Published online May 26, 2025. doi: 10.4330/wjc.v17.i5.107320
Revised: April 10, 2025
Accepted: May 7, 2025
Published online: May 26, 2025
Processing time: 64 Days and 2.6 Hours
This narrative review examines osteosarcopenia, characterized by the concurrent loss of muscle mass and bone density, as a pivotal marker of frailty in older adults. Its implications for patients undergoing transcatheter aortic valve re
Core Tip: Osteosarcopenia, the co-occurrence of muscle atrophy and bone density loss, is increasingly recognized as a potent marker of frailty in older adults undergoing transcatheter aortic valve replacement (TAVR) for severe aortic stenosis. This review synthesizes evidence demonstrating that osteosarcopenia affects 15%-20% of TAVR patients and independently predicts 1-year mortality (P < 0.05). Compared to isolated sarcopenia or osteoporosis, osteosarcopenia correlates with higher risks of post-procedural complications, prolonged hospitalization, and functional decline. Key imaging biomarkers—psoas muscle cross-sectional area (muscle mass) and lumbar trabecular attenuation (bone density)—derived from pre-TAVR computed tomography scans provide objective frailty metrics. Integrating these parameters into risk stratification models
- Citation: Li P, Zhang HP. Osteosarcopenia in older adults undergoing transcatheter aortic valve replacement: A narrative review of mortality and frailty implications. World J Cardiol 2025; 17(5): 107320
- URL: https://www.wjgnet.com/1949-8462/full/v17/i5/107320.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i5.107320
Aortic stenosis (AS) is a leading form of valvular heart disease in older adults, with a prevalence that rises sharply with age. Among individuals over 75 years, AS affects approximately 12.4%, and severe symptomatic AS is present in 3.4% of this population[1]. Untreated severe AS carries a grave prognosis, with a two-year mortality rate exceeding 50% once symptoms develop[2]. Transcatheter aortic valve replacement (TAVR) has revolutionized the management of severe AS, providing a less invasive alternative to surgical aortic valve replacement (SAVR). TAVR has significantly reduced procedural risk and improved survival, particularly in patients with high or prohibitive surgical risk[3]. However, despite technological advancements, osteosarcopenia or frailty continue to contribute to suboptimal outcomes, underscoring the need to refine risk stratification and perioperative care strategies[4].
Osteosarcopenia represents a critical intersection of frailty, where the combined deficits in muscle and bone health exacerbate the vulnerability to stressors, such as TAVR. Osteosarcopenia, first conceptualized by Binkley et al[5] in 2009 (sarco-osteopenia), defined by the concomitant presence of low bone mineral density (BMD) and reduced skeletal muscle mass. It is increasingly recognized as a critical determinant of poor prognosis in older adults undergoing cardiovascular interventions. While frailty have been established as predictor of adverse events post-TAVR, osteosarcopenia represents an amplified risk state that predisposes patients to falls, fractures, prolonged hospital stays, and increased mortality. Recent studies show that osteosarcopenia triples 1-year mortality, doubles postoperative disability, increases heart failure, renal injury, depression and stroke risk[6-8]. This emerging syndrome reflects the interplay between musculoskeletal aging and cardiovascular pathology, providing valuable insight into the biological vulnerability of patients with severe AS. Unlike isolated sarcopenia or osteoporosis, osteosarcopenia captures a broader spectrum of frailty, making it a compelling prognostic marker in the TAVR population.
The purpose of this review is to examine the relationship between osteosarcopenia and mortality in older adults undergoing TAVR. We highlight the epidemiological significance of osteosarcopenia, delineate its pathophysiological mechanisms, and discuss its impact on clinical outcomes in patients with severe AS. By integrating insights from current literature, this review aims to advocate for the routine incorporation of osteosarcopenia assessment into TAVR pre
Osteosarcopenia as a biological substrate of frailty: Osteosarcopenia represents a maladaptive intersection of musculoskeletal decline and systemic frailty. Mechanistically, chronic inflammation [elevated interleukin-6 (IL-6), tumour necrosis factor alpha] and hormonal dysregulation (vitamin D deficiency, insulin resistance) drive concurrent muscle atrophy and bone resorption, exacerbating physical frailty. Clinically, osteosarcopenic patients exhibit amplified vulnerability to the 'frailty cycle': (1) Reduced mobility; (2) Increased falls; (3) Fracture risk; (4) Prolonged immobility; and (5) Further muscle/bone loss[9]. This bidirectional relationship positions osteosarcopenia as both a biomarker and accelerator of frailty in TAVR populations. Therefore, the interplay between muscle and bone deterioration in osteosarcopenia not only reflects the physical aspects of frailty but also contributes to its progression, making it a key target for intervention in frail older adults.
