin has a positive effect on coronary endothelial function in patients who have chest pain but angiographically normal coronary arteries. The favorable effect of aspirin on coronary endothelial function as well as the prevention of thrombus formation may be involved in the mechanisms responsible for the preventive effects of aspirin against cardiovascular disease.
We would like to thank Dr. Yuichi Fujii, Dr. Noritaka Fujimura, and Dr. Tatsuya Maruhashi for their help with coronary catheterization. We would also like to thank Ms. Michiko Aoyama, Yuka Shiroshita-Tanaka, and Ryoko Tachiyama for their administrative assistance.
Aspirin, an inhibitor of cyclooxygenase-1, helps prevent cardiovascular disease. This preventive effect of aspirin may be primarily due to its prevention of thrombus formation. In addition, several studies have reported a relationship between aspirin and endothelial function. However, it has not been fully elucidated whether aspirin has a positive influence on coronary endothelial function.
Aspirin has a preventive effect against cardiovascular disease, mainly mediated by its anti-platelet effect. Clarifying the relationship between aspirin and endothelial function may reveal other mechanisms responsible for the preventive effect of aspirin against cardiovascular diseases.
The results showed that acetylcholine (ACh)-induced coronary artery dilation was higher in patients who took aspirin compared with patients who did not take aspirin, whereas nitroglycerin (NTG)-induced coronary artery dilatation and coronary blood flow increase in response to ACh or coronary flow reserve did not differ significantly between the 2 groups. Furthermore, aspirin-induced coronary artery dilation in response to ACh was higher in patients who took low-dose aspirin, compared with patients who took high-dose aspirin. These findings suggest that only low-dose aspirin has a positive effect on coronary endothelial function in such patients.
Aspirin should be used in primary prevention, but in consideration of the balance between atherosclerotic burden and bleeding risks because it can cause gastrointestinal bleedings. However, if taking aspirin, a low dose should be recommended for improving endothelial function.
There are two components of coronary vascular functions: at the level of conduit vessels (epicardial coronary artery) and at the level of resistance vessels (microvascular coronary artery). In addition, there are two factors of coronary artery vasodilation: endothelium-dependent and -independent. In the present study, using quantitative coronary angiography and Doppler velocity measurements, the authors defined the percent changes in epicardial coronary diameter in response to ACh and NTG infusions as the endothelium-dependent and -independent functions, respectively, of the coronary artery at the level of conduit vessels, and the authors defined the percent change in coronary blood flow in response to ACh infusion and coronary flow reserve as the endothelium-dependent and -independent functions, respectively, of the coronary artery at the level of resistance vessels.
The research is important in that it provides new evidence of aspirin effect on the endothelial function of the coronary artery. The experiments are well designed with good controls matched with age, gender, body mass index, coronary risk factors, medications, left ventricular function, as well as many biochemical parameters. The study also excluded many apparent heart diseases, making the sampled population more homogenous. Paper is well organized.
P- Reviewers: Chang YC, Gurevich VV, Pasceri V S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2851] [Cited by in RCA: 2616] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 2. | Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Raju N, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med. 2011;124:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 652] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 564] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 6. | Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 484] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 7. | McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. [PubMed] |
| 9. | Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Smith SC, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Amin AR, Attur MG, Pillinger M, Abramson SB. The pleiotropic functions of aspirin: mechanisms of action. Cell Mol Life Sci. 1999;56:305-312. [PubMed] |
| 12. | Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716-720. [PubMed] |
| 13. | Noon JP, Walker BR, Hand MF, Webb DJ. Impairment of forearm vasodilatation to acetylcholine in hypercholesterolemia is reversed by aspirin. Cardiovasc Res. 1998;38:480-484. [PubMed] |
| 14. | Monobe H, Yamanari H, Nakamura K, Ohe T. Effects of low-dose aspirin on endothelial function in hypertensive patients. Clin Cardiol. 2001;24:705-709. [PubMed] |
| 15. | Magen E, Viskoper JR, Mishal J, Priluk R, London D, Yosefy C. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. J Hum Hypertens. 2005;19:667-673. [PubMed] |
| 16. | Furuno T, Yamasaki F, Yokoyama T, Sato K, Sato T, Doi Y, Sugiura T. Effects of various doses of aspirin on platelet activity and endothelial function. Heart Vessels. 2011;26:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001;86:212-216. [PubMed] |
| 18. | Teragawa H, Fukuda Y, Matsuda K, Ueda K, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart. 2004;90:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Teragawa H, Mitsuba N, Nishioka K, Ueda K, Kono S, Higashi Y, Chayama K, Kihara Y. Impaired coronary microvascular endothelial function in men with metabolic syndrome. World J Cardiol. 2010;2:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Teragawa H, Mitsuba N, Ishibashi K, Nishioka K, Kurisu S, Kihara Y. Evaluation of coronary microvascular function in patients with vasospastic angina. World J Cardiol. 2013;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Shiode N, Morishima N, Nakayama K, Yamagata T, Matsuura H, Kajiyama G. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol. 1996;27:304-310. [PubMed] |
| 22. | Kato M, Shiode N, Yamagata T, Matsuura H, Kajiyama G. Coronary segmental responses to acetylcholine and bradykinin in patients with atherosclerotic risk factors. Am J Cardiol. 1997;80:751-755. [PubMed] |
| 23. | Matsuda K, Teragawa H, Fukuda Y, Nakagawa K, Higashi Y, Chayama K. Leptin causes nitric-oxide independent coronary artery vasodilation in humans. Hypertens Res. 2003;26:147-152. [PubMed] |
| 24. | Lüscher TF, Richard V, Tschudi M, Yang ZH, Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol. 1990;15:519-527. [PubMed] |
| 25. | Joannides R, Richard V, Haefeli WE, Benoist A, Linder L, Lüscher TF, Thuillez C. Role of nitric oxide in the regulation of the mechanical properties of peripheral conduit arteries in humans. Hypertension. 1997;30:1465-1470. [PubMed] |
| 26. | Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol. 1999;31:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Baggish JS, Bhatt DL, Topol EJ. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Grosser N, Schröder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol. 2003;23:1345-1351. [PubMed] |
| 29. | Taubert D, Berkels R, Grosser N, Schröder H, Gründemann D, Schömig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Bode-Böger SM, Martens-Lobenhoffer J, Täger M, Schröder H, Scalera F. Aspirin reduces endothelial cell senescence. Biochem Biophys Res Commun. 2005;334:1226-1232. [PubMed] |
| 31. | Bulckaen H, Prévost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, Garçon G, Corman B, Gosset P, Shirali P, Creusy C, Puisieux F. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. Am J Physiol Heart Circ Physiol. 2008;294:H1562-H1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |