TO THE EDITOR
Atrial fibrillation (AF) is the most common arrhythmia[1], affecting more than 40 million people worldwide[2]. This pathological condition is more frequently found in the elderly[3] and is related to a high risk for thromboembolic events and mortality[4]. AF also represents an important global health issue with substantial economic burden. AF healthcare costs are primarily due to hospitalizations, treatments, and management of complications such as stroke[5]. The prevalence of AF is increasing, particularly among the aging population, leading to substantial healthcare costs[6]. The rising prevalence of AF underscores the urgent need for improved risk stratification methods and advanced treatment strategies to effectively manage this condition and reduce its economic burden.
While several medical treatments are available as a first line of therapy to restore sinus rhythm, the use of radiofrequency catheter ablation (RFCA) is particularly indicated in symptomatic patients (palpitations, fatigue, shortness of breath, or exercise intolerance) in cases of AF refractory to medication[4]. RFCA is more effective in paroxysmal AF, while success rates in persistent AF are lower and variable[7]. Furthermore, RFCA can be especially useful in younger patients to avoid possible life-long medical treatment and AF-related or treatment-related complications. However, the rates of AF recurrence can be up to 45% within 12 months after RFCA[8], with higher recurrence rates in persistent AF compared to paroxysmal AF[9].
Advancements in bioinformatics have enabled the development of new immune scoring systems for medical applications, enhancing diagnosis, prognosis, and therapeutic options for various diseases, particularly in oncology, such as the classification of glioma subtypes[10]. Similarly, the development of high-quality predictive tools in cardiology could be invaluable for closely monitoring patients at higher risk of recurrence.
Risk scores, such as the CHADS-VASc[11], are widely used to assess stroke risk in patients with AF. However, these scores have limitations in fully capturing the multifactorial nature of AF. For instance, the CHADS2-VASc score primarily focuses on clinical factors like age, hypertension, and diabetes, but it does not account for other important variables such as inflammation. This limitation can lead to suboptimal risk predictions and management strategies. Incorporating novel biomarkers like the systemic immune-inflammation index (SII)[12] could enhance the predictive accuracy of these risk scores. SII has shown promise in identifying patients at higher risk of AF recurrence and adverse outcomes[13]. By integrating such biomarkers, clinicians can achieve a more comprehensive risk assessment, leading to better-tailored treatment plans and improved clinical outcomes.
BACKGROUND AND PRESENTATION OF THE PAPER
Here, we discuss the original article by Wang et al[14] entitled “Role of a new inflammation predictor in predicting recurrence of atrial fibrillation after radiofrequency catheter ablation” that was recently published in World Journal of Cardiology.
RFCA was introduced as a treatment for AF by Haïssaguerre M in the late 1990s and completely changed the outcome of many patients[15]. However, not all patients achieve long-term sinus rhythm maintenance after this procedure, and some may experience a recurrence of AF [8]. The recurrence of AF following RFCA is likely due to multiple factors. RFCA is a highly precise and technically demanding procedure, and even the most skilled cardiologists can sometimes perform an incomplete ablation if trigger areas are difficult to locate. This challenge is particularly pronounced when triggers are situated outside the pulmonary veins in regions that are not easily accessible during the initial ablation. Atrial remodeling, such as fibrosis and enlargement, can favor the recurrence of AF[16]. Obese[17], sleep apnea[18], hypertensive[19], and diabetes[20] patients have a higher risk of AF recurrence after RFCA. Inflammation is widely recognized as a significant factor contributing to AF recurrence[21]. Post-ablation inflammation often results from the natural healing process of heart tissue, which can create a temporary pro-inflammatory environment that may promote arrhythmogenicity.
Inflammation plays a critical role in the initiation and perpetuation of AF[22]. The underlying mechanisms include atrial remodeling, fibrosis, and autonomic dysfunction, which are central contributors to AF pathophysiology[23]. Inflammation can lead to structural changes in the atria, known as atrial remodeling. This process involves the enlargement and alteration of atrial tissue, which can disrupt normal electrical conduction and promote the development of AF[24]. Additionally, inflammatory processes contribute to the formation of fibrotic tissue within the atria. Fibrosis creates a substrate for AF by causing heterogeneous conduction and reentry circuits, which sustain the arrhythmia[25]. Furthermore, inflammation can affect the autonomic nervous system, leading to imbalances that favor AF. Autonomic dysfunction can increase atrial excitability and trigger abnormal electrical activity[26]. However, AF recurrence is also frequently linked to chronic, low-grade inflammation, particularly common in elderly patients. This persistent inflammatory state often stems from a phenomenon known as “inflammaging”—a progressive, age-related inflammatory condition.
