Revised: October 10, 2010
Accepted: October 16, 2010
Published online: February 26, 2011
AIM: To evaluate the left ventricular structure and function in isolated metabolic syndrome.
METHODS: One hundred and fifty six consecutive adults with metabolic syndrome were enrolled in the study. Fifty nine had isolated metabolic syndrome (group A) and 97 had metabolic syndrome with hypertension and/or diabetes (group B). There was a control group of 34 healthy adults. In addition to classic echocardiographic assessment of myocardial structural and functional changes, the Tei index was used to evaluate global left ventricular performance.
RESULTS: There were no statistically significant differences between group A and controls in all parameters of left ventricular structural, systolic, and diastolic function except global myocardial performance (Tei index). On the other hand, significant differences were observed between group B and the control group in most of the parameters of left ventricular structural and global performance.
CONCLUSION: The early identification of isolated metabolic syndrome in non-diabetic, non-hypertensive adults may be an indication that aggressive preventive measures should not be postponed until overt obesity, hypertension or diabetes mellitus has developed.
- Citation: Sliem H, Nasr G, Ibrahiem D. Global left ventricular performance in non-diabetic non-hypertensive metabolic syndrome adults. World J Cardiol 2011; 3(2): 48-53
- URL: https://www.wjgnet.com/1949-8462/full/v3/i2/48.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i2.48
Metabolic syndrome is a condition characterized by the accumulation of multiple risk factors (insulin resistance, hyperglycemia, dyslipidemia, hypertension, visceral obesity) for cardiovascular disease (CVD) in an individual with a background of obesity and/or lack of exercise[1]. However, it is not known whether isolated metabolic syndrome (hyperglycemia or elevated blood pressure but not diabetes mellitus or hypertension)[2] is also associated with abnormal cardiac structure and function. If isolated metabolic syndrome indicates persons who have already developed abnormal left ventricular (LV) structure and function, early recognition of isolated metabolic syndrome would be important.
The new International Diabetes Federation (IDF) definition of metabolic syndrome, when compared to the National Cholesterol Education Program Adult Treatment Panel III (ATPIII) criteria, allows for better applicability to different ethnic groups, because of ethnic-specific cutoffs for identification of visceral obesity[3,4].
The Tei index was introduced by Tei et al[5] as a Doppler-derived index that combines both systolic and diastolic function to separate those with normal ventricular function from those with ventricular dysfunction. This index has been found to correlate well with invasive measures of systolic and diastolic LV function[6]. Measurement of the Tei index is non-invasive and easily obtained, does not require the presence of an experienced echocardiographer, and it does not significantly prolong the time required for the examination[7].
The goal of the current study was to examine the echocardiographic parameters of LV structural and global performance using the Tei index in metabolic syndrome patients with and without hypertension and/or diabetes and in healthy controls.
A case-control study was performed. One hundred and fifty six consecutive adults with metabolic syndrome were enrolled in the study. All were recruited from the outpatient cardiology, diabetes and general medicine clinics of Suez Canal University Hospital from November 2007 to April 2010. Fifty nine patients fulfilling the hyperglycemia or elevated blood pressure criteria for metabolic syndrome, but not the criteria for diabetes mellitus or hypertension, were considered as having isolated metabolic syndrome (group A). Ninety seven patients had metabolic syndrome with hypertension and/or diabetes, (group B). Thirty four healthy adults with no metabolic syndrome and matched for age and gender comprised the control group. Metabolic syndrome was diagnosed according to the International Diabetes Federation (IDF) definition: waist circumference ≥ 94/80 cm (men/women) plus any 2 of the following 4 factors: increased triglyceride level ≥ 150 mg/dL or a specific treatment for this lipid abnormality; reduced high density lipoprotein cholesterol < 40/
50 mg/dL (men/women) or a treatment specific for this lipid abnormality; raised blood pressure (BP): systolic BP ≥ 130 or diastolic BP ≥ 85 mm Hg or treatment of previously diagnosed hypertension; raised fasting plasma glucose ≥ 100 mg/dL or treatment of previously diagnosed type 2 diabetes[3].
Exclusion criteria were the following: chronic kidney disease; a history or findings of cardiovascular disease including heart failure symptoms or systolic dysfunction; coronary artery disease; significant valvular heart disease (i.e. greater than mild valvular insufficiency, or stenosis) and/or hypertrophic cardiomyopathy; pregnancy or lactation; and/or major systemic illness.
All groups had a full medical history and clinical examination including BP measurement, anthropometric measures, systemic examination, biochemical tests including lipid profile, fasting plasma glucose (FPG), glycosylated hemoglobin (Hb) A1c, and echocardiographic studies. Body mass index (BMI) was calculated as weight/height2 (kg/m2) and was used as an estimate of overall adiposity. Waist circumference, a validated estimate of visceral adiposity, was measured to the nearest 0.5 cm[8]. Systemic hypertension was defined according to the Joint National Committee VII (JNC VII) criteria, as a BP > 140/90 mmHg and/or under current antihypertensive therapy[9]. Diabetes was defined according to revised American Diabetes Association criteria as (a) fasting serum glucose level ≥ 126 mg%, or HbA1c ≥ 6.5 and/or (b) current medical therapy with an oral hypoglycemic agent and/or insulin[10].
M-Mode and 2D echocardiographic studies were performed with a Hewlett-Packard phased array ultrasonoscope (Sonos 1800, USA, model: DR 53 15) using a 2.5 and 3.5 MHz transducer.
Parameters of LV structure: LV dimensions (systolic diameter (SD) and diastolic diameter (DD), LV diameter (LVD), interventricular septum (IVS) and posterior wall (PW) thicknesses were measured at end diastole (R wave of electrocardiogram) and end systole (maximum posterior motion of septum) and were indicated by d and s, respectively. All were detected in the parasternal long-axis view during M-mode tracing according to the recommendation of the American Society of Echocardiography[11]. LV mass (LVM) was calculated according to the modified cube formula of Mayosi et al[12] as follows: LVM = 1.01[(IVSd + PWd + DD)3 - (DD)3] - 13.6 g.
LVM index (LVMI) was then calculated as follows: LVMI=LVM/m2, where m was the height of the patient in meters. Relative wall thickness (RWT) was calculated as the ratio (IVSd+PWd)/LVDd. LV geometric pattern was considered normal if LVMI was < 50 g/m2 and RWT was < 0.44. Concentric remodeling was diagnosed when LVMI was < 50 g/m2 and RWT was > 0.44; concentric hypertrophy was defined as LVMI > 50 g/m2 and RWT > 0.44; eccentric hypertrophy was diagnosed when LVMI was > 50 g/m2 and RWT was < 0.44.
Parameters of systolic function: LV end-diastolic and end-systolic volumes (EDV and ESV) were calculated according to Abraham et al[13]; stroke volume was calculated as the difference between EDV and ESV; cardiac output (CO) was obtained as the product of stroke volume and heart rate (HR). Ejection fraction (EF) was calculated as EF% = 100 × (EDV–ESV)/EDV[14].
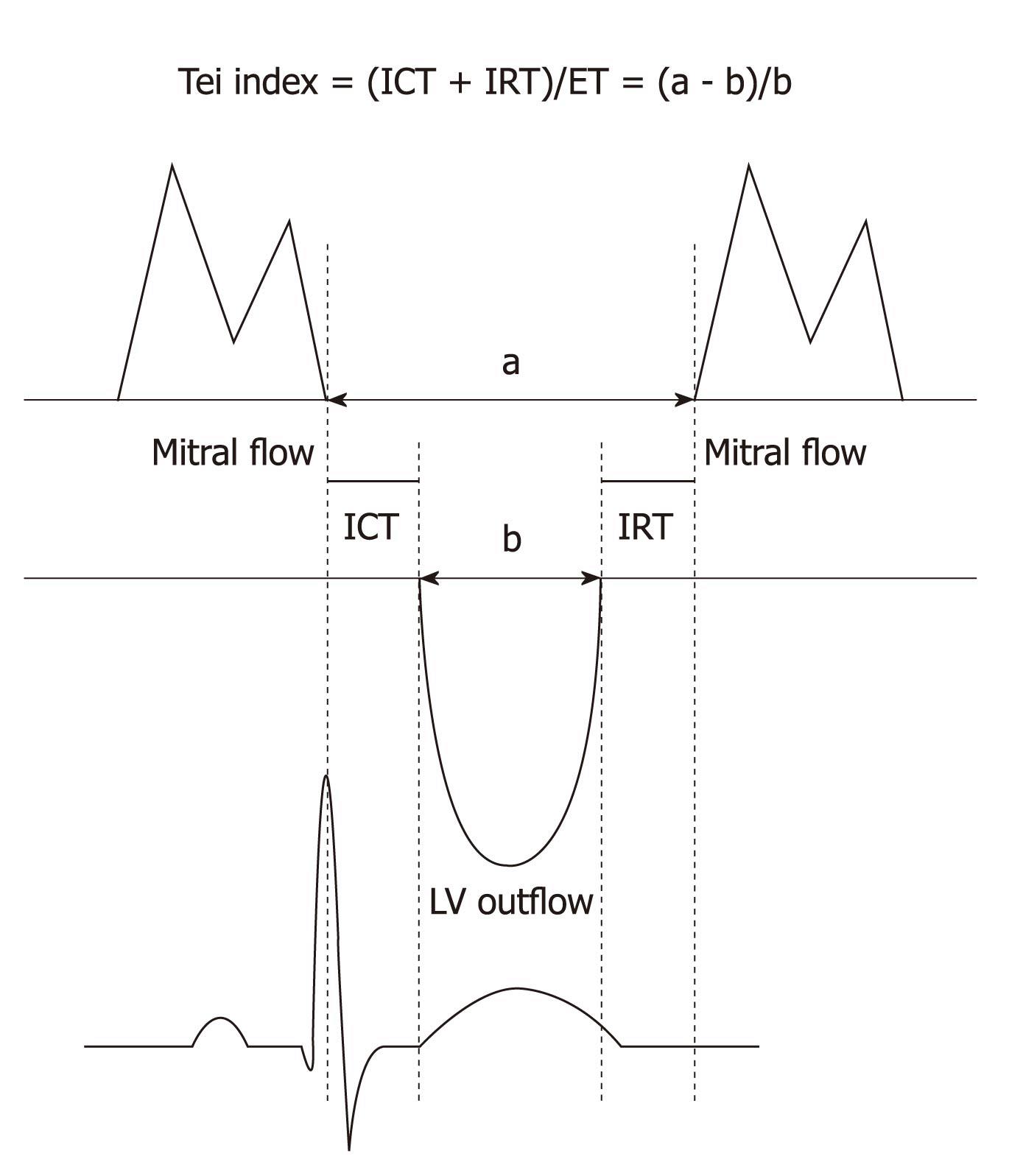