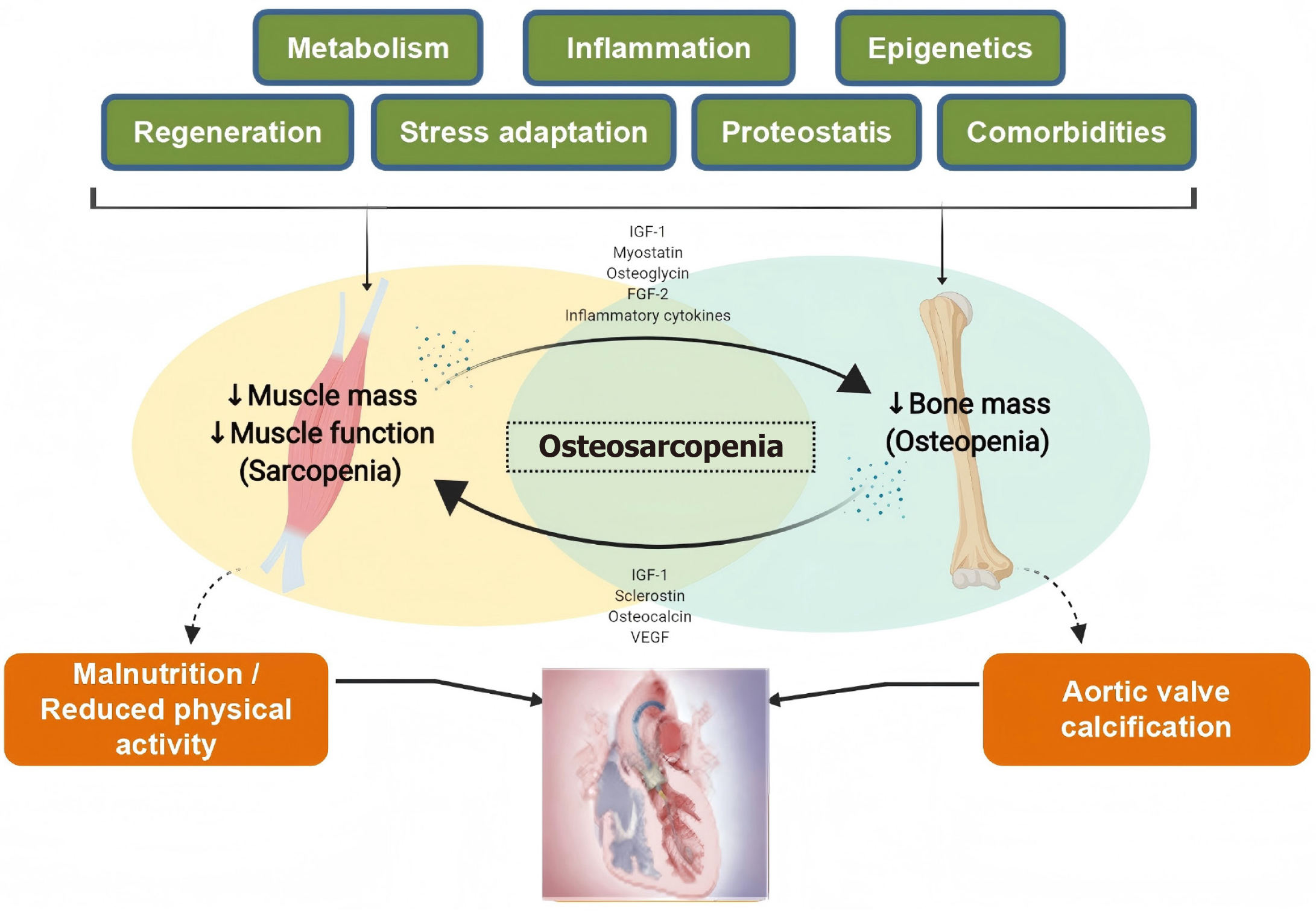
Molecular crosstalk in the muscle-bone axis and aortic valve stenosis: These two age-related conditions (sarcopenia and osteoporosis) share fundamental biological pathways and clinical consequences (Figure 1). Both disorders demonstrate parallel trajectories of tissue mass loss (muscle and bone respectively), driven by common mechanisms including chronic inflammation, hormonal changes, metabolic dysregulation, and comorbidites (heart failure, myocardial infarction, diabetes, etc.)[10]. The muscle-bone axis maintains systemic homeostasis through bidirectional signaling mediated by myokines and osteokines. Myocytes secrete regulatory proteins including IL-6, myostatin, irisin, insulin-like growth factor 1, and fibroblast growth factor 21, while osteocytes produce osteocalcin, sclerostin, prostaglandin E2, transforming growth factor beta, and receptor activator of nuclear factor kappa B ligand[11-13]. These molecules create a biochemical feedback loop that coordinates muscle protein synthesis with bone remodeling[14]. Adipose tissue amplifies this interplay through adipokines (leptin, resistin, adiponectin) that modulate both osteoclastic activity and skeletal muscle catabolism[15]. The endocrine effects of these factors operate through autocrine, paracrine, and systemic pathways, forming a tripartite regulatory network among muscle, bone, and fat.
Osteosarcopenia is diagnosed through concurrent confirmation of osteoporosis and sarcopenia using validated criteria. For osteoporosis diagnosis in postmenopausal women and men ≥ 50 years, the World Health Organization recommends central dual-energy X-ray absorptiometry (DXA) T-scores ≤ -2.5 at lumbar spine (L1-L4), femoral neck, total hip, or non-dominant distal radius. This threshold also applies to fragility fractures at hip/vertebrae (regardless of T-score) or proximal humerus/pelvis/distal forearm fractures with osteopenia (T-score -2.5 to -1.0)[16]. Sarcopenia assessment follows the Asian Working Group (AWGS) criteria requiring: (1) Low muscle mass [DXA: < 7.0 kg/m² (men)/< 5.4 kg/m² (women); bioelectrical impedance analysis (BIA): < 7.0 kg/m²/< 5.7 kg/m²]; (2) Reduced grip strength (< 28 kg/< 18 kg); and (3) Impaired function [gait speed ≤ 1 m/second, Short Physical Performance Battery (SPPB) ≤ 9, or sit-to-stand ≥ 12 seconds][17].
Additionally, five international consensus frameworks (European Working Group on Sarcopenia in Older People 2, Foundation for the National Institutes of Health, AWGS, Sarcopenia Definition and Outcome Consortium, International Working Group on Sarcopenia) guide sarcopenia evaluation, with AWGS specifically defining severe sarcopenia when all three components-low mass, weak strength, and poor function-coexist. The diagnostic integration requires meeting both systems' criteria: Osteoporosis (T-score ≤ -2.5 or fragility fracture patterns) plus sarcopenia (AWGS muscle mass thre
Osteosarcopenia, the concurrent loss of muscle mass and bone density, is a prevalent yet underrecognized condition in older adults, with particular relevance for patients undergoing TAVR. In community-dwelling older populations, prevalence rates vary widely, ranging from 5% to 37%, due to differences in diagnostic criteria and assessment methods[18]. A comprehensive meta-analysis by Huang et al[19], which pooled data from 31 studies and 15062 patients, estimated an overall prevalence of 21.0% (95%CI: 0.16–0.26). This risk was significantly higher among women [odds ratio (OR) = 5.10, 95%CI: 2.37–10.98], older age groups (OR = 1.12, 95%CI: 1.03–1.21), and those with prior fractures (OR = 2.92, 95%CI: 1.62–5.25)[19]. In TAVR candidates, such as those evaluated in large prospective cohorts, the incidence of osteosarcopenia is approximately 15%, highlighting its clinical significance in this vulnerable group[7].
Emerging data suggest significant geographic and ethnic variability in osteosarcopenia prevalence among TAVR populations. For instance, Asian cohorts report lower rates of osteosarcopenia (8%–12%) compared to European/North American populations (15%–20%), potentially reflecting differences in body composition norms, genetic predisposition, or lifestyle factors[20,21]. Notably, the FRAILTY-AVR cohort (multinational enrollment) demonstrated higher osteosarcopenia rates in White (14.9%) patients[7], though this may be confounded by regional sarcopenia diagnostic thresholds. Racial disparities are further compounded by inequities in access to TAVR—underrepresented minorities often present with advanced frailty at younger ages due to delayed referrals. Future studies must standardize osteosarcopenia criteria across diverse populations to clarify these patterns. Additionally, osteosarcopenia often coexists with frailty, further complicating accurate prevalence estimates[21]. Despite the heterogeneity in definitions, osteosarcopenia remains a pervasive condition in the TAVR population, underscoring its significance as a critical risk factor for adverse outcomes.