Inflammaging arises from a complex interplay of factors, including metabolic dysregulation, mitochondrial dysfunction, and oxidative stress. Metabolic dysregulation in AF leads to an imbalance in energy supply and demand, causing cellular stress and promoting inflammatory pathways[27]. Mitochondrial dysfunction further exacerbates this condition by impairing adenosine triphosphate production and increasing the generation of reactive oxygen species, which damage cellular components and trigger inflammatory responses[28]. Oxidative stress, resulting from excessive reactive oxygen species, contributes to structural and electrical remodeling of the atria, perpetuating inflammation and creating a vicious cycle that sustains chronic inflammation in AF[29]. Indeed, pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, promote atrial remodeling in AF via structural and electrical changes in the atrial tissue, which can perpetuate the arrhythmia and complicate its management[30]. Genetic predisposition also plays a role along with chronic low-grade endotoxemia—a condition where bacterial endotoxins from the gut enter circulation, causing persistent immune activation[31]. Additionally, immunosenescence, or the gradual decline of the immune system with age, further contributes to the chronic inflammatory state, as does epigenetic alteration, which can modify gene expression patterns related to inflammation and immune response over time[32].
Together, these factors create a chronic inflammatory environment that facilitates AF recurrence and is also implicated in the pathogenesis of other cardiovascular diseases. In fact, inflammation is now regarded as one of the primary drivers of cardiovascular disease progression, influencing the development of conditions like atherosclerosis, hypertension, and heart failure[33]. Therefore, addressing inflammation is increasingly recognized as a critical component of AF management and cardiovascular disease prevention.
The SII is a novel marker of systemic inflammation that has shown promise in various clinical settings. The SII, calculated as the product between neutrophil and platelets count divided by lymphocytes count, is increasingly used to assess the inflammatory state of the body[12]. SII is simply derived from a complete blood count, making it easily accessible, cost-effective, and can be measured repeatedly to monitor inflammation over time. The SII has been employed in several clinical settings, such as cancer, showing a possible prediction of patients’ prognosis[12]. In the cardiovascular field, it can be used in patients undergoing percutaneous coronary intervention to assess those at high risk of complications[34]. However, its sensitivity and specificity can be influenced by acute infections, chronic inflammatory conditions, and other systemic diseases. Factors such as medication use, comorbidities, and individual variations in immune response can affect SII levels, potentially confounding its interpretation. Although SII is a valuable tool, its clinical applicability may be limited in certain scenarios where more specific biomarkers are required.
On the other hand, the APPLE score is a clinical tool specifically designed to evaluate the risk of AF recurrence after catheter ablation[35]. This score showed higher predictive efficacy compared to other scores like the CHADS2 and CHA2DS2-VASc scores[35].
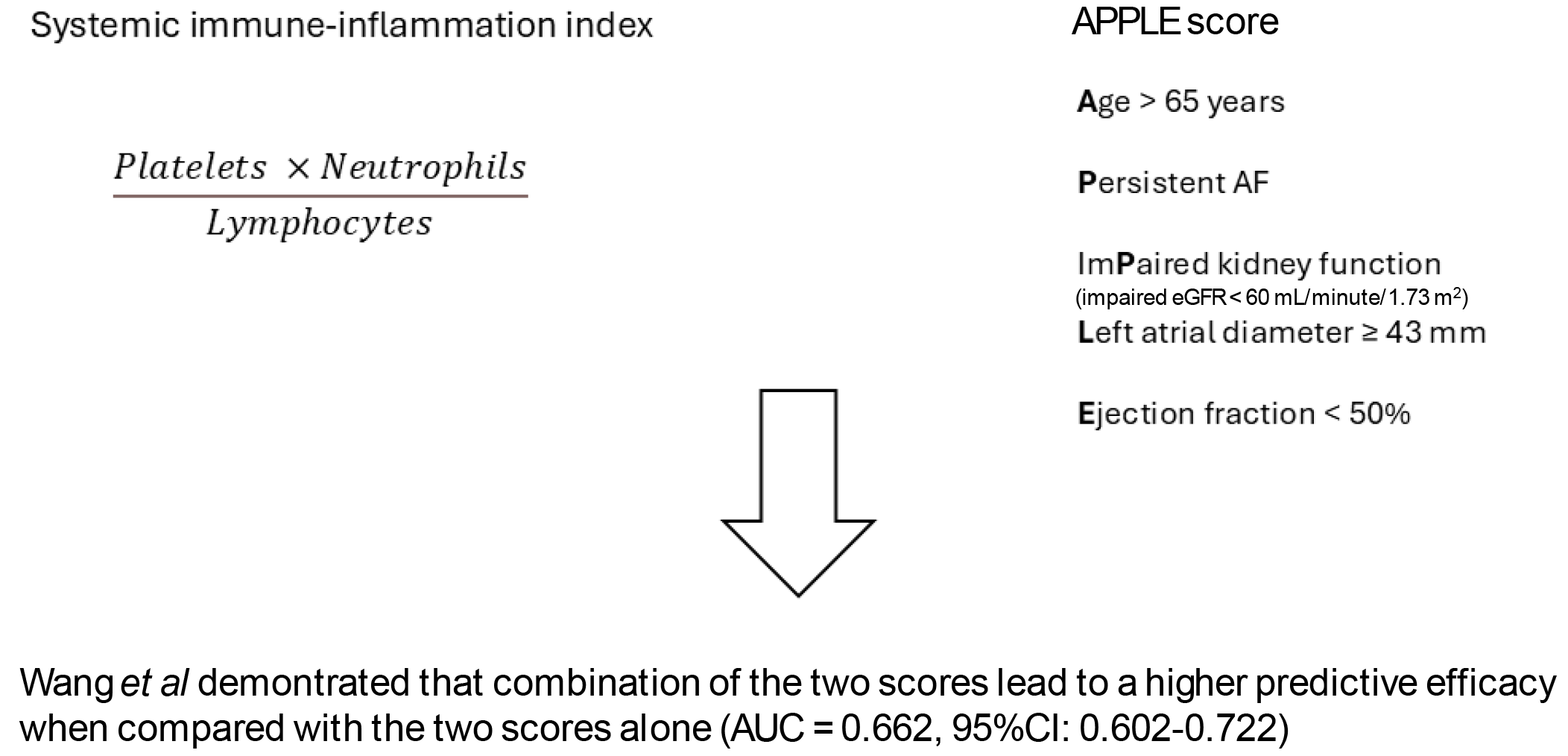
In their paper, Wang et al[14] investigated the possible predictive role of the SII on AF recurrence in patients that underwent RFCA. The authors retrospectively evaluated 457 patients with non-valvular AF that underwent a first RFCA. After subdivision of these patients according to the AF recurrence into two groups (recurrence, n = 113 and non-recurrence, n = 344), the authors evaluated the possible predictive role of SII and its additional predictive effect in combination with the APPLE score. They found that about a quarter of patients in the study experienced a recurrence of AF in a 12-month follow-up