Parameters of diastolic function: assessment of diastolic function was obtained by pulsed-wave Doppler of both transmitral and pulmonary venous flow patterns recorded in the apical 4-chamber view. Peak flow velocity in early diastole (E wave) and during atrial contraction (A wave) and peak E/A ratio were measured. LV isovolumetric relaxation time (IRT) was also measured in ms as the interval between the aortic valve closure click and the start of mitral flow[15].
The following time intervals were measured for Tei index calculation: IRT (ms: from the end of the S wave to the beginning of the E wave); isovolumetric contraction time (ICT ms: from the beginning of the first positive deflection after the Q wave to the onset of the S wave; ejection time (ET) ms: From the beginning to the end of the S wave. The Tei index was calculated as (ICT+IRT)/ET (Figure 1)[16]. A Tei index < 0.40 is considered normal. Higher index values correspond to more pathological states with overall cardiac dysfunction[17].
Informed consent was obtained from all adults. The aim and the value of the work were explained in a simplified manner for them. There was no harm inflicted on them. On the contrary all showed a benefit in the follow-up and in the final results of the study. The study was approved by the Ethics Committee of Faculty of Medicine, Suez Canal University. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s Human Research Committee.
According to the type of data, the Student unpaired t test and the χ2 test were used for statistical comparisons between 2 groups. Descriptive statistics were obtained including mean, standard deviation, mode and median for quantitative variables and frequency and percent for qualitative variables. The analysis was carried out by a computer program (SPSS Version 11). The P value was set at < 0.05 for statistically significant results and at < 0.0001 for highly significant results.
Baseline characteristics of 59 patients (33 male, 26 female, mean age 44.4 years) with isolated metabolic syndrome (group A), 97 patients (51 male, 46 female, mean age
49.9 years) with metabolic syndrome (group B) and 34 healthy controls (19 male, 15 female, mean age 46.9 years) are shown in Table 1. In group B, 20 patients had diabetes mellitus, 36 patients had hypertension, and 41 patients had both. The average durations of diabetes and hypertension were 5.4 and 7.2 years, respectively. The majority of diabetics were taking secretogogues, 75% of the hypertensive patients were taking angiotensin converting enzyme inhibitors, but only 40% of patients with dyslipidemia were taking statins. No patient in group A was taking any medications for elevated blood sugar or high blood pressure. No significant difference was observed among the groups regarding age. There were significant differences in BMI, waist circumference, BP, FBS, and triglycerides between group B and the control group. There were significant differences between group A and group B regarding BP, FPG and HbA1c.