The epidemiological burden of osteosarcopenia extends beyond prevalence, as it is strongly linked to adverse outcomes. A meta-analysis of 14429 participants across prospective cohort studies demonstrated a 53% increased risk of mortality over a mean follow-up of 6.6 years (risk ratio = 1.53; 95%CI: 1.28–1.78)[22]. Among the TAVR patients, the presence of osteosarcopenia or frailty in TAVR patients is associated with an increased risk of perioperative complications, prolonged hospital stays, and higher 30-day and 1-year mortality rates (Table 1)[4,7,23-33]. This heightened risk arises from increased susceptibility to falls[30], fractures, and functional decline—key considerations for TAVR patients[31-35]. Given the variability in prevalence estimates and the profound impact on survival, there is an urgent need for standardized diagnostic criteria and routine screening in older adults, especially those with severe AS. These broad epidemiological trends provide a foundation for understanding the specific clinical implications of osteosarcopenia, as elucidated by prospective studies like the FRAILTY-AVR cohort.
| Ref. | Year | Study type | Key inclusion/exclusion criteria | Patient’s characteristics | Assessment method | Key findings |
| Green et al[23] | 2012 | Single-center prospective study | Inclusion: Age ≥ 60 years, severe calcific AS, with advanced cardiac symptoms, TAVR candidates. Exclusion: Inoperability | 159 patients, mean age 86 years, 50% male | Frailty score derived from gait speed, grip strength, serum albumin, and ADL | Frailty was associated with a 35-fold increase in 1-year mortality but not with procedural complications |
| Green et al[24] | 2015 | Post hoc analysis | Inclusion: Age ≥ 60 years, severe symptomatic AS requiring TAVR, frailty assessed at 3 high-enrolling sites. Exclusion: Missing baseline frailty assessment | 244 patients, mean age 86 years, 51.6% male | Frailty assessed using serum albumin, grip strength, gait speed, and Katz ADL survey | Frail individuals had higher 1-year mortality (32.7% vs 15.9% nonfrail) and poor outcomes (50% vs 31.5%) |
| Afilalo et al[4] | 2017 | Prospective multicenter cohort study | Inclusion: Age ≥ 70, severe AS, TAVR/surgical aortic valve replacement planned. Exclusion: Dementia, metastatic cancer, acute myocardial infarction < 30 days | 1020 patients, mean age 82 years, 59% male | Fried, Fried+, Rockwood, and EFT etc. | EFT strongly predicted 1-year mortality (aOR = 3.72; 95%CI: 2.54-5.45), 1-year disability (aOR = 2.13; 95%CI: 1.57-2.87), and death at 30 days (aOR = 3.29; 95%CI: 1.73-6.26) |
| Kundi et al[25] | 2019 | CMS MedPAR database | Inclusion: Age ≥ 70 years, referred for TAVI (2011-2015), severe symptomatic AS. Exclusion: Declined consent | 28531patients, mean age 815 years, 53.6% male | Hospital Frailty Risk Score | 1-year mortality rates were 76% in low-risk patients, 17.6% in intermediate-risk patients, and 30.1% in high-risk patients (log rank P < 0.001) |
| Skaar et al[26] | 2019 | Prospective observational study | Inclusion: Age ≥ 65 years, undergoing transcatheter mitral valve repair/TAVR. Exclusion: Age < 65 years | 142 patients, 54% women, mean age 83 years | A novel geriatric assessment frailty score | Geriatric assessment frailty score predicted mortality within 2 years, with an estimated HR of 1.79 (95%CI: 1.34–2.36, P < 0.001) |
| Goudzwaard et al[27] | 2020 | Prospective, observational study | Inclusion: Severe symptomatic AS patients referred for TAVI | 239 patients, mean age 808 years, 49.8% male | Erasmus Frailty Score | Frailty was an independent predictor of deteriorated HR quality of life 1 year after TAVI (OR = 2.24, 95%CI: 1.07–4.70, P = 0.003) |
| Seoudy et al[28] | 2021 | Retrospective cohort study | Inclusion: Symptomatic AS, underwent transfemoral TAVR, Geriatric Nutritional Risk Index | 1930 patients, mean age 82 years, 52.5% female | GNRI | After a mean follow-up of 21.1 months, all-cause mortality was significantly increased in the low-GNRI group compared with the normal-GNRI group (P < 0.001) |
| Arnold et al[29] | 2022 | PARTNER 2A trial, SAPIEN 3 intermediate-risk registry, and PARTNER 3 trial | Inclusion: Intermediate-surgical-risk or low-surgical-risk patients, Severe symptomatic AS, Enrolled in PARTNER 2A trial, SAPIEN 3 intermediate-risk registry, or PARTNER 3 trial | 3025 patients, mean age 793 years, 61.6% men | Frailty was examined as a continuous variable based on grip strength, gait speed, albumin, and ADL | Increasing frailty (none vs prefrail vs frail) was associated with higher 2-year mortality (5.5% vs 11.1% vs 22.8%; log-rank P < 0.001) and worse 2-year health status among survivors (Kansas City Cardiomyopathy Questionnaire scores adjusted for baseline: 84.8 vs 79.6 vs 77.4, P < 0.001) |
| Strange et al[30] | 2023 | Danish nationwide registries study | Inclusion: Undergoing first-time TAVR, Valid Hospital Frailty Risk Score | 5971 patients, median age 81 years, and 55.4% men | Hospital Frailty Risk Score | 1-year risk of death was 5.8% of patients in the low frailty group compared with 10.3% of patients in the intermediate frailty group and 15.6% of patients in the high frailty group |
| Stein et al[31] | 2024 | Multicenter prospective registry | Inclusion: Pre-TAVR CT available. Exclusion: Prior valve surgery, end-stage renal disease | 445 patients, median age 829 years, 41% female | 3-part definition psoas muscle area indexed to height; handgrip strength; and gait speed | Among the 3 components of sarcopenia, only slower gait speed (muscle performance) was independently associated with increased post-TAVR mortality (aHR = 1.38 per 1 SD decrease (95%CI: 1.11–1.72); P = 0.004) |
| Persits et al[32] | 2024 | Retrospective cohort study | Inclusion: aged ≥ 70 years, undergoing TAVR, Pre-TAVR CT scans available. Exclusion: Emergent TAVR procedures, unstable vital signs, CT scans with fields of view excluding the abdomen | 184 patients, average age 806 years, 41.8% female | Sarcopenia was defined as having both low muscle mass and either muscle weakness or poor physical performance. Frailty status was assessed using Green score | There were higher rates of the postoperative adverse events in patients with sarcopenia (54.8%) and frailty (41.9% with the Adapted Green and 50.5% with the Green-SMI score) compared to their nonsarcopenic (30.3%) and nonfrail counterparts (25.4% with the Adapted Green and 18.8% with the Green-SMI score) |