period. Patients with AF recurrence had significantly higher SII values compared to those without recurrence (516.11 ± 260.91 vs 428.37 ± 221.24). At the multivariate regression, the SII and APPLE score independently predicted AF recurrence [SII odds ratio (OR) = 2.257, 95%CI: 1.219-4.179, P = 0.001; and APPLE score: OR = 1.723, 95%CI: 1.338-2.219, P < 0.001], after adjustment for non-paroxysmal AF, left atrial diameter, B-type natriuretic peptide levels, estimated glomerular filtration rate, neutrophils and lymphocytes levels. In addition, combining SII with the APPLE score led to a higher predictive efficacy as showed by the receiver operating characteristic curve analyses [combined model area under the curve (AUC) = 0.662 with 95%CI: 0.602–0.722, P < 0.001; SII alone: AUC = 0.597 with 95%CI: 0.535–0.659, P < 0.001; APPLE score alone: AUC = 0.624 with 95%CI: 0.563-0.684, P < 0.001]. Overall, this study confirms the possible role of inflammation in contributing to AF recurrence after RFCA.
CONCLUSION
Implementing the SII in the APPLE score enhances the predictive efficacy for AF recurrence after catheter ablation, underscoring the role of inflammation as a crucial co-determinant of cardiovascular diseases (Figure 1). By incorporating SII into clinical practice, healthcare providers could adopt more intensive monitoring protocols for patients identified as high-risk. This could involve regular follow-up visits, frequent blood tests to monitor SII levels, and the use of advanced imaging techniques to detect early signs of atrial remodeling or fibrosis. Additionally, introducing prophylactic therapies based on SII levels could be beneficial. For instance, anti-inflammatory medications or lifestyle interventions aimed at reducing systemic inflammation might be considered for patients with elevated SII. These approaches could lead to better management and outcomes for patients at risk of AF recurrence by enabling early intervention and personalized treatment plans.
Figure 1 The implementation of the systemic immune-inflammation index in the APPLE score leads to a better prediction of atrial fibrillation recurrency after radiofrequency catheter ablation.
Atrial fibrillation (AF) recurrence can occur in up to 45% of patients after radiofrequency catheter ablation. The recurrence of AF in these patients is likely multifactorial. Inflammation is thought to play a key role in this setting. The systemic immune-inflammation index has been recently developed to evaluate the inflammatory status of the body. Conversely, the APPLE score has been developed to evaluate the risk of AF recurrence after radiofrequency catheter ablation. The study by Wang et al[14] proved that using the two scores together leads to a more precise evaluation of the AF recurrence risk. AF: Atrial fibrillation; AUC: Area under the curve; EGFR: Estimated glomerular filtration rate.
Moreover, integrating SII into the APPLE score has been shown to further enhance the prediction of AF recurrence. This combined approach leverages the strengths of both the APPLE score and SII, providing a more comprehensive risk assessment. Clinically, this integration could lead to personalized follow-up strategies, allowing for more tailored patient management and early intervention. However, the use of SII as an inflammatory marker has limitations, such as variability due to acute infections or other systemic conditions, which may affect its reliability. Large-scale studies are needed to confirm the clinical relevance of SII and to ensure its applicability across diverse patient populations. Such studies should aim to include a wide range of participants, considering different age groups, comorbidities, and ethnic backgrounds. Additionally, prospective studies with long-term follow-up are crucial to determine the consistency and reliability of SII as a predictive marker. By conducting comprehensive research, the medical community can better understand the potential of SII and refine its use in clinical practice, ultimately improving patient care and outcomes.