| Characteristics | Controls (n = 34) | Group A (n = 59) | Group B (n = 97) | P | P1 | P2 |
| Age (yr) | 46.9 ± 7.4 | 44.4 ± 7.3 | 49.9 ± 8.3 | NS | NS | NS |
| SBP (mmHg) | 117.5 ± 6.1 | 134.4 ± 5.6 | 140.1 ± 8.1 | NS | 0.01 | < 0.05 |
| DBP (mmHg) | 78.9 ± 6.4 | 85.3 ± 4.3 | 91.9 ± 7.9 | NS | 0.05 | < 0.05 |
| BMI (%) | 22.2 ± 1.2 | 28.7 ± 3.6 | 31.8 ± 4.7 | < 0.5 | < 0.01 | NS |
| W. Circum. (cm) | 82.2 ± 4.6 | 98.1 ± 6.5 | 105.1 ± 7.1 | < 0.05 | < 0.05 | NS |
| HDL (mg/dL) | 45.7 ± 6.9 | 40.8 ± 6.5 | 43.2 ± 7.6 | NS | NS | NS |
| Triglyceride (mg/dL) | 138.9 ± 16.1 | 174.7 ± 23.3 | 181.9 ± 24.1 | < 0.05 | < 0.05 | NS |
| FPG (mg/dL) | 91.7 ± 10.6 | 98.6 ± 15.2 | 118.4 ± 43.6 | < 0.05 | < 0.01 | < 0.05 |
| HbA1c (%) | 5.10 ± 0.41 | 6.20 ± 0.30 | 7.40 ± 1.10 | < 0.05 | < 0.01 | < 0.05 |
Echocardiographic and Doppler data are shown in Table 2. There were no statistically significant differences between group A and controls in all parameters of LV structural, systolic, and diastolic function except the global myocardial performance (Tei index). On the other hand, significant differences were observed between group B and the control group in most of the parameters of LV structural and global performance. Comparing echocardiographic parameters between group A and B, no significant differences were observed except for LVMI, RWT, and E/A ratio.
| Characteristics | Controls (n = 34) | Group A (n = 59) | Group B (n = 97) | P | P1 | P2 |
| End SD (mm) | 28.8 ± 1.5 | 29.1 ± 1.4 | 28.8 ± 1.4 | NS | NS | NS |
| End DD (mm) | 48.7 ± 4.6 | 50.6 ± 4.4 | 49.9 ± 4.5 | NS | NS | NS |
| EF (%) | 63.9 ± 7.9 | 63.5 ± 6.5 | 62.7 ± 7.4 | NS | NS | NS |
| IVS thickness (mm) | 8.6 ± 0.5 | 8.4 ± 0.6 | 9.2 ± 0.7 | NS | < 0.05 | NS |
| PW thickness (mm) | 8.6 ± 0.7 | 8.6 ± 0.6 | 9.3 ± 0.9 | NS | < 0.05 | NS |
| RWT (%) | 36.3 ± 3.6 | 37.9 ± 3.8 | 47.7 ± 5.8 | NS | < 0.05 | < 0.05 |
| LVMI (g/m2) | 88.2 ± 7.4 | 93.2 ± 4.1 | 120.8 ± 26.8 | NS | < 0.05 | < 0.01 |
| E/A ratio | 1.41 ± 0.19 | 1.26 ± 0.12 | 1.17 ± 0.08 | NS | < 0.01 | < 0.05 |
| Tei index | 0.36 ± 0.07 | 0.64 ± 0.17 | 0.87 ± 0.12 | < 0.01 | < 0.001 | NS |
The value of a diagnosis of metabolic syndrome has been challenged because it includes persons with established hypertension and diabetes mellitus, components already known to be CVD risk factors. Metabolic syndrome also includes persons with mild hyperglycemia, but not diabetes, who are at an increased risk of developing overt diabetes[18]. Established hypertension is a powerful risk factor for CVD, but those with pre-hypertension may already manifest detrimental changes in cardiac structure and function[19]. To investigate this further, the current study was undertaken to evaluate whether isolated metabolic syndrome is also associated with abnormal cardiac structure and function. If isolated metabolic syndrome identifies persons who have already developed abnormal LV structural and functional changes, the importance of early recognition of isolated metabolic syndrome would be enhanced.