| Petrovic et al[33] | 2024 | Prospective multicentre WIN-TAVI registry | Inclusion: Women, with symptomatic severe AS, Intermediate or high surgical risk, pre-TAVR Fried frailty criteria assessment. Exclusion: Missing frailty assessment data | 1019 women, mean age 82 years | Fried frailty criteria | 1-year risk of the primary outcome was significantly higher in prefrail and frail (20.2%) than in nonfrail (14.9%) women (aHR = 1.51). The risk of major bleeding was higher in prefrail or frail (19.9%) than in nonfrail (10.0%) women (aHR = 2.06) |
| Solla-Suarez et al[7] | 2024 | Prospective multicenter cohort study | Inclusion: Undergoing TAVR, pre-TAVR CT scans available, Availability of HGS or gait speed measurements. Exclusion: Incomplete CT imaging, missing HGS or gait speed data | 605 patients, mean age 826 years, 45% female | Osteosarcopenia was defined as a combination of low PMA and low VBD | One-year mortality was highest in osteosarcopenia (32%) followed by low PMA alone (14%), low VBD alone (11%), and normal bone and muscle status (9%) (P < 0.001) |
The FRAILTY-AVR studies, a multicenter prospective cohort investigation, have significantly advanced our under
In 2017, Afilalo et al[4] carried out a prospective cohort study involving 1020 older adults with a median age of 82 years who were undergoing TAVR or SAVR[4]. The study revealed that frailty prevalence ranged from 26% to 68%, depending on the assessment tool employed, highlighting the necessity for a standardized approach. The Essential Frailty Toolset (EFT), a concise four-item scale evaluating lower-extremity weakness, cognitive impairment, anemia, and hypoalbuminemia, emerged as the most robust predictor of adverse outcomes. The authors found that EFT-defined frailty was linked to a 3.72-fold increase in 1-year mortality [adjusted OR (aOR) = 3.72; 95%CI: 2.54–5.45], a 2.13-fold increase in worsening disability at 1 year (aOR = 2.13; 95%CI: 1.57–2.87), and a 3.29-fold increase in 30-day mortality (aOR = 3.29; 95%CI: 1.73–6.26).
Building on the 2017 findings, the 2024 FRAILTY-AVR study shifted attention to osteosarcopenia—a condition defined by the simultaneous loss of muscle mass and bone density—as a potentially more precise frailty marker in TAVR candidates. This multicenter study enrolled 605 patients aged 70 years or older with severe AS[7]. Preoperative computed tomography (CT) was used to measure psoas muscle area (PMA) and vertebral bone density (VBD), identifying osteosarcopenia in 15% of participants. The results showed that the osteosarcopenic group faced a 1-year mortality rate of 32%, compared to 9% in those with normal muscle and bone metrics (P < 0.001), while patients with isolated low PMA or low VBD showed intermediate mortality rates of 14% and 11%, respectively. Osteosarcopenia independently predicted a 3.18-fold increase in 1-year mortality (aOR = 3.18; 95%CI: 1.54–6.57) and doubled the risk of worsening disability, even after adjusting for traditional frailty measures such as the EFT and the Clinical Frailty Scale. These findings underscore the compounded prognostic impact of combined muscle and bone deterioration, suggesting that osteosarcopenia may provide greater precision in risk stratification for TAVR patients than frailty alone.
Clinically, the 2017 study introduced EFT as a practical tool for identifying at-risk patients, while the 2024 study advanced this framework by incorporating osteosarcopenia as a targeted indicator, leveraging advanced imaging to enhance outcome prediction (Table 2)[4,7]. This evolution emphasizes the value of integrating general frailty assessments with specific syndromes like osteosarcopenia into clinical practice[26], optimizing patient selection and management in both TAVR and SAVR settings.
| Characteristic | 2017 study (Afilalo et al[4]) | 2024 study (Solla-Suarez et al[7]) |
| Study population | 1020 patients, median age 82 years (interquartile range: 77–86 years), undergoing TAVR (n = 646) or surgical aortic valve replacement (n = 374) | 605 patients, aged 70 years or older (mean age 826 years ± 6.2 years), undergoing TAVR |
| Grouping method | Comparison of seven frailty scales: Fried, Fried+, Rockwood, Short Physical Performance Battery, Bern, Columbia, EFT | Assessment of osteosarcopenia using computed tomography scans to measure psoas muscle area and vertebral bone density |
| Follow-up period | One year for primary outcome; 30 days for secondary outcomes | One year for primary outcome; 30 days for secondary outcomes |
| Primary outcome | One-year all-cause mortality; 145 deaths (14%), EFT was the strongest predictor (aOR = 3.72; 95%CI: 2.54–5.45) | One-year all-cause mortality; 84 deaths (13.9%), osteosarcopenia associated with a 318-fold increase (aOR = 3.18; 95%CI: 1.54–6.57) |
| Secondary outcomes | 30-day mortality: 4.2% (43 deaths), EFT aOR = 3.29 (95%CI: 1.73–6.26). Composite of death or worsening disability at one year: 35% incidence (357/1,020), EFT aOR = 2.13 (95%CI: 1.57–2.87) | 30-day mortality: 3.6% (21 deaths), hospital length of stay: Mean 6.5 days ± 8.0 days, discharge not to home: 20.5% (120/585), worsening disability at one year: 40.6% (215/529), osteosarcopenia OR = 2.11 (95%CI: 1.19–3.74) |
Although isolated sarcopenia is a known risk factor for poor outcomes following TAVR, its impact is significantly amplified when combined with osteoporosis as osteosarcopenia. The FRAILTY-AVR cohort demonstrates that osteosarcopenia triples the mortality risk compared to isolated deficits. This synergistic effect likely reflects the combined contributions of muscle and bone loss, which exacerbate frailty, heighten fall and fracture risk, and hinder postoperative recovery[36]. In contrast, patients with isolated sarcopenia may retain sufficient bone integrity to partially offset functional decline, whereas osteosarcopenia signals a more severe state of musculoskeletal compromise, reducing resilience to surgical stress[37]. These observations highlight the need for comprehensive assessments that integrate both sarcopenic and osteoporotic parameters into preoperative evaluations, allowing for the identification of high-risk subgroups who may benefit from tailored interventions such as prehabilitation, nutritional support, and post-TAVR rehabilitation programs.
Accurate identification of sarcopenia and osteosarcopenia prior to TAVR is essential for risk stratification and optimizing clinical outcomes. Routine preoperative imaging, specifically CT, offers a valuable opportunity to assess musculoskeletal health without the need for additional tests[38]. Cross-sectional imaging of the PMA at the L3-L4 vertebral level is widely used to evaluate muscle mass, while VBD is assessed through Hounsfield unit (HU) measurements of trabecular bone on the same CT scan[39]. These opportunistic assessments allow for the simultaneous evaluation of sarcopenia and osteo
Thresholds for diagnosing sarcopenia typically include PMA values of < 22 cm² in men and < 12 cm² in women, while low VBD is often defined by measurements below 90 HU[40]. Notably, osteosarcopenia—characterized by concurrent low PMA and VBD—has demonstrated superior predictive value for postoperative mortality and disability compared to isolated measures of sarcopenia or osteoporosis[40]. Incorporating these assessments into routine TAVR workups enhances risk prediction models, such as the TAVI2-SCORE, by providing a more comprehensive evaluation of frailty. In addition to CT, DXA and BIA remain viable alternatives for assessing muscle mass and bone density. However, these modalities are less frequently utilized in the TAVR setting due to limited availability and logistical constraints. Emerging automated algorithms for CT-based sarcopenia and osteosarcopenia assessment are anticipated to streamline preoperative screening, further integrating musculoskeletal evaluation into standard cardiovascular workflows.
While CT provides precise musculoskeletal phenotyping, its routine use for osteosarcopenia screening raises cost-effectiveness concerns. In the United States, a dedicated abdominopelvic CT costs approximately 500$–1200$, whereas opportunistic measurements during pre-TAVR CT angiography add minimal expense. A Markov model analysis estimated that CT-based osteosarcopenia screening could be cost-effective if it reduces 1-year mortality by ≥ 5% (current data: 12% absolute reduction), though this assumes universal access to post-TAVR rehabilitation—a limitation in resource-constrained settings[41]. For clinics without advanced imaging, pragmatic alternatives like strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire + FRAIL scale may suffice, albeit with lower sensitivity. Future value-based analyses should weigh CT’s prognostic benefits against implementation barriers in low-income regions.
While imaging provides a structural assessment of muscle and bone health, functional evaluations are critical for quantifying the clinical impact of sarcopenia. The SPPB is one of the most widely used tools for assessing lower extremity function and frailty[42]. It comprises gait speed, balance, and chair rise tests, with scores ≤ 8 indicating poor physical performance and heightened procedural risk. Low gait speed (< 0.8 m/second) has been independently associated with increased mortality and morbidity following TAVR[43].
Handgrip strength, measured using a dynamometer, serves as an additional indicator of sarcopenia, with thresholds of < 27 kg for men and < 16 kg for women representing diminished muscle strength[44]. This simple, cost-effective test correlates strongly with overall frailty and predicts adverse outcomes in the perioperative period. The SARC-F questionnaire, a self-reported screening tool assessing strength, ambulation, rising from a chair, stair climbing, and falls, offers a practical method for identifying patients at risk for sarcopenia[45]. Although less precise than direct muscle measurements, SARC-F facilitates large-scale screening and prioritizes patients for further evaluation.
Combining imaging with functional assessments provides a multidimensional perspective on frailty, enhancing the ability to identify high-risk patients and personalize perioperative management strategies. Integrating these evaluations into routine TAVR protocols holds promise for improving long-term outcomes and reducing procedural complications.
Multiple studies from the Franconian Osteopenia and Sarcopenia Trial demonstrate that high-intensity resistance training (HIRT), often combined with whey protein, vitamin D, and calcium supplementation, significantly improves skeletal muscle mass index (SMI), BMD, and functional strength in older men with osteosarcopenia. For instance, a 12-month HIRT intervention maintained lumbar spine BMD and increased SMI, with significant between-group differences compared to controls [standardized mean difference (SMD) = 0.90 and SMD = 1.95, respectively][46]. Extending HIRT to 18 months further enhanced lumbar spine and total hip BMD (SMD = 0.72 for both) and sarcopenia Z-scores (SMD = 1.40)[47]. These benefits were supported by high adherence (95%) and no reported injuries, underscoring HIRT’s feasibility and safety[48]. Detraining effects highlight the need for continuous intervention. After 6 months of detraining following 18 months of HIRT, significant losses in SMI, leg strength, lean body mass, and cardiometabolic health (metabolic syndrome Z-score) were observed, though some overall benefits persisted (P ≤ 0.004)[48]. This suggests that intermitted training with prolonged breaks may undermine long-term efficacy.
Nutritional augmentation, particularly with whey protein (1.5–1.6 g/kg/day), amplified HIRT’s effects on muscle mass and strength. After 28 weeks, HIRT plus protein significantly improved sarcopenia Z-scores (P < 0.001), SMI, and handgrip strength compared to controls[49]. However, HIRT’s impact on fat infiltration varied by muscle group. While thigh muscle intermuscular adipose tissue volume stabilized after 16 months of HIRT (P = 0.004 vs increase in controls)[50], no significant reduction in paraspinal muscle fat infiltration was observed after 16 months, despite BMD gains. Similarly, visceral adipose tissue (VAT) decreased significantly (-7.7%, P < 0.001) after 18 months of HIRT, but abdominal aortic calcification progressed independently of exercise.
Beyond HIRT, leucine-enriched whey protein supplementation (1.5 g/kg/day) alone or with resistance exercise im
Pharmacological interventions targeting sarcopenia and osteosarcopenia remain an area of ongoing investigation. Currently, no Food and Drug Administration-approved medications specifically address sarcopenia; however, several agents have shown promise in clinical trials. Selective androgen receptor modulators (SARMs) have demonstrated potential in increasing lean muscle mass and enhancing physical function, although their long-term safety profile warrants further evaluation. Myostatin inhibitors, which prevent muscle degradation by blocking the myostatin pathway, represent another novel therapeutic avenue[54]. Testosterone replacement therapy has been explored in men with low endogenous levels, yielding modest improvements in muscle mass and strength[55]. However, its use is limited by potential cardiovascular risks and the need for careful monitoring. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have also been investigated for their role in mitigating muscle wasting, with evidence suggesting that they may enhance mitochondrial function and reduce inflammation[56]. Bisphosphonates and denosumab, commonly used in the treatment of osteoporosis, are critical in managing the bone component of osteosarcopenia. Additionally, depression and cognitive dysfunction are common in patients that undergo TAVR and are associated with increased mortality and worse quality of life. Statin, which are commonly used among TAVR patients, could safely and effectively improve the symptoms of depression and inflammation status among depressive patients[57]. These agents reduce fracture risk and preserve BMD, complementing exercise and nutritional interventions aimed at preventing falls and disability.
Therefore, HIRT consistently emerges as an effective, safe intervention for improving BMD, muscle mass, and strength in osteosarcopenic men, with nutritional support enhancing outcomes. However, its limited effect on muscle fat infiltration and VAT suggests that additional strategies (e.g., aerobic exercise) may be needed to address metabolic compo
A major limitation in the management of sarcopenia and osteosarcopenia in TAVR patients is the absence of standardized diagnostic criteria. Current definitions and cutoffs for sarcopenia and low bone density vary across studies, leading to inconsistencies in prevalence estimates and prognostic assessments[58]. Harmonizing diagnostic thresholds for PMA and VBD using CT or DXA is essential to ensure uniform identification of high-risk patients[59]. International collaboration aimed at developing consensus guidelines will enable more accurate stratification and targeted interventions, ultimately improving clinical outcomes.
Ongoing and future clinical trials are critical for advancing the management of osteosarcopenia in TAVR patients. Trials such as the PERFORM-TAVR (NCT03522454) are investigating the efficacy of prehabilitation programs incorporating exercise and nutritional supplementation. Additionally, randomized controlled studies assessing pharmacologic agents, including myostatin inhibitors and SARMs[60] and stem cell therapy[61], are expected to yield insights into novel thera
The optimal intervention strategy for AS patients with comorbidites (coronary artery disease, hypertrophic cardiomyopathy) is a research hot topic. The optimal timing for percutaneous coronary intervention (PCI) in patients undergoing TAVR is debatable[64,65]. Searching the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy Registry and Medicare Linkage, a recently published study showed patients undergoing concomitant PCI and TAVR had higher major vascular complications, and higher risk of all-cause mortality and stroke compared with those who underwent PCI within 90 days before the TAVR procedure[66]. However, meta-analysis demonstrated that among patients undergoing TAVR who required PCI, no significant differences were observed in the early and long-term outcomes between those receiving concurrent TAVR and PCI versus staged surgery[67]. Furthermore, the co-existence of AS and obstructive hypertrophic cardiomyopathy is not uncommon, and treatment with aficamten could result in a significantly greater improvement in quality of life[68]. Surgical intervention is the gold standard management, and it is important to measure the quality of life and functional improvement among patients with severe AS and HCM undergoing TAVR[69]. However, TAVR outcomes are unclear in this population. Continued research focusing on integrating imaging biomarkers and functional assessments will refine perioperative risk models, fostering precision medicine approaches in the management of frail older adults undergoing TAVR.
Osteosarcopenia represents a critical, yet underrecognized, predictor of adverse outcomes in older adults undergoing TAVR. Its dual impact on muscle and bone health amplifies frailty, increasing the risk of mortality and disability. Integrating standardized diagnostic criteria, prehabilitation strategies, and nutritional support into routine care can improve quality of life and heart function, and improve postoperative recovery after cardiac surgery[70]. Future research and clinical trials will integration in biomedical science[71], further refine therapeutic approaches, enhancing patient selection and outcomes. Addressing osteosarcopenia holistically within cardiovascular care is essential to optimizing long-term success in this vulnerable population. By operationalizing osteosarcopenia as a measurable frailty phenotype, clinicians can better risk-stratify TAVR candidates and tailor interventions to break the cycle of musculoskeletal decline.
| 1. | Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 927] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 2. | Coisne A, Montaigne D, Aghezzaf S, Ridon H, Mouton S, Richardson M, Polge AS, Lancellotti P, Bauters C; VALVENOR Investigators. Association of Mortality With Aortic Stenosis Severity in Outpatients: Results From the VALVENOR Study. JAMA Cardiol. 2021;6:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Ya’qoub L, Arnautovic J, Faza NN, Elgendy IY. Sex Differences in Transcatheter Structural Heart Disease Interventions: How Much Do We Know? CVIA. 2023;8. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 574] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 5. | Binkley N, Buehring B. Beyond FRAX: it's time to consider "sarco-osteopenia". J Clin Densitom. 2009;12:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Li H, Ren Y, Duan Y, Li P, Bian Y. Association of the longitudinal trajectory of urinary albumin/creatinine ratio in diabetic patients with adverse cardiac event risk: a retrospective cohort study. Front Endocrinol (Lausanne). 2024;15:1355149. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Solla-Suarez P, Arif SG, Ahmad F, Rastogi N, Meng A, Cohen JM, Rodighiero J, Piazza N, Martucci G, Lauck S, Webb JG, Kim DH, Kovacina B, Afilalo J. Osteosarcopenia and Mortality in Older Adults Undergoing Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2024;9:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 8. | Wang R, Wang Y, Wei Z, Wang J, Tang H, Gao X, Wang J, Zhang C, Chen X. The association between HDL-c levels and computed tomography-based osteosarcopenia in older adults. BMC Musculoskelet Disord. 2024;25:932. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Vinciguerra M, D’abramo M, Sciuccati B, Bruno N, Wretschko E, Totaro M, Barretta A, Miraldi F, Greco E. Prognostic Implications of Degenerative Mitral Regurgitation on Eligibility for TAVI. CVIA. 2024;9. [DOI] [Full Text] |
| 10. | Ong P, Hill S, Bierbaum D, Geiger N, Wunder C, Franke U, Ahad S, Bekeredjian R. Successful TAVI Despite Sudden Low Output and Ventricular Fibrillation in a Patient with Cardiac Amyloidosis. CVIA. 2023;8. [DOI] [Full Text] |
| 11. | Bakker AD, Jaspers RT. IL-6 and IGF-1 Signaling Within and Between Muscle and Bone: How Important is the mTOR Pathway for Bone Metabolism? Curr Osteoporos Rep. 2015;13:131-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Liu S, Gao F, Wen L, Ouyang M, Wang Y, Wang Q, Luo L, Jian Z. Osteocalcin Induces Proliferation via Positive Activation of the PI3K/Akt, P38 MAPK Pathways and Promotes Differentiation Through Activation of the GPRC6A-ERK1/2 Pathway in C2C12 Myoblast Cells. Cell Physiol Biochem. 2017;43:1100-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Wu M, Chen J, Kuang X, Chen Y, Wang Y, Huang L, Su M, Chen Y, Qu E, Zhang X. Sarcopenia-related Traits, Body Mass Index and Ovarian Cancer Risk: Investigation of Causal Relationships Through Multivariable Mendelian Randomization Analyses. BIO Integration. 2024;5. [DOI] [Full Text] |