In the current study, we found structural modifications of the heart in patients with metabolic syndrome. The associated established hypertension and/or diabetes could be thought to be responsible factors in the induction of the structural cardiac changes. Analysis of the current data revealed that concentric hypertrophy appears to be the most obvious morphological change (LVMI > 50 g/m2 and RWT > 44). Similar results were recorded in various studies[2,20-22]. However, in the current study we observed that, in the metabolic syndrome group, the increase in LVM could be attributed to an increase in septal and posterior wall thickness without changes in LV diastolic diameters, compared with no increase in LVM in the isolated metabolic syndrome group. The concentric hypertrophy may be the result of a lack of increase in LV end-diastolic dimensions, whereas wall thickness increases under the stimulus of the elevated total vascular resistance.
The E/A ratio exhibited a stepwise decrease from the control group to the isolated metabolic syndrome group to the metabolic syndrome group, primarily as a result of increased A-wave velocity. The deceleration time and isovolumic relaxation time were significantly longer in the metabolic syndrome group. These findings suggest that there is a progressive impairment in LV relaxation depending on the component of the metabolic syndrome. The present data showed a significant decrease in diastolic function for group B vs control, indicating impairment in diastolic function with the increasing burden of metabolic syndrome.
The results of the present study are consistent with those of prior studies that identified hypertension, diabetes mellitus, and obesity as independent predictors of impaired LV structure and function[23-29]. Increased LV mass, RWT, and deceleration time have been reported in hypertensive subjects with metabolic syndrome compared with a hypertensive cohort without metabolic syndrome[22]. In the Strong Heart Study, those with metabolic syndrome had greater LVM and RWT and significantly lower E/A ratio[21]. Similarly, in the current study, LV diastolic function was not found in the isolated metabolic group but was present in the metabolic syndrome group.
The above altered geometric pattern was associated in the current study with a non significant depressed systolic function in both groups, and significantly altered diastolic function in the metabolic syndrome group. These different findings might be due to the variability in metabolic diagnostic criteria and subsequently the total vascular resistance.
Tei Chuwa devised and published an index of myocardial performance (the Tei index) in 1995 that evaluated LV systolic and diastolic function in combination[16]. The index has proved to be a reliable method for the evaluation of LV systolic and diastolic performance, with clear advantages over older established indexes and has prognostic value in many kinds of heart disease[30,31].
In the current study LV global function was assessed using the Tei index. The Tei index exhibited a stepwise increase from the control group to the isolated metabolic syndrome group to the metabolic syndrome group. In spite of the apparent normal LV systolic function (as shown by a normal EF), the high Tei index predicted the presence of early combined systolic and diastolic function in isolated metabolic syndrome.
Other studies have previously shown that visceral obesity is associated with diastolic dysfunction, an effect that may be mediated by an obesity-related pro-inflammatory state and/or by suppression of adiponectin expression[24,26]. In the current study, even in the isolated metabolic group who were non-diabetic and non-hypertensive and had a BMI significantly lower than that the metabolic syndrome group, the altered global ventricular performance may be mediated by other potential mechanisms. This may contribute to insulin resistance, hypertriglyceridemia with subsequent impaired endothelial dysfunction, abnormalities in myocardial perfusion and/or metabolic substrate utilization, inflammation and oxidative stress, interstitial fibrosis, impaired ventricular–vascular interaction, etc.
Finally, as the Tei index is capable of estimating combined systolic and diastolic performance, it could be more advantageous than the isolated measurement of either systolic or diastolic parameters in the early evaluation of global LV function in isolated metabolic syndrome patients. It is simple, noninvasive, easy to use and reproducible. Moreover, the calculation of the Tei index is independent of age, arterial pressure, heart rate, ventricular geometry, atrioventricular valve regurgitation, afterload, and preload in patients who are in a supine position[5,17,28,31,32].
In conclusion, the current study shows that metabolic syndrome groups (those with or without hypertension and or/diabetes) have an associated abnormal LV global performance. The functional changes are independent of and precede the structural changes. In metabolic syndrome the increase in LVMI is physiologically consistent with an increase in LV diastolic dysfunction. The identification of isolated metabolic syndrome in non-diabetic, non-hypertensive adults may be an indication that aggressive preventive measures should not be postponed until overt obesity, hypertension or diabetes mellitus have developed.