| 14. | Collins KH, Gui C, Ely EV, Lenz KL, Harris CA, Guilak F, Meyer GA. Leptin mediates the regulation of muscle mass and strength by adipose tissue. J Physiol. 2022;600:3795-3817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 16. | Osteoporosis Group of Chinese Orthopaedic Association. [Guideline for diagnosis and treatment of osteoporotic fractures]. Zhonghua Guke Zazhi. 2022;42:1473-1491. [DOI] [Full Text] |
| 17. | Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300-307.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2739] [Cited by in RCA: 3689] [Article Influence: 737.8] [Reference Citation Analysis (0)] |
| 18. | Nielsen BR, Abdulla J, Andersen HE, Schwarz P, Suetta C. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med. 2018;9:419-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Huang T, Li C, Chen F, Xie D, Yang C, Chen Y, Wang J, Li J, Zheng F. Prevalence and risk factors of osteosarcopenia: a systematic review and meta-analysis. BMC Geriatr. 2023;23:369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Saeki C, Kanai T, Ueda K, Nakano M, Oikawa T, Torisu Y, Saruta M, Tsubota A. Osteosarcopenia predicts poor survival in patients with cirrhosis: a retrospective study. BMC Gastroenterol. 2023;23:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Shimada H, Suzuki T, Doi T, Lee S, Nakakubo S, Makino K, Arai H. Impact of osteosarcopenia on disability and mortality among Japanese older adults. J Cachexia Sarcopenia Muscle. 2023;14:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Veronese N, Ragusa FS, Sabico S, Dominguez LJ, Barbagallo M, Duque G, Al-Daghri N. Osteosarcopenia increases the risk of mortality: a systematic review and meta-analysis of prospective observational studies. Aging Clin Exp Res. 2024;36:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 24. | Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, Rihal CS, Xu K, Lei Y, Hawkey MC, Kim RJ, Alu MC, Leon MB, Mack MJ. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 25. | Kundi H, Popma JJ, Reynolds MR, Strom JB, Pinto DS, Valsdottir LR, Shen C, Choi E, Yeh RW. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Skaar E, Eide LSP, Norekvål TM, Ranhoff AH, Nordrehaug JE, Forman DE, Schoenenberger AW, Hufthammer KO, Kuiper KK, Bleie Ø, Packer EJS, Langørgen J, Haaverstad R, Schaufel MA. A novel geriatric assessment frailty score predicts 2-year mortality after transcatheter aortic valve implantation. Eur Heart J Qual Care Clin Outcomes. 2019;5:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Goudzwaard JA, de Ronde-Tillmans MJAG, van Hoorn FED, Kwekkeboom EHC, Lenzen MJ, van Wiechen MPH, Ooms JFW, Nuis RJ, Van Mieghem NM, Daemen J, de Jaegere PPT, Mattace-Raso FUS. Impact of frailty on health-related quality of life 1 year after transcatheter aortic valve implantation. Age Ageing. 2020;49:989-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Seoudy H, Al-Kassou B, Shamekhi J, Sugiura A, Frank J, Saad M, Bramlage P, Seoudy AK, Puehler T, Lutter G, Schulte DM, Laudes M, Nickenig G, Frey N, Sinning JM, Frank D. Frailty in patients undergoing transcatheter aortic valve replacement: prognostic value of the Geriatric Nutritional Risk Index. J Cachexia Sarcopenia Muscle. 2021;12:577-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Arnold SV, Zhao Y, Leon MB, Sathananthan J, Alu M, Thourani VH, Smith CR, Mack MJ, Cohen DJ. Impact of Frailty and Prefrailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ Cardiovasc Interv. 2022;15:e011375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Strange JE, Christensen DM, Sindet-Pedersen C, Schou M, Falkentoft AC, Østergaard L, Butt JH, Graversen PL, Køber L, Gislason G, Olesen JB, Fosbøl EL. Frailty and Recurrent Hospitalization After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2023;12:e029264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Stein EJ, Neill C, Nair S, Terry JG, Carr JJ, Fearon WF, Elmariah S, Kim JB, Kapadia S, Kumbhani DJ, Gillam L, Whisenant B, Quader N, Zajarias A, Welt FG, Bavry AA, Coylewright M, Piana R, Mallugari RR, Vatterott A, Jackson N, Huang S, Lindman BR. Associations of Sarcopenia and Body Composition Measures With Mortality After Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2024;17:e013298. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Persits I, Mirzai S, Sarnaik KS, Volk MC, Yun J, Harb S, Puri R, Kapadia S, Krishnaswamy A, Chen PH, Reed G, Tang WHW. Sarcopenia and frailty in patients undergoing transcatheter aortic valve replacement. Am Heart J. 2024;276:49-59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Petrovic M, Spirito A, Sartori S, Vogel B, Tchetche D, Petronio AS, Mehilli J, Lefevre T, Presbitero P, Capranzano P, Pileggi B, Iadanza A, Sardella G, van Mieghem NM, Meliga E, Feng Y, Dumonteil N, Cohen R, Fraccaro C, Trabattoni D, Mikhail G, Ferrer-Gracia MC, Naber C, Sharma SK, Watanabe Y, Morice MC, Dangas GD, Chieffo A, Mehran R. Prognostic Impact of Prefrailty and Frailty in Women Undergoing TAVR: Insights From the WIN-TAVI Registry. Can J Cardiol. 2024;40:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 34. | O'Gara PT, Guduguntla V, Bonow RO. Osteosarcopenia and Mortality After Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2024;9:618-619. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Romeo FJ, Chiabrando JG, Seropian IM, Raleigh JV, de Chazal HM, Garmendia CM, Smietniansky M, Cal M, Agatiello CR, Berrocal DH. Sarcopenia index as a predictor of clinical outcomes in older patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2021;98:E889-E896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Damluji AA, Bernacki G, Afilalo J, Lyubarova R, Orkaby AR, Kwak MJ, Hummel S, Kirkpatrick JN, Maurer MS, Wenger N, Rich MW, Kim DH, Wang RY, Forman DE, Krishnaswami A. TAVR in Older Adults: Moving Toward a Comprehensive Geriatric Assessment and Away From Chronological Age: JACC Family Series. JACC Adv. 2024;3:100877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Damluji AA, Alfaraidhy M, AlHajri N, Rohant NN, Kumar M, Al Malouf C, Bahrainy S, Ji Kwak M, Batchelor WB, Forman DE, Rich MW, Kirkpatrick J, Krishnaswami A, Alexander KP, Gerstenblith G, Cawthon P, deFilippi CR, Goyal P. Sarcopenia and Cardiovascular Diseases. Circulation. 2023;147:1534-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 237] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 38. | Persits I, Mirzai S, Sarnaik KS, Volk MC, Yun J, Harb S, Puri R, Kapadia S, Krishnaswamy A, Chen PH, Reed G, Tang WHW. Low Muscle Mass by Preprocedural Computed Tomography Is Associated With Worse Short-Term Outcomes in Transcatheter Aortic Valve Replacement Recipients. Am J Cardiol. 2024;217:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Graffy PM, Lee SJ, Ziemlewicz TJ, Pickhardt PJ. Prevalence of Vertebral Compression Fractures on Routine CT Scans According to L1 Trabecular Attenuation: Determining Relevant Thresholds for Opportunistic Osteoporosis Screening. AJR Am J Roentgenol. 