Peer reviewer: Jun Ren, MD, PhD, FAHA, Associate Dean for Research and Professor of Pharmacology, University of Wyoming College of Health Sciences,Director, Wyoming INBRE Program, Laramie, WY 82071, United States
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683-689. |
| 2. | Aijaz B, Ammar KA, Lopez-Jimenez F, Redfield MM, Jacobsen SJ, Rodeheffer RJ. Abnormal cardiac structure and function in the metabolic syndrome: a population-based study. Mayo Clin Proc. 2008;83:1350-1357. |
| 3. | Balkau B, Valensi P, Eschwège E, Slama G. A review of the metabolic syndrome. Diabetes Metab. 2007;33:405-413. |
| 4. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-421. |
| 5. | Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357-366. |
| 6. | Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658-664. |
| 7. | Møller JE, Søndergaard E, Poulsen SH, Appleton CP, Egstrup K. Serial Doppler echocardiographic assessment of left and right ventricular performance after a first myocardial infarction. J Am Soc Echocardiogr. 2001;14:249-255. |
| 8. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. |
| 9. | The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413-2446. |
| 10. | Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167. |
| 11. | Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072-1083. |
| 12. | Mayosi BM, Keavney B, Kardos A, Davies CH, Ratcliffe PJ, Farrall M, Watkins H. Electrocardiographic measures of left ventricular hypertrophy show greater heritability than echocardiographic left ventricular mass. Eur Heart J. 2002;23:1963-1971. |
| 13. | Abraham TP, Laskowski C, Zhan WZ, Belohlavek M, Martin EA, Greenleaf JF, Sieck GC. Myocardial contractility by strain echocardiography: comparison with physiological measurements in an in vitro model. Am J Physiol Heart Circ Physiol. 2003;285:H2599-H2604. |
| 14. | Heatlie GJ, Giles M. Echocardiography and the general physician. Postgrad Med J. 2004;80:84-88. |
| 15. | Myerson SG, Montgomery HE, World MJ, Pennell DJ. Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension. 2002;40:673-678. |
| 16. | Harjai KJ, Scott L, Vivekananthan K, Nunez E, Edupuganti R. The Tei index: a new prognostic index for patients with symptomatic heart failure. J Am Soc Echocardiogr. 2002;15:864-868. |
| 17. | Kuwahara E, Otsuji Y, Takasaki K, Yuasa T, Kumanohoso T, Nakashima H, Toyonaga K, Yoshifuku S, Miyata M, Hamasaki S. Increased Tei index suggests absence of adequate coronary reperfusion in patients with first anteroseptal acute myocardial infarction. Circ J. 2006;70:248-253. |
| 19. | Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102-107. |
| 20. | de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323-331. |
| 21. | Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). A. m J Cardiol. 2004;93:40-44. |
| 22. | Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vaudo G, Mannarino E. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension. 2006;47:881-886. |
| 23. | Mureddu GF, Greco R, Rosato GF, Cella A, Vaccaro O, Contaldo F, de Simone G. Relation of insulin resistance to left ventricular hypertrophy and diastolic dysfunction in obesity. Int J Obes Relat Metab Disord. 1998;22:363-368. |
| 24. | Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12-S8. |
| 25. | Grandi AM, Maresca AM, Giudici E, Laurita E, Marchesi C, Solbiati F, Nicolini E, Guasti L, Venco A. Metabolic syndrome and morphofunctional characteristics of the left ventricle in clinically hypertensive nondiabetic subjects. Am J Hypertens. 2006;19:199-205. |
| 26. | Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Dávila-Román VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399-1404. |
| 27. | Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066-3072. |
| 28. | Schillaci G, Vaudo G, Pasqualini L, Reboldi G, Porcellati C, Verdecchia P. Left ventricular mass and systolic dysfunction in essential hypertension. J Hum Hypertens. 2002;16:117-122. |
| 29. | Voulgari C, Moyssakis I, Papazafiropoulou A, Perrea D, Kyriaki D, Katsilambros N, Tentolouris N. The impact of metabolic syndrome on left ventricular myocardial performance. Diabetes Metab Res Rev. 2010;26:121-127. |
| 30. | Ajami G, Borzouee M, Amoozgar H, Ashnaee F, Kashef S, Nesar MS, Nesar MS. Evaluation of myocardial function using the Tei index in patients with Kawasaki disease. Cardiol Young. 2010;20:44-48. |
| 31. | Lakoumentas JA, Panou FK, Kotseroglou VK, Aggeli KI, Harbis PK. The Tei index of myocardial performance: applications in cardiology. Hellenic J Cardiol. 2005;46:52-58. |
| 32. | Poulsen SH, Nielsen JC, Andersen HR. The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr. 2000;13:379-384. |