2017;209:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Bertschi D, Kiss CM, Schoenenberger AW, Stuck AE, Kressig RW. Sarcopenia in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI): A Systematic Review of the Literature. J Nutr Health Aging. 2021;25:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Tokuda T, Yamamoto M, Kagase A, Koyama Y, Otsuka T, Tada N, Naganuma T, Araki M, Yamanaka F, Shirai S, Mizutani K, Tabata M, Ueno H, Takagi K, Higashimori A, Watanabe Y, Hayashida K; OCEAN-TAVI Investigators. Importance of combined assessment of skeletal muscle mass and density by computed tomography in predicting clinical outcomes after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020;36:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Wang R, Tang H, Wang J, Chen X. Computed tomography (CT)-based osteosarcopenia evaluation during chest CT scans. J Endocrinol Invest. 2025;48:501-502. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Goel K, O'Leary JM, Barker CM, Levack M, Rajagopal V, Makkar RR, Bajwa T, Kleiman N, Linke A, Kereiakes DJ, Waksman R, Allocco DJ, Rizik DG, Reardon MJ, Lindman BR. Clinical Implications of Physical Function and Resilience in Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2020;9:e017075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Ribeiro GS, Melo RD, Deresz LF, Dal Lago P, Pontes MR, Karsten M. Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: Systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Nashimoto S, Inoue T, Hotta K, Sugito Y, Iida S, Tsubaki A. The safety of exercise for older patients with severe aortic stenosis undergoing conservative management: A narrative review. Physiol Rep. 2022;10:e15272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Imamura T. Implication of Exercise Training in Patients With Aortic Stenosis. Circ Rep. 2021;3:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Arai H, Nozoe M, Matsumoto S, Morimoto T. Exercise Training for Patients With Severe Aortic Stenosis in a Convalescent Rehabilitation Ward - A Retrospective Cohort Study. Circ Rep. 2021;3:361-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1043] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 49. | Cailleaux PE, Déchelotte P, Coëffier M. Novel dietary strategies to manage sarcopenia. Curr Opin Clin Nutr Metab Care. 2024;27:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Jang YJ. The Effects of Protein and Supplements on Sarcopenia in Human Clinical Studies: How Older Adults Should Consume Protein and Supplements. J Microbiol Biotechnol. 2023;33:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 51. | Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 801] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 52. | Iwamoto SJ, Rothman MS, T'Sjoen G, Defreyne J. Approach to the Patient: Hormonal Therapy in Transgender Adults With Complex Medical Histories. J Clin Endocrinol Metab. 2024;109:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Cho MR, Lee S, Song SK. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J Korean Med Sci. 2022;37:e146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 54. | Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, Swerdloff RS, Traish A, Zitzmann M, Cunningham G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 56. | Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2034] [Cited by in RCA: 1963] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 57. | Xiao X, Deng H, Li P, Sun J, Tian J. Statin for mood and inflammation among adult patients with major depressive disorder: an updated meta-analysis. Front Psychiatry. 2023;14:1203444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 58. | Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 2022;51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 59. | Stefanini G, Tartaglia F. See, Touch, Feel: The Need for Modern PCI in TAVR Patients. JACC Cardiovasc Interv. 2025;18:255-259. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL 2nd, DeVon HA, Alexander KP; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 145] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 61. | Li P. Comparative breakthrough: Umbilical cord mesenchymal stem cells vs bone marrow mesenchymal stem cells in heart failure treatment. World J Cardiol. 2024;16:776-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 62. | Chong ETJ, Ng JW, Lee P. Classification and Medical Applications of Biomaterials–A Mini Review. BIO Integration. 2023;4. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Li P, Jia N, Liu B, He Q. Effect of cardiac shock wave therapy plus optimal medical therapy on rehospitalization in patients with severe coronary artery disease: A meta-analysis and trial sequential analysis. Front Cardiovasc Med. 2022;9:1010342. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Dhoble A, Ahmed T, Mckay RG, Kliger C, Beohar N, Baron SJ, Hermiller JB. Timing and Outcomes of PCI in Conjunction With TAVR With Balloon-Expandable Valves. JACC Cardiovasc Interv. 2025;18:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Zendjebil S, Akodad M, Iung B, Dumonteil N, Cuisset T, le Breton H, Beurtheret S, du Chayla F, Leclère M, Sanguineti F, Hovasse T, Chevalier B, Neylon A, Eltchaninoff H, Garot P, Gilard M, Benamer H, Lefèvre T; STOP-AS and FRANCE-TAVI Investigators. Coronary Events After Transcatheter Aortic Valve Replacement: Insights From the France TAVI Registry. JACC Cardiovasc Interv. 2025;18:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Zhang X, Geng W, Yan S, Zhang K, Liu Q, Li M. Comparison of the outcomes of concurrent versus staged TAVR combined with PCI in patients with severe aortic stenosis and coronary artery disease: a systematic review and meta-analysis. Coron Artery Dis. 2024;35:481-489. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Ahmed A, Kaddoura R, Aggarwal A, Zinyandu T, Webber F, Davila C, Zarich S. Transcatheter Aortic Valve Replacement in Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Catheter Cardiovasc Interv. 2025;105:754-760. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Li P. Aficamten for Obstructive Hypertrophic Cardiomyopathy. N Engl J Med. 2024;391:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 69. | Biermann J, Horack M, Kahlert P, Schneider S, Nickenig G, Zahn R, Senges J, Erbel R, Wasem J, Neumann T. The impact of transcatheter aortic valve implantation on quality of life: results from the German transcatheter aortic valve interventions registry. Clin Res Cardiol. 2015;104:877-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Liu R, Fu Z, Yao J, Yan Y, Song G. Transcatheter Aortic Valve Replacement for Aortic Regurgitation–A Review. CVIA. 2023;8. [DOI] [Full Text] |
| 71. | Chen Z, Er Saw P. Integration in Biomedical Science 2024: Emerging Trends in the Post-Pandemic Era. BIO Integration. 2024;5. [DOI] [Full